Abstract
Objective: Determine diagnostic accuracy of a quantitative faecal immunochemical haemoglobin test (QuikRead go® FIT, Orion Diagnostica Oy) in symptomatic patients referred for colonoscopy, at various cut-offs and for one or two tests.
Methods: Patients referred to four endoscopy units in mid-Sweden between 2013 and 2017 provided information on lower abdominal symptoms and faecal samples from two separate days prior to colonoscopy.
Results: In all, 5.4% (13/242) patients had colorectal cancer (CRC). For one FIT at cut-off 10 µg Hb/g faeces, sensitivity for CRC was 92% (95% CI 78–100%) and specificity 77% (95% CI 72–83%); equal to 74%; 95% CI 68–80 (178/242) colonoscopies potentially avoidable and one CRC missed. Based on the maximal outcome of two FITs, sensitivity was 100%, specificity 71% (66–77%) and 68%; 95% CI 62–74 (160/237) colonoscopies potentially avoidable. Among 17% (42/242) patients with one FIT of >200 µg Hb/g faeces, 85% (11/13) had CRC. Positive predictive values of FIT varied 16.9–26.2% depending on cut-off and one or two FITs, whereas NPVs were 99% and above in all scenarios.
In 60 patients reporting rectal bleeding, one FIT at cut-off 10 µg Hb/g discriminated well between CRC and other conditions (p = .001). In regression models, FIT was more important than age, sex and all symptoms.
Conclusion: One or two FITs in symptomatic patients referred for colonoscopy imply powerful risk stratification abilities for CRC, even among patients reporting rectal bleeding. Larger studies in various settings will clarify how to make the best use of this opportunity.
Trial registration: Clinicaltrails.gov NCT 02491593
Introduction
Colorectal cancer (CRC) is the third most common cancer diagnosed in 1.8 million patients annually and it is the second cause of cancer-related deaths worldwide [Citation1]. Most CRC patients are diagnosed after a thorough workup of symptoms, but the association between lower abdominal symptoms and CRC is generally weak. The positive predictive values for constipation, diarrhoea and abdominal pain in primary care is estimated to 1% or even lower [Citation2], whereas the association with rectal bleeding is stronger and the PPV 3–4% among elderly men [Citation2,Citation3]. The pressure on diagnostic capacities to investigate all low risk but not no-risk patients are high. Recent observations suggest an increasing incidence of CRC at younger age [Citation4] and consequently, age will be a less reliable factor for selection purposes for bowel examinations.
Lately, quantitative faecal immunochemical tests (FIT) have emerged as a promising tool for selection purposes and several studies have reported on low faecal hemoglobin (f-Hb) concentration as a potential rule-out test for colorectal cancer in symptomatic patients referred for colonoscopy [Citation5–8]. The objective of this study was to determine the diagnostic accuracy of a new quantitative FIT in such a population of patients and, secondly, to estimate the relative importance of lower abdominal symptoms in relation to the FIT outcome in patients referred for colonoscopy.
Material and methods
Participants, inclusion and sample collection
This is a prospective study in symptomatic patients referred for colonoscopy at four endoscopy units (Eskilstuna General district hospital, Örebro University hospital, Aleris Handen and Hötorget Endoscopy centre, Stockholm) in mid-Sweden. Patients were included from November 2013 to March 2017, but recruitment periods differed between the sites. Adult patients (>18 years) referred from primary care or local hospitals for a colonoscopy investigating lower abdominal symptoms according to the referral letter were eligible. Eligible patients were sent an invitation to participate in the study along with the appointment letter for their colonoscopy. Invitations had to be interrupted during clinically busy periods and the study participants therefore represent a convenience series. Invited patients were contacted by phone a few days later by a research nurse who provided further information and ascertained patients fulfilled the inclusion criteria of reporting symptoms associated with the colonoscopy. For those who accepted participation, a specific case report form was used by the nurse to collect information on the nature of recent symptoms (abdominal pain, bloating, rectal bleeding and rectal mucus, change in bowel habits, weight loss). Patients could report one or several symptoms. They were also asked about medication with aminosalicylic acid or any anticoagulants. The research nurse was blinded to all other aspects of the study.
Consenting patients were sent two FIT sampling devices (QuikRead go® FOB Sampling set, Orion Diagnostica Oy), transportation tubes, pre-paid envelopes, written and pictorial sampling instructions and a consent form to be signed and returned. No recommendations on diet or the use of drugs were given. Patients collected two samples from bowel movements of separate days. They returned the samples on the day of collection, or were instructed to keep them in the refrigerator for at most three days.
