Abstract
Background and aim: Clinical guidelines recommend endoscopy surveillance at given intervals or endoscopic therapy for Barrett’s esophagus with low-grade dysplasia (LGD) and high-grade dysplasia (HGD). Whether these guidelines are followed in clinical practice is unknown and was assessed in this study.
Methods: This nationwide Swedish cohort study included patients with Barrett’s esophagus with histologically verified LGD or HGD from 50 centers in 2006–2013. These patients were followed up using nationwide registers. Adherence to clinical guidelines was explored. Eight potential risk factors for deviation from guidelines were assessed using multivariable logistic regression, providing adjusted odds ratios (OR) with 95% confidence intervals (95%CI).
Results: Among 211 patients with Barrett’s esophagus (mean age 67.0 years, standard deviation 9.7 years, 81% male), 71% had LGD and 29% had HGD. During median 3.9 years of follow-up, 84% underwent a follow-up endoscopy, 17% received endoscopic therapy and 8% underwent esophagectomy. The clinical management deviated from guidelines in 60% of all patients (69% in LGD and 39% in HGD), which was mainly due to under-surveillance (86%). Risk factors for deviation from guidelines were LGD compared to HGD (OR 3.4, 95%CI 1.7–6.8), longer Barrett’s segment length (OR 2.0, 95%CI 1.0–3.9, comparing ≥3 cm with <3 cm), and treatment at gastroenterology compared to surgery departments (OR 2.3, 95%CI 1.2–4.4). Age, sex, calendar period and university hospital status were not associated with deviation from surveillance guidelines.
Conclusions: Adherence to guidelines for dysplastic Barrett’s esophagus is poor, particularly for LGD. Efforts to implement clinical guideline recommendations are needed.
Introduction
Barrett’s esophagus is the precursor lesion to esophageal adenocarcinoma, a highly lethal tumor with rapidly increasing incidence [Citation1]. Esophageal adenocarcinoma develops in an orderly sequence from gastroesophageal reflux disease, to non-dysplastic Barrett’s esophagus, low-grade dysplasia (LGD), high-grade dysplasia (HGD) and finally to adenocarcinoma [Citation2]. Patients with Barrett’s esophagus should be regularly monitored by endoscopy with biopsies to assess whether LGD or HGD is present in order to prevent adenocarcinoma [Citation3]. Clinical guidelines recommend endoscopy surveillance at given intervals or endoscopic eradication therapy for patients with LGD or HGD without focal lesions [Citation3,Citation4], and endoscopic surveillance of Barrett’s esophagus prevents mortality in esophageal adenocarcinoma [Citation5]. Non-dysplastic Barrett’s may be monitored at longer intervals because the absolute risk of esophageal adenocarcinoma is low in these patients [Citation6,Citation7]. Adherence to guidelines for non-dysplastic Barrett’s esophagus have been reported to be poor, but no study has evaluated how well the guidelines specifically for dysplastic Barrett’s esophagus are followed in clinical practice, where the cancer risk is substantially higher than for non-dysplastic Barrett’s esophagus. This study aimed to help clarify the clinical adherence of recommended guidelines in patients with LGD or HGD and to identify risk factors for deviations from these guidelines.
Methods
Study design
This was a nationwide Swedish population-based cohort study during the study period 1 January 2006 to 31 December 2013. Study participants were recruited from all centers managing patients with dysplastic Barrett’s esophagus in Sweden during the study period. Data on risk factors and outcomes were collected from medical records and four nationwide health data registers. Ethical approval was obtained from The Regional Ethical Review Board in Stockholm, Sweden.
Study cohort
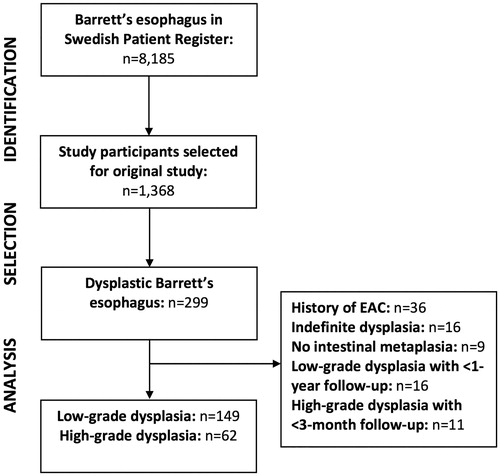
shows a flowchart of the identification and selection of the study participants. The source cohort included all 8185 patients aged >18 years with Barrett’s esophagus identified in the Swedish Patient Register (by the International Classification of Diseases [ICD] code K227) during the study period. As part of a case-control study nested within the source cohort, endoscopy and histopathology reports from 1368 patients with Barrett’s esophagus at 83 endoscopy centers were retrieved and reviewed [Citation8]. Based on this review, 299 patients with dysplastic Barrett’s esophagus, according to the histopathology reports, were considered for this study, identified from 50 centers. Among these 299 patients, we excluded those with a history of esophageal adenocarcinoma (n = 36), indefinite dysplasia (n = 16), dysplasia without evidence of specialized intestinal metaplasia (n = 9), and those ineligible for surveillance because of a too short follow-up due to death, early diagnosis of esophageal adenocarcinoma, or end of study period (n = 27). After these exclusions, 211 patients remained for final analysis.
Data sources
The endoscopy and histopathology reports provided data regarding indication for endoscopy, hiatal hernia, esophagitis, Barrett’s segment length, type of metaplasia and degree of dysplasia (LGD or HGD).
The Swedish Patient Register was used to identify all patients with Barrett’s esophagus for the source cohort as explained above, and to identify comorbidities, endoscopies, treating departments and hospitals. The Patient Register contains demographic and clinical data of all in-patient healthcare and all specialized out-patient healthcare in Sweden from 2001 onwards. The clinical data of interest included diagnoses, surgical and diagnostic procedures and hospitalizations. Data from the Swedish Patient Register have been validated for their usefulness in research [Citation9].
The Swedish Cancer Register was used to identify patients with esophageal adenocarcinoma. This register contains data of all incident cancers in Sweden since 1958, including information regarding site, tumor stage and histopathology. The register has been validated for the recording of esophageal adenocarcinoma with 98% completeness for recording, 98% completeness for tumor staging and 100% for histological confirmation [Citation10,Citation11].
The Swedish Cause of Death Register was used to assess mortality for descriptive and censoring purposes. The register records date and cause of all deaths of Swedish residents from 1952 onwards. The completeness of the register is almost 100% for both date of death and cause of death [Citation12].
The Swedish Prescribed Drug Register was used to identify participants using proton pump inhibitors. The register records dispensations, package sizes and doses of all prescribed medications in Sweden. The registration is automated, making the completeness and quality of the data excellent [Citation13].
Risk factors
Eight variables were considered as possible risk factors for deviation from guidelines: age, sex, comorbidity, degree of dysplasia, Barrett’s segment length, calendar period, type of hospital and type of department. Data on age were available from the endoscopy report. Data on sex and comorbidity were available from the Patient Register. Comorbidity was assessed based on the most recent version of the well-validated Charlson Comorbidity Index, a score based on specific ICD codes [Citation14]. Data on degree of dysplasia (LGD or HGD) were retrieved from the histopathology reports. Information about Barrett’s segment length was available from the endoscopy reports. Calendar period data were available from the endoscopy reports. Information about type of hospital and type of department was available from the Patient Register.
Outcomes
The outcome was deviation from recommended guidelines for dysplastic Barrett’s, which was defined depending on the degree of dysplasia. The recommended surveillance intervals and treatment regimens were derived from published guidelines just before or during the study period, which were all very uniform for dysplastic Barrett’s esophagus (Appendix) [Citation15–19]. For LGD, adherence to guidelines was defined as repeat endoscopy with biopsies within 6–12 months of the baseline assessment. For HGD, adherence to guidelines was defined as repeated endoscopy with biopsies, endoscopic eradication therapy or surgical resection (esophagectomy) within 3 months of baseline assessment. Any management other than this was classified as deviation from guidelines. Deviation was further categorized into under-surveillance and over-surveillance. Under-surveillance was defined as lack of surveillance or treatment after the recommended time interval, while over-surveillance was defined as surveillance before the recommended time interval (relevant for LGD only). Data on endoscopy with biopsies, endoscopic eradication therapy and esophagectomy were retrieved from the medical records or the Patient Register (details are given in the Appendix).
Statistical analysis
Follow-up started from the date of index endoscopy with biopsies and ended at death, diagnosis of esophageal adenocarcinoma or end of the study period, whichever occurred first. The data were explored for adherence to guidelines and also stratified by degree of dysplasia (LGD or HGD). The eight potential risk factors presented above were assessed using logistic regression to calculate crude and adjusted odds ratios (OR) with 95% confidence intervals (CI). In the analysis of a specific risk factor, all other seven potential risk factors were adjusted for in the model, with the following categorizations: age was categorized into <65 or ≥65 years, Barrett’s segment length into <3 or ≥3 cm, comorbidity as Charlson Comorbidity Index 0 or ≥1, calendar period of index endoscopy into <2010 or ≥2010, type of hospital into university hospital or non-university hospital, and type of department into surgical, gastroenterological or other. All statistical analyses adhered to a detailed study protocol that was agreed upon by all authors before the data collection. The data management and statistical analyses were performed in STATA (version IC 15.1, StataCorp, College Station, TX).
Results
Study participants
Among the 211 study participants with dysplastic Barrett’s esophagus, 149 (71%) had LGD and 62 (29%) had HGD. These patients were followed up for a median of 3.9 person-years (interquartile range 2.2–5.6 years). The mean age at baseline was 67.0 years (standard deviation 9.7 years, range 28.8–89.3 years) and 81% were male. Maximum (Prague M) and circumferential (Prague C) segment length were reported in 90 and 76%, respectively. Compared to patients with LGD, those with HGD were more likely to be older, male, have concomitant hiatal hernia or esophagitis, have longer circumferential Barrett’s segments, have more comorbidity, and use antireflux medication with a proton pump inhibitor ().
Table 1. Characteristics of 211 patients with dysplastic Barrett’s esophagus.
Adherence to guidelines
Deviation from guidelines occurred in 60% of all participants (). Deviation was more common in patients with LGD (69%) than in those with HGD (39%). Under-surveillance was the most common cause of deviation from guidelines (86%), while over-surveillance was less frequent (14%). Endoscopic eradication therapy was performed in 39% of participants with HGD and 8% of participants with LGD, while 19% of participants with HGD and 3% of participants with LGD underwent esophagectomy. Esophageal adenocarcinoma developed in four patients in the LGD group (6.5 cases per 1000 person-years) and four patients in the HGD group (16.3 cases per 1000 person-years). Inadequate surveillance was not associated with death during follow-up (OR 0.8, 95%CI 0.4–1.8).
Table 2. Clinical management of 211 patients with dysplastic Barrett’s esophagus.
Risk factors for deviation from guidelines
The results of the analyses of risk factors for deviation from guidelines are presented in . Age was not associated with deviation from guidelines (OR 1.1, 95% CI 0.6–2.1, comparing ages ≥65 and <65 years). Women had an increased point estimate of deviation from guidelines compared to men (OR 1.4, 95% CI 0.6–3.1), but this was not statistically significant. Likewise, the OR of deviation from guidelines was non-significantly increased in patients with more comorbidity (OR 1.7, 95% CI 0.8–3.5, comparing Charlson score ≥1 with 0). LGD was associated with an increased OR of deviation from guidelines compared to HGD (OR 3.4, 95% CI 1.7–6.8). Barrett’s maximum segment length was associated with increased OR of deviation from guidelines (OR 2.0, 95% CI 1.0–3.9, comparing ≥3 cm length with <3 cm). The OR of deviation from guidelines was non-significantly increased in those endoscope prior to 2010 compared to 2010 or later (OR 1.5, 95% CI 0.8–2.8). Management at a non-university hospital was associated with a non-significantly increased point estimate of deviation from guidelines compared to university hospitals (OR 1.3, 95% CI 0.5–2.8). Management at a gastroenterology department was associated with a 2-fold increased OR of deviation from guidelines compared to management in surgery departments (OR 2.3, 95% CI 1.2–4.4).
Table 3. Risk factors for deviation from guidelines in 211 patients with dysplastic Barrett’s esophagus.
Risk factors for under- and over-surveillance
Among the eight tested potential risk factors, management at a gastroenterology department was associated with an increased risk of under-surveillance (OR 1.8, 95% CI 1.1–3.4) (Supplementary Table 1) and age ≥65 years was associated with a decreased risk of over-surveillance (OR 0.3, 95% CI 0.1–0.9) (Supplementary Table 2). No other risk factors were statistically significantly associated specifically with under- or over-surveillance.
Discussion
This study indicates that adherence to clinical guidelines of dysplastic Barrett’s esophagus is poor, particularly in patients with LGD, who often were under-surveyed. Guidelines were more strictly enforced in surgery departments compared to gastroenterology departments, and patients with long-segment Barrett’s esophagus more often received inappropriate surveillance. The other studied potential risk factors were not statistically significantly associated with deviations from guidelines.
Methodological strengths of the study include the population-based design and the extensive data collection from medical records and histopathology reports, combined with data from nationwide high-quality health data registers. The diagnosis of dysplastic Barrett’s esophagus was validated through manual review of histopathology reports. The nationwide design ensured that patients who switched healthcare provider or moved house were still followed up. The study also has weaknesses. Despite the nationwide approach, the sample size was limited, which might explain why several increased point estimates were statistically non-significant. Some deviation from guidelines might have been due to factors impossible to assess in this study, e.g., patient frailty and refusal by the patient to participate. In addition, although the recording of data was prospective, the study was conducted after all data collection were completed, why some variables of interest were incompletely recorded, e.g., Barrett’s segment length.
This was to our knowledge the first study to evaluate adherence to clinical guidelines specifically of patients with dysplastic Barrett’s esophagus. Previous studies examining adherence to guidelines have mainly considered non-dysplastic Barrett’s esophagus, which represents the vast majority of Barrett’s patients. A large cohort study from the United States indicated that only a minority (23%) of non-dysplastic Barrett’s patients had regular surveillance, but could not identify any specific risk factor for deviation from guidelines [Citation20]. A recent United States single-center study found that only 16% of patients with non-dysplastic Barrett’s received appropriate surveillance, and risk factors for under-surveillance were male sex and comorbidity, while long-segment Barrett’s was associated with over-surveillance [Citation21]. Another study from the United States found that poor health insurance coverage was a risk factor for under-surveillance. This latter factor is not applicable to Sweden, where healthcare insurance is paid by taxes and equally distributed among all residents [Citation22]. A recent Danish study indicated poor endoscopic reporting and sampling of suspected Barrett’s esophagus [Citation23], and limited use of the widely recommended Prague criteria for assessing segment length and the Seattle protocol for tissue mapping [Citation24,Citation25].
The results of this study extend the findings of poor adherence to guidelines also to include patients with dysplastic Barrett’s esophagus. Yet, compared to studies investigating non-dysplastic Barrett’s esophagus, the guidelines were followed to a greater extent in dysplastic Barrett’s esophagus. Indeed, as participants with LGD were at increased risk of deviation from guidelines compared to HGD, adherence seems to improve with degree of dysplasia. This pattern is likely due to the higher incidence of esophageal adenocarcinoma for each of these conditions, from 0.2% annually in non-dysplastic Barrett’s esophagus, to 0.5% in LGD, and 5–10% in HGD [Citation26,Citation27]. Likewise, because Barrett’s segment length is a risk factor for neoplastic progression it may lead to over-surveillance [Citation21]. Also in this study, long-segment Barrett’s esophagus was more frequently associated with deviation from guidelines, indicating that surgeons and gastroenterologists manage these patients differently to patients with short-segment Barrett’s esophagus. Questionnaire data have indicated that most gastroenterologists (particularly at university hospitals) largely follow guideline recommendations for Barrett’s esophagus [Citation28], but that gastroenterologists often use sub-selection based on age, Barrett’s segment length, or presence of an ulcer or stricture [Citation29]. However, in a large cohort study from the United States, only 45% patients received a follow-up endoscopy, indicating a discrepancy between reported and actual management [Citation20]. Because gastroenterologists are often medically responsible for surveillance of Barrett’s esophagus, they might more often consciously deviate from guidelines, which was observed in this study.
Several of the studied potential risk factors for deviation from guidelines indicated increased point estimates but were statistically non-significant. Whether the lack of statistical significance was due to limited power or a lack of association remains to be clarified in further original studies or meta-analyses. Speculatively, deviations from guidelines might be more common in women than men because esophageal adenocarcinoma develops more often in men, and comorbidity associated with frailty and decreased life expectancy might decrease compliance. The tendency for improvement during later calendar periods is encouraging, but efforts are needed to improve compliance with recommended guidelines. While guidelines regarding surveillance interval and treatment remained largely unchanged during the study period, in recent years guidelines have advocated more use of endoscopic eradication therapy for HGD and in selected cases of LGD [Citation30]. This may help increase the adherence to guidelines.
The poor adherence to surveillance guidelines is troubling given the potentially lethal outcome of patients with dysplastic Barrett’s esophagus. Several observational studies suggest that surveillance of Barrett’s esophagus improves the prognosis of esophageal adenocarcinoma, although the improved outcomes may be a result of lead and length time bias [Citation31,Citation32]. However, high-quality data from randomized studies are lacking. Based on the premise that surveillance endoscopy for Barrett’s esophagus prevents death in esophageal adenocarcinoma, the results from this study highlight a potential to improve survival in this cancer.
Although this study does not provide any definitive area to target to improve the adherence to guidelines, it is worth noting that most patients were treated in low-volume departments. Given that dysplastic Barrett’s esophagus is uncommon, these patients should be referred to dedicated centers with equipment for detecting mucosal abnormalities and with the most updated treatment modalities available, including radiofrequency ablation and endoscopic resection. The quality of treatment improves with endoscopist volume: volume of radiofrequency ablation is associated with both decreased risk of incomplete eradication of the metaplasia [Citation33] and fewer number of sessions needed for complete eradication [Citation34]. It is likely that centralization of these patients will improve adherence to surveillance guidelines.
In conclusion, this first study evaluating the adherence to clinical guidelines in patients with dysplastic Barrett’s esophagus found that the guidelines were poorly followed, particularly for LGD and for long-segment Barrett’s, mainly due to under-surveillance. Management at surgery departments decreased the risk of deviation from guidelines. These findings indicate that the clinical management of patients with dysplastic Barrett’s esophagus need to be improved and efforts to better implement guidelines in clinical practice are warranted. By extension, improved adherence to guidelines may reduce the mortality in esophageal adenocarcinoma.
Author contributions
Dag Holmberg: Study concept and design, statistical analysis, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content.
Eivind Ness-Jensen: Study concept and design, critical revision of the manuscript for important intellectual content.
Fredrik Mattsson: Study concept and design, statistical analysis, analysis and interpretation of data.
Jesper Lagergren: Study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtained funding, administrative, technical, or material support, and study supervision.
Supplemental Material
Download PDF (85.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;22:31462–31469.
- Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371:836–845.
- Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol. 2016;111:30–50.
- Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63:7–42.
- Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068–2086.
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375–1383.
- Holmberg D, Ness-Jensen E, Mattsson F, et al. Risk of oesophageal adenocarcinoma in individuals with Barrett’s oesophagus. Eur J Cancer. 2017;75:41–46.
- Holmberg D, Ness-Jensen E, Mattsson F, et al. Clinical prediction model for tumor progression in Barrett's esophagus. Surg Endosc. 2018;1–8. https://doi.org/10.1007/s00464-018-6590-5
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:1–16.
- Lindblad M, Ye WM, Lindgren AS, et al. Disparities in the classification of esophageal and cardia adenocarcinomas and their influence on reported incidence rates. Ann Surg. 2006;243:479–485.
- Brusselaers N, Vall A, Mattsson F, et al. Tumour staging of oesophageal cancer in the Swedish cancer registry: a nationwide validation study. Acta Oncol. 2015;54:903–908.
- Brooke H, Talbäck M, Hörnblad J, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–773.
- Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register – opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–735.
- Brusselaers N, Lagergren J. The Charlson comorbidity index in registry-based research. Methods Inf Med. 2017;56:401–406.
- Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 2002;97:1888–1895.
- Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570.
- Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s Esophagus. Am J Gastroenterol. 2008;103:788–797.
- Spechler SJ, Sharma P, Souza RF, et al. American gastroenterological association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18–1091.
- Evans JA, Early DS, Fukami N, et al. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087–1094.
- El-Serag HB, Duan Z, Hinojosa-Lindsey M, et al. Practice patterns of surveillance endoscopy in a veterans affairs database of 29,504 patients with Barrett's esophagus. Gastrointest Endosc. 2012;76:743–755.
- Tavakkoli A, Appelman HD, Beer DG, et al. Use of appropriate surveillance for patients with non-dysplastic Barrett’s esophagus. Clin Gastroenterol Hepatol. 2018;16:862–869.
- Faqih A, Broman KK, Huang LC, et al. Frequency of endoscopic surveillance for Barrett’s esophagus is influenced by health insurance status: results from a population-based analysis. Dis Esophagus. 2017;30:1.
- Vogt JS, Larsen AC, Sommer T, et al. Quality of endoscopic surveillance of Barrett’s esophagus. Scand J Gastroenterol. 2018;53:256–259.
- Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392–1399.
- Levine DS, Blount PL, Rudolph RE, et al. Safety of a systematic endoscopic biopsy protocol in patients with Barrett’s esophagus. Am J Gastroenterol. 2000;95:1152–1157.
- Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394–398.
- Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79:897–909.
- Amamra N, Touzet S, Colin C, et al. Current practice compared with the international guidelines: endoscopic surveillance of Barrett’s esophagus. J Eval Clin Pract. 2007;13:789–794.
- Mandal A, Playford RJ, Wicks AC. Current practice in surveillance strategy for patients with Barrett’s oesophagus in the UK. Aliment Pharmacol Ther. 2003;17:1319–1324.
- di Pietro M, Fitzgerald RC. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett's oesophagus with low-grade dysplasia. Gut. 2018;2:392–393.
- El-Serag HB, Naik AD, Duan ZG, et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett’s oesophagus. Gut. 2016;65:1252–1260.
- Tramontano AC, Sheehan DF, Yeh JM, et al. The impact of a prior diagnosis of Barrett’s esophagus on esophageal adenocarcinoma survival. Am J Gastroenterol. 2017;4:82.
- Fudman DI, Lightdale CJ, Poneros JM, et al. Positive correlation between endoscopist radiofrequency ablation volume and response rates in Barrett's esophagus. Gastrointest Endosc. 2014;80:71–77.
- Pasricha S, Cotton C, Hathorn KE, et al. Effects of the learning curve on efficacy of radiofrequency ablation for Barrett’s esophagus. Gastroenterology. 2015;149:890–896.
Appendix 1 – diagnostic and procedural codes used in the study
Endoscopy
Nordic Medico-Statistical Committee Classification of Surgical Procedures:
Esophagoscopy: UJC02, UJC05, UJC12, UJC15
Gastroscopy: UJD02, UJD05
International Classification of Diseases, 10th Revision
Myocardial infarction: I21-I22, I12, I252
Heart failure: I11, I13, I255, I43, I50, I517
Peripheral vascular disease: I70-I73, I770, I771, K558, K559, Z958, Z959, K551, R02
Cerebrovascular disease: G45-G46, I60-I69
Dementia: F00-F03, G30-G31, A810, F051
Chronic obstructive pulmonary disease: I26-I27, J40-J47, J60-J67, J684, J701, J703
Rheumatic disease: M05-M06, M32-M36, M09, M120, M315
Liver disease: K70-K71, B18, I85, I864, I982, K721, K29, K76, R162, Z944
Diabetes (type 1 and 2): E10-E14
Hemi-/paraplegia: G81-G83, G114
Renal disease: N01, N03, N05, N07-N08, N171, N172, N18, N19, N25, Z49, Z940, Z992
AIDS/HIV: B20-B24
Malignancy: C00-C26, C30-C34, C37-C41, C43, C45-C58, C60-C76, C80-C85, C88, C90-C97
Metastatic tumors: C77-C79
International Classification of Diseases, 7th Revision
Esophageal cancer: 1500–1509
Cardia cancer: 1511
C24 Histology code
096 (adenocarcinoma)
Nordic Medico-Statistical Committee Classification of Surgical Procedures:
Endoscopic contact coagulation in esophagus: JCA35
Other endoscopic hemostatic procedure in esophagus JCA42
Endoscopic mucosal or submucosal resection in esophagus: JCA45
Other endoscopic procedure using diathermy or heat in esophagus, incl. destruction of lesion and
Incision of stenosis: JCA 52
Nordic Medico-Statistical Committee Classification of Surgical Procedures:
Esophagectomy: JCC00, JCC10, JCC11, JCC20, JCC30, JCC96 and JCC97
Partial gastrectomy: JDC00, JDC10, JDC11, JDC20, JDC30, JDC40, JDC96 and JDC97
Total gastrectomy: JDD00, JDD96
Appendix 2 – guidelines during the study period
Published guidelines for surveillance intervals and treatment regimens just before or during the study period.