Abstract
Duodenal perforation is a rare, but potentially life-threatening injury. Multiple etiologies are associated with duodenal perforations such as peptic ulcer disease, iatrogenic causes and trauma. Computed tomography with intravenous and oral contrast is the most valuable imaging technique to identify duodenal perforation. In some cases, surgical exploration may be necessary for diagnosis. Specific treatment depends upon the nature of the disease process that caused the perforation, the timing, location and extent of the injury and the clinical condition of the patient. Conservative management seems to be feasible in stable patients with sealed perforations. Immediate surgery is required for patients presenting with peritonitis and/or intra-abdominal sepsis. Minimally invasive techniques are safe and effective alternatives to conventional open surgery in selected patients with duodenal perforations. Here we review the current literature on duodenal perforations and discuss the outcomes of different treatment strategies.
Introduction
Duodenal perforation represents a rare but potentially life-threatening condition. The mortality rate ranges from 8% to 25% in published studies [Citation1–3]. The first description of a perforated duodenal ulcer was made in 1688 by Muralto and reported by Lenepneau [Citation4]. In 1894, Dean [Citation5] reported the first successful surgical closure of a perforated duodenal ulcer. Surgery is still the mainstay of treatment for duodenal perforation. Many perforations are repaired using an omental patch, a technique that was first described by Cellan-Jones in 1929 [Citation6] and was later modified by Graham in 1937 [Citation7]. The first laparoscopic repair for a perforated duodenal ulcer was reported in 1990 [Citation8].
The incidence of peptic ulcer disease has decreased in recent years [Citation9]. This can partly be explained by the use of proton pump inhibitors (PPIs) and eradication treatment for Helicobacter pylori. However, peptic ulcer complications, including perforation, still remain a substantial healthcare problem. This may be related to increased use of non-steroidal anti-inflammatory drugs (NSAIDs) and to the aging population [Citation3,Citation10]. Furthermore, iatrogenic duodenal perforations are becoming more common following the widespread use of endoscopic procedures, such as endoscopic retrograde cholangiopancreatography (ERCP) [Citation11].
Optimal methods for the management of duodenal perforations remain controversial. The diagnosis is often delayed leading to decreased survival. There are few randomized controlled studies and management strategies often rely on data from observational studies, or even case reports. One area of controversy includes the role of non-operative management. In patients that need surgery, there is still ongoing debate regarding type of repair, open or laparoscopic technique and the role of gastric diversion procedures, such as pyloric exclusion.
In this review, we provide an overview of duodenal perforations and potential management strategies based on available data.
Etiology
Underlying duodenal pathology
Peptic ulcer disease is a leading cause of duodenal perforation. Acute perforations of the duodenum are estimated to occur in 2–10% of patients with ulcers [Citation12]. The two major causes of peptic ulceration and perforation are H. pylori infection and NSAIDs. In patients with recurrent ulcers despite active treatment, hypersecretory states such as Zollinger-Ellison syndrome need to be considered.
Duodenal perforations can also occur in people with conditions such as duodenal diverticula [Citation13], duodenal ischemia [Citation14,Citation15], infectious disease [Citation16–18] and autoimmune conditions, including Crohn’s disease [Citation19], scleroderma [Citation20] and vasculitis (e.g., abdominal polyarteritis nodosa [Citation21]). Tumors may penetrate the duodenal wall directly or cause obstruction [Citation22]. Perforations can also be related to chemotherapy [Citation23,Citation24]. Impacted gallstones in the duodenum have also been associated with perforations [Citation25].
Iatrogenic perforations
Endoscopic perforations
Upper endoscopy may lead to iatrogenic perforations to the duodenum. The incidence of endoscopic perforations is higher for therapeutic procedures. The rate of duodenal perforations after ERCP ranges from 0.09 to 1.67% [Citation26,Citation27]. The Stapfer classification has been developed to categorize ERCP-related perforations [Citation28]. Type I perforations are large lateral or medial duodenal wall perforations usually caused by the endoscope itself. Type II perforations, also known as peri-Vaterian injuries, are related to the sphincterotomy. Type III perforations represent distal bile duct injuries caused by wire or basket instrumentation, while type IV perforations represent retroperitoneal air alone on imaging and are often asymptomatic. Risk factors for ERCP-related perforations have been reported to include old age, sphincter of Oddi dysfunction, precut, intramural injection of contrast medium and anatomical abnormalities, such as Billroth II gastrectomy [Citation29,Citation30].
Operative injury
Duodenal injuries may be caused by surgical instrumentation. They may go unnoticed during the initial operation and manifest themselves several days later as a delayed perforation a consequence of coagulation necrosis of the duodenal wall. Laparoscopic cholecystectomy is one of the most common surgical procedures in general surgery. In a series of 77,604 patients undergoing laparoscopic cholecystectomy, a total of 12 duodenal injuries (0.015%) were reported [Citation31]. In the world literature, 74 cases of duodenal injury after laparoscopic cholecystectomy have been identified [Citation32]. The mechanisms of injury were mainly related to thermal burns by electrocautery or by sharp or blunt dissection.
Trauma
Traumatic injuries to the duodenum are uncommon, representing less than 2% of all abdominal injuries [Citation33]. The majority of these traumatic lesions are due to penetrating mechanisms. Isolated duodenal injuries are rare. Duodenal injuries often occur together with other organ injuries and damages to large vessels [Citation34].
Foreign bodies
Ingested foreign bodies generally pass through the gastrointestinal tract without complications. Less than 1% cause perforations [Citation35–38]. Sharp and thin foreign bodies have been associated with a higher perforation risk. Implanted foreign bodies such as endoprosthesis [Citation39] or artificial vascular grafts [Citation40,Citation41] can cause erosion into the duodenum leading to fistula and abscess formation or vasculo-enteric fistulas.
Spontaneous perforations
This type of perforation occurs in neonates. The underlying cause remains unknown [Citation42].
Diagnosis
Perforation of the duodenum is defined as a transmural injury to the duodenal wall. A partial thickness laceration may over time develop into a transmural injury. Duodenal perforation can cause acute pain associated with free perforation, or less acute symptoms associated with abscess or fistula formation.
Perforation of the duodenum with spillage of intraluminal contents into the peritoneal cavity causes acute chemical peritonitis. This is followed by a systemic inflammatory response syndrome (SIRS), which can progress to secondary bacterial peritonitis and sepsis. Patients with retroperitoneal perforation may lack peritoneal signs and present more indolently.
Double-contrast computed tomography (CT) scan is the most valuable method for diagnosing duodenal perforation. It should be performed whenever there is a clinical suspicion and the patient does not need immediate surgery. CT features of perforation include discontinuity of the duodenal wall and the presence of extraluminal air or extravasated oral contrast. Other CT findings include duodenal wall thickening, fat stranding and periduodenal fluid collection [Citation43].
Treatment
Management of duodenal perforations includes conservative, endoscopic and surgical strategies (). The main goals of treatment are resuscitation, control of infection, nutritional support and restoration of gastrointestinal tract continuity.
Figure 1. A general management algorithm for duodenal perforations. Abbreviations: NG: nasogastric; NPO: nil per os; OTSC: over-the-scope clip; PPI: proton pump inhibitor; SEMS: self-expandable metal stent; TTSC: through-the-scope clip.
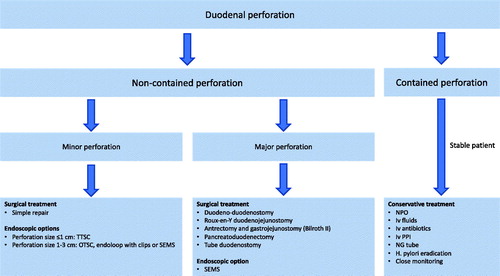
Conservative treatment
Initial conservative management consists of nil per os, intravenous fluid therapy, broad-spectrum antibiotics, intravenous PPIs, nasogastric tube insertion and H. pylori eradication. The added value of somatostatin remains controversial. However, there are some data to support the benefit of somatostatin for enterocutaneous fistula closure [Citation44].
Non-operative management of perforated duodenal ulcers is feasible in selected patients. Perforated ulcers may seal spontaneously with fibrin, omentum or by fusion of the duodenum to the underside of the liver between the gallbladder and the falciform ligament [Citation45]. Approximately, 50–70% of patients with perforated peptic ulcers respond to conservative treatment without surgery [Citation46,Citation47]. For patients undergoing conservative treatment, a gastroduodenogram may be performed soon after admission to investigate if there is any contrast extravasation. Conservative management seems to be safe if the gastroduodenogram shows self-sealing [Citation48].
Operative management is usually recommended if there is free leakage of contrast medium into the peritoneal cavity. Progressive abdominal signs or intra-abdominal sepsis should warrant surgery.
In high-risk patients, who cannot tolerate surgical treatment, conservative management may also include percutaneous drainage of fluid collections [Citation49].
Endoscopic management
Endoscopic treatment is an attractive treatment modality due to its minimally invasive nature. Early endoscopic closure (<24 h) is considered to be technically easier because the inflammatory changes are less pronounced [Citation50].
TTSC
Through-the-scope clips (TTSC) can be used for endoscopic closure of small duodenal perforations. Linear perforations <1 cm are most suitable for the use of TTSC [Citation50,Citation51].
OTSC
In contrast to common endoscopic clips, the over-the-scope clips (OTSC) are able to compress larger quantities of tissue. The OTSC system is shaped like a bear trap to enable a full-thickness closure of the tissue. The OTSC technique can be used for perforations ranging from 1 to 3 cm. OTSC treatment has been shown to be effective for peptic ulcer perforations, with few complications [Citation52].
Endoloop with clips
A combined technique using TTSC and an endoloop can be used if the OTSC technique is unavailable [Citation53].
SEMS
Self-expandable metal stents (SEMS) are alternative endoscopic treatment options for duodenal perforations [Citation50,Citation51,Citation54].
Surgical treatment
The choice of surgical treatment depends on the size and localization of the perforation, the viability of the duodenal walls, the degree of local contamination and underlying etiology.
Simple surgical repair
The main surgical treatment is simple repair of the perforation site. This can be performed as a primary closure with or without the addition of an omental patch. Alternatively, a pedicled omental flap (Cellan–Jones repair) [Citation6] or free omental plug (Graham patch) [Citation7] can be sutured into the perforation. Sutureless techniques have also been developed using a gelatin sponge and fibrin glue to seal off the perforation [Citation55]. There seem to be no significant differences in terms of postoperative morbidity and mortality rates when comparing primary closure, omentopexy or tegmentation (without closure) [Citation55–57]. Surgical repair can be performed either with conventional open surgery or with laparoscopy. The results of a recent meta-analysis including seven randomized controlled trials showed a significant benefit for the laparoscopic approach for the treatment of perforated peptic ulcer disease with a significant reduction in postoperative complications and hospital stay [Citation58].
Abdominal drains
The routine placement of abdominal drains after surgical repair is controversial. The literature suggests no benefit in preventing postoperative fluid collections or abscesses [Citation59]. Furthermore, drains may be associated with increased morbidity such as drain wound site infection.
Pyloric exclusion
Pyloric exclusion involves surgical repair of the duodenum, gastrotomy and closure of the pylorus from within and finally the formation of a gastrojejunostomy. The rationale behind this procedure is to divert all gastric and biliary secretions from the duodenum. The added benefit of using a gastric diversion procedure such as pyloric exclusion for duodenal perforations has been questioned in recent years. Importantly, the procedure has been associated with more postoperative complications and longer hospital stay compared to simple repair without pyloric exclusion [Citation60–62].
Reconstructive surgery
For large duodenal perforations, a duodeno-duodenostomy may be necessary [Citation33]. If this is not possible, a Roux-en-Y duodenojejunostomy may be performed over the perforation. A Billroth II operation may be necessary if the perforation is to the first or proximal second portion of the duodenum. If the duodeno-pancreatic head complex is destroyed, a pancreaticoduodenectomy may be necessary [Citation63].
Tube duodenostomy
Tube duodenostomy is a damage control procedure for large duodenal perforations when other repair techniques are not possible due to the magnitude of duodenal damage, hemodynamic instability of the patient or the lack of surgical expertise for complex reconstruction [Citation64]. The perforation is sutured around a catheter inserted into the perforation to enhance directed fistulation of the perforation. The catheter is removed after a minimum of 6 weeks. A feeding jejunostomy may be placed for enteral nutritional support.
Prognostic factors
The main prognostic factor remains the time interval between the perforation and treatment. Mortality increases when the delay is greater than 24 h [Citation3,Citation11,Citation65]. Other prognostic factors have been reported but are mainly related to clinical signs of sepsis, such as increased Acute Physiology and Chronic Health Evaluation II (APACHE II) score [Citation65,Citation66]. Old age and co-morbidity are also strong adverse prognostic factors [Citation65].
Conclusion
Duodenal perforation is caused by a variety of different mechanisms. Some duodenal perforations can be managed conservatively, while others require prompt surgical treatment. The type of treatment should be individualized and depends on the mechanism of injury, the timing, location and extent of the injury and the clinical state of the patient. Open surgery is still the gold standard for patients that need surgical intervention and most duodenal perforations can be managed with a simple repair of the defect. Gastric diversion procedures such as pyloric exclusion have been used for many years to treat duodenal perforations, but there is little evidence to support any benefit. Minimally invasive treatments are slowly emerging as alternative methods to open surgery in the treatment of duodenal perforation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Machado NO. Management of duodenal perforation post-endoscopic retrograde cholangiopancreatography. When and whom to operate and what factors determine the outcome? A review article. JOP. 2012;13:18–25.
- Moller MH, Adamsen S, Thomsen RW, et al. Multicentre trial of a perioperative protocol to reduce mortality in patients with peptic ulcer perforation. Br J Surg. 2011;98:802–810.
- Lau JY, Sung J, Hill C, et al. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84:102–113.
- Lenepneau I. Cas de perforation der duodenum de lien d’une ancienne cicatrice de cet intestina. Gaz Hop. 1839;35:137.
- Dean HP. A case of perforation of a chronic ulcer of the duodenum successfully treated by excision: death two months later from acute intestinal obstruction by a band. Br Med J. 1894;1:1014–1015.
- Cellan-Jones CJ. A rapid method of treatment in perforated duodenal ulcer. Br Med J. 1929;1:1076–1077.
- Graham RR. The treatment of perforated duodenal ulcers. Surg Gynecol Obstet. 1937;64:235–238.
- Mouret P, Francois Y, Vignal J, et al. Laparoscopic treatment of perforated peptic ulcer. Br J Surg. 1990;77:1006.
- Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009;29:938–946.
- Soreide K, Thorsen K, Soreide JA. Strategies to improve the outcome of emergency surgery for perforated peptic ulcer. Br J Surg. 2014;101:e51–e64.
- Cirocchi R, Kelly MD, Griffiths EA, et al. A systematic review of the management and outcome of ERCP related duodenal perforations using a standardized classification system. Surgeon. 2017;15:379–387.
- Behrman SW. Management of complicated peptic ulcer disease. Arch Surg. 2005;140:201–208.
- Thorson CM, Paz Ruiz PS, Roeder RA, et al. The perforated duodenal diverticulum. Arch Surg. 2012;147:81–88.
- Haruna L, Aber A, Rashid F, et al. Acute mesenteric ischemia and duodenal ulcer perforation: a unique double pathology. BMC Surg. 2012;12:21.
- Kim SI, Jin YJ, Cho SG, et al. Duodenal perforation and esophageal ischemia following transarterial chemoembolization for hepatocellular carcinoma: a case report. Medicine. 2016;95:e3987.
- Ueda N. Gastroduodenal perforation and ulcer associated with rotavirus and norovirus infections in japanese children: a case report and comprehensive literature review. Open Forum Infect Dis. 2016;3:ofw026.
- Berney T, Badaoui E, Totsch M, et al. Duodenal tuberculosis presenting as acute ulcer perforation. Am J Gastroenterol. 1998;93:1989–1991.
- Sarmast AH, Parray FQ, Showkat HI, et al. Duodenal perforation with an unusual presentation: a case report. Case Rep Infect Dis. 2011;2011:512607.
- Katz S, Talansky A, Kahn E. Recurrent free perforation in gastroduodenal Crohn’s disease. Am J Gastroenterol. 1983;78:722–725.
- Ebert EC, Ruggiero FM, Seibold JR. Intestinal perforation. A common complication of scleroderma. Dig Dis Sci. 1997;42:549–553.
- Tun M, Malik AK. Massive small bowel infarction and duodenal perforation due to abdominal polyarteritis nodosa: a case report. Malays J Pathol. 1994;16:75–78.
- Negoi I, Paun S, Hostiuc S, et al. Most small bowel cancers are revealed by a complication. Einstein. 2015;13:500–505.
- Chao TC, Jeng LB, Jan YY, et al. Spontaneous gastroduodenal perforation in cancer patients receiving chemotherapy. Hepatogastroenterology. 1998;45:2157–2160.
- Vaidya R, Habermann TM, Donohue JH, et al. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 2013;24:2439–2443.
- Thomas TL, Jaques PF, Weaver PC. Gallstone obstruction and perforation of the duodenal bulb. Br J Surg. 1976;63:131–132.
- Dubecz A, Ottmann J, Schweigert M, et al. Management of ERCP-related small bowel perforations: the pivotal role of physical investigation. Can J Surg. 2012;55:99–104.
- Rabie ME, Mir NH, Al Skaini MS, et al. Operative and non-operative management of endoscopic retrograde cholangiopancreatography-associated duodenal injuries. Ann R Coll Surg Engl. 2013;95:285–290.
- Stapfer M, Selby RR, Stain SC, et al. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. 2000;232:191–198.
- Enns R, Eloubeidi MA, Mergener K, et al. ERCP-related perforations: risk factors and management. Endoscopy. 2002;34:293–298.
- Loperfido S, Angelini G, Benedetti G, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1–10.
- Deziel DJ, Millikan KW, Economou SG, et al. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9–14.
- Machado NO. Duodenal injury post laparoscopic cholecystectomy: incidence, mechanism, management and outcome. World J Gastrointest Surg. 2016;8:335–344.
- Malhotra A, Biffl WL, Moore EE, et al. Western trauma association critical decisions in trauma: diagnosis and management of duodenal injuries. J Trauma Acute Care Surg. 2015;79:1096–1101.
- Pandey S, Niranjan A, Mishra S, et al. Retrospective analysis of duodenal injuries: a comprehensive overview. Saudi J Gastroenterol. 2011;17:142–144.
- Velitchkov NG, Grigorov GI, Losanoff JE, et al. Ingested foreign bodies of the gastrointestinal tract: retrospective analysis of 542 cases. World J Surg. 1996;20:1001–1005.
- Cho EA, Lee du H, Hong HJ, et al. An unusual case of duodenal perforation caused by a lollipop stick: a case report. Clin Endosc. 2014;47:188–191.
- Gardner AW, Radwan RW, Allison MC, et al. Double duodenal perforation following foreign body ingestion. BMJ Case Rep. 2017;2017.
- Dalrymple RA, Berry K, Jester I. A sharp lesson: duodenal perforation 2 months after ingestion of a pin. J Indian Assoc Pediatr Surg. 2017;22:179–180.
- Kusters PJ, Keulen ET, Peters FP. Duodenal perforation following bile duct endoprosthesis placement. Endoscopy. 2014;46:E646–E647.
- Saratzis N, Saratzis A, Melas N, et al. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther. 2008;15:441–448.
- Malgor RD, Labropoulos N. A systematic review of symptomatic duodenal perforation by inferior vena cava filters. J Vasc Surg. 2012;55:856–861.e3.
- Nazzal M, Kaidi A, Lee YM. Spontaneous duodenal perforation in neonates: a case report and review of literature. Am Surg. 1996;62:706–708.
- Kim SH, Shin SS, Jeong YY, et al. Gastrointestinal tract perforation: MDCT findings according to the perforation sites. Korean J Radiol. 2009;10:63–70.
- Rahbour G, Siddiqui MR, Ullah MR, et al. A meta-analysis of outcomes following use of somatostatin and its analogues for the management of enterocutaneous fistulas. Ann Surg. 2012;256:946–954.
- Donovan AJ, Vinson TL, Maulsby GO, et al. Selective treatment of duodenal ulcer with perforation. Ann Surg. 1979;189:627–636.
- Crofts TJ, Park KG, Steele RJ, et al. A randomized trial of nonoperative treatment for perforated peptic ulcer. N Engl J Med. 1989;320:970–973.
- Songne B, Jean F, Foulatier O, et al. Non operative treatment for perforated peptic ulcer: results of a prospective study. Ann Chir. 2004;129:578–582.
- Berne TV, Donovan AJ. Nonoperative treatment of perforated duodenal ulcer. Arch Surg. 1989;124:830–832.
- Saber A, Gad MA, Ellabban GM. Perforated duodenal ulcer in high risk patients: is percutaneous drainage justified? North Am J Med Sci. 2012;4:35–39.
- Paspatis GA, Dumonceau JM, Barthet M, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2014;46:693–711.
- Jung Y. Management of gastrointestinal tract perforations. Gastrointest Interv. 2017;6:157–161.
- Wei JJ, Xie XP, Lian TT, et al. Over-the-scope-clip applications for perforated peptic ulcer. Surg Endosc. 2019. [Epub ahead of print].
- Nakagawa Y, Nagai T, Soma W, et al. Endoscopic closure of a large ERCP-related lateral duodenal perforation by using endoloops and endoclips. Gastrointest Endosc. 2010;72:216–217.
- Bergstrom M, Arroyo Vazquez JA, Park PO. Self-expandable metal stents as a new treatment option for perforated duodenal ulcer. Endoscopy. 2013;45:222–225.
- Lau WY, Leung KL, Kwong KH, et al. A randomized study comparing laparoscopic versus open repair of perforated peptic ulcer using suture or sutureless technique. Ann Surg. 1996;224:131–138.
- Lin BC, Liao CH, Wang SY, et al. Laparoscopic repair of perforated peptic ulcer: simple closure versus omentopexy. J Surg Res. 2017;220:341–345.
- Abd Ellatif ME, Salama AF, Elezaby AF, et al. Laparoscopic repair of perforated peptic ulcer: patch versus simple closure. Int J Surg. 2013;11:948–951.
- Quah GS, Eslick GD, Cox MR. Laparoscopic repair for perforated peptic ulcer disease has better outcomes than open repair. J Gastrointest Surg. 2019;23:618–625.
- Pai D, Sharma A, Kanungo R, et al. Role of abdominal drains in perforated duodenal ulcer patients: a prospective controlled study. Aust N Z J Surg. 1999;69:210–213.
- Seamon MJ, Pieri PG, Fisher CA, et al. A ten-year retrospective review: does pyloric exclusion improve clinical outcome after penetrating duodenal and combined pancreaticoduodenal injuries? J Trauma. 2007;62:829–833.
- Cruvinel Neto J, Pereira BM, Ribeiro MA, Jr., et al. Is there a role for pyloric exclusion after severe duodenal trauma? Rev Col Bras Cir. 2014;41:228–231.
- DuBose JJ, Inaba K, Teixeira PG, et al. Pyloric exclusion in the treatment of severe duodenal injuries: results from the National Trauma Data Bank. Am Surg. 2008;74:925–929.
- Cogbill TH, Moore EE, Feliciano DV, et al. Conservative management of duodenal trauma: a multicenter perspective. J Trauma. 1990;30:1469–1475.
- Kutlu OC, Garcia S, Dissanaike S. The successful use of simple tube duodenostomy in large duodenal perforations from varied etiologies. Int J Surg Case Rep. 2013;4:279–282.
- Moller MH, Adamsen S, Thomsen RW, et al. Preoperative prognostic factors for mortality in peptic ulcer perforation: a systematic review. Scand J Gastroenterol. 2010;45:785–805.
- Lee FY, Leung KL, Lai BS, et al. Predicting mortality and morbidity of patients operated on for perforated peptic ulcers. Arch Surg. 2001;136:90–94.