Faecal haemoglobin concentration
Samples were sent to Unilabs hospital laboratory, Eskilstuna, Sweden and analysed by the laboratory staff on the day of arrival using the QuikRead go® instrument (Orion Diagnostica Oy). Calibration and analyses were performed according to instructions provided by the manufacturer. Results were provided in the range of 15- >200 µg Hb/g faeces [Citation9]. A supplementary analysis of the obtained haemoglobin absorbances was performed by Orion Diagnostica to provide a numerical assessment of f-Hb concentration ranging between 10–15 µg Hb/g faeces. Concentrations below this interval were reported as <10 µg Hb/g. The laboratory staff was blinded to all clinical information. A checklist for reporting on faecal immunochemical tests is provided in Appendix A [Citation10].
Reference standard
Colonoscopy reports for included patients were collected and scrutinized by one researcher (GT), blinded to the FIT outcome. The endoscopists were also blinded to the FIT outcome and in most cases not aware of the patient participating in the study. The overwhelming majority of colonoscopies were performed by specialists in gastroenterology, followed by endoscopically experienced surgeons and some 5% by fellowship gastroenterologists under surveillance of their senior colleagues. The study relied on clinical judgement insofar that any examination would be interrupted if bowel cleansing was considered too poor. Information on bowel cleansing was extracted from the written colonoscopy reports.
Information on intraluminal findings as well as completeness of the examination (intubation of caecum or ileum) was extracted from records. Biopsy specimens were sent for pathology assessment and the outcome extracted from pathology reports. The pathologists were not aware of the study. Any endoscopic finding of CRC was confirmed by pathology reports. Findings of adenoma were categorized based on the number and size approximation by endoscopists, and grade of dysplasia and cellular architecture as assessed by the pathologists. Advanced adenoma (AA) was classified as; 3 or more adenomas, one adenoma 10 mm or larger, and adenoma with high-grade dysplasia or villous architecture. All other patients with a microscopically confirmed adenoma were classified as ‘non-advanced adenoma’ (NAA). If a patient had several neoplastic findings, they were categorized according to the most advanced neoplastic lesion. The findings of colitis were based on the macroscopic appearance as described undoubtedly in the colonoscopy reports.
Sample size
We initially performed a pilot study at the Endoscopy unit, Eskilstuna GDH in 2013 on twenty patients (not included in the present study). We found that almost all, 12/13 patients with no abnormalities at colonoscopy had <10 µg Hb/g faeces, whereas two CRC patients both had >200 µg Hb/g, and for five adenoma patients there was a mixed outcome. At that time, there was only one study published on quantitative FIT in symptomatic patients using another FIT (Eiken Chemical Co., Tokyo, Japan) including only six CRC patients [Citation6]. As it showed very promising results and encouraged by our pilot study, we decided to set up a prospective study. In order to detect a statistically significant sensitivity of at least 0.90, at a statistical significance level α = 0.05 and power = 0.80, 400 patients would be needed at 3% prevalence of CRC and 240 patients at 5% prevalence of CRC.
Statistical analysis
F-Hb concentration of the first faecal sample provided by each patient was denoted as “one FIT” and the highest numerical outcome of FIT of any of two samples was denoted as ‘max/2 FITs’. F-Hb concentration (µg Hb/g faeces) were presented in four commonly used categories; <10, 10–14.9, 15–19.9 and ≥20. Sensitivity, specificity and predictive values were calculated with corresponding 95% confidence intervals (CIs). Positive and negative likelihood ratios for various cut-offs and number of tests were calculated. The statistical significance of age, sex, and symptoms in addition to the FIT outcome >10 µg Hb/g was evaluated in a binary logistic regression model, and odds ratios were estimated with corresponding 95% CIs. Receiver operating characteristic (ROC) curves were constructed and area under the curve (AUC) calculated. Youden´s J statistic was used to find cut-off on the ROC curve and relevant performance of FIT at certain cut-offs was calculated accordingly [Citation11]. All analyses were performed in Stata 15.1 (StataCorp; College station, TX, USA) and SPSS 22 (IBM, Chicago, IL, USA). A two-sided p-value <.05 was considered statistically significant.
A checklist for reporting according to the STARD guidelines is in Appendix B [Citation12]. The study was approved of by the Ethical review board in Stockholm in 2013-03-04 and registered in clinicaltrials.gov (NCT 02491593).
Results
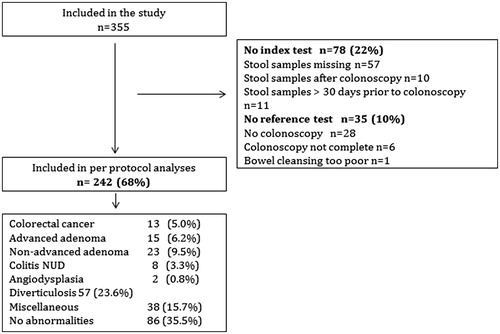
In all, 355 patients were recruited but only 68.2% (242) provided complete information on both index and reference test and included in the final analysis (). Excluded patients were 22% (78) with no information on the index test and 10% (35) patients with no information on the reference test (further details in ).
Median age of included patients was 65 (range 20–87) years, and 140 (57.9%) were females (). Most patients (67%) were included at the site recruiting patients for the longest time period (Eskilstuna GDH). In all, 13 (5.4%) were diagnosed with CRC, whereas 86 (35.5%) had no abnormalities at colonoscopy. Abdominal pain was reported by every second patient and rectal bleeding had the highest PPV (10%, 6/60) for CRC ().
Table 1. Basic characteristics of patients in the analyses (n = 242).
Table 2. Reported symptoms and positive predictive value (PPV) for colorectal cancer among patients referred for colonoscopy (n = 242).
In all, 98% (237/242) provided a second FIT (), median one (IQR 1–2) day after the first sample. The distribution of f-Hb concentration of both one and max/2 FITs was U-shaped, that is, among 86 patients with no abnormalities at colonoscopy, one FIT showed <10 µg Hb/g in 78 (91%) cases and among 42/242 (17%) patients with a FIT outcome >200 µg Hb/g, 23/42 (55%) patients had clinically important findings including eleven CRC, six AA, five colitis and one patient with angiodysplasia. As expected, FIT was <10 µg Hb/g in almost half of all patients with AA, and adding a second FIT did not change this (). There was a statistically significant difference in FIT >10 µg Hb/g between patients with larger (15-20 mm, n = 7) and smaller adenomas (<10 mm, n = 8) (86% vs. 25%, chi-square 5.5; p = .02). There were only eight IBD (colitis) patients but 6/8 had FIT >10 µg Hb/g of one FIT, and 8/8 of max/2 FITs.
Table 3. Faecal haemoglobin concentrations (µg Hb/g) of one FIT and max value/2 FITs by colonoscopy findings. Values in parenthesis are row percentages.
Poor bowel cleansing was reported for 22 patients and in this group FIT was >10 µg Hb/g in five (23%) patients compared to 59/220 (27%) in all others (p = .7). Among patients under treatment of warfarin and other anticoagulants, FIT was >10 µg Hb/g in 6/20 (30%) vs. 58/222 (26%) in all others (p = .7) (further data not shown).
Accuracy for colorectal cancer and avoidable colonoscopies
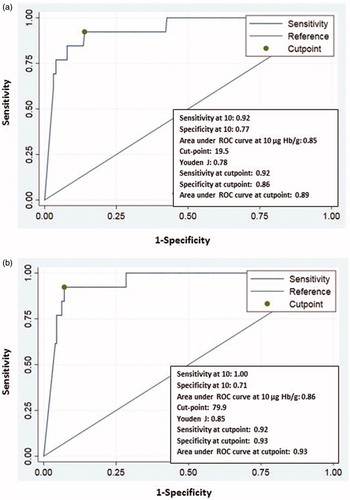
Accuracy for CRC in the investigated population of one FIT and max/2 FITs at cut-off 10, 15 and 20 µg Hb/g is presented in . At cut-off 10 µg Hb/g and one FIT, sensitivity was 92.3% (12/13) (95% CI 77.8–1.00) and specificity 77.3% (177/229) (95% CI 71.9–82.7). For max/2 FITs, sensitivity was 100% (13/13) and specificity decreased to 71.4% (160/224) (95% CI 65.5–77.3). Positive predictive values were in the range 17.1–26.2% for one FIT and NPVs were very high (99–100%) in all scenarios. The corresponding positive likelihood ratios were in the range of 3 to 6, that is, a slight to moderate effect for an increased post-test probability of disease and the negative likelihood ratio were 0.1 or less in all scenarios (with the exception for one FIT at cut-off 20 µg Hb/g), i.e. a large effect for a reduced post-test probability of disease.
Table 4. Accuracy and predictive values for colorectal cancer of one FIT and max value/2 FITs at cut-off 10, 15 and 20 µg Hb/g.
If selection for colonoscopy in this population had been based on one FIT and cut-off 10 µg Hb/g, 73.6% (178/242) (95% CI 68–80) colonoscopies could have been avoided and one CRC, seven AA, 17 NAA, and two patients with colitis would have been missed. Based on max/2 FITs at cutoff 10 µg Hb/g, 67.5% (160/237) (95% CI 62–74) colonoscopies could have been avoided with no cancer and colitis, albeit seven AA and 14 NAA patients would have been missed.
Area under the curve for CRC at cutoff 10 µg Hb/g for one FIT and max/2 FITs was 0.85 and 0.86 respectively (). Youden´s J index of one FIT was 0.78 and for max/2 FITs 0.85; that is, again a small difference between one and two tests.
Association with symptoms
Among 60 patients reporting rectal bleeding, one FIT was >10 µg Hb/g in 20 patients, including all 6 patients with CRC, and FIT was <10 µg Hb/g in all 40 patients with no CRC (chi-square 13.3; p < .001). This discriminatory ability of FIT was similar through all symptoms (further data not shown).
Univariate odds for CRC were estimated and, unlike all symptoms, only age and one FIT >10 µg Hb/g were significant, whereas in a multivariate model, only FIT remained as a significant covariate ().
Table 5. Estimated odds ratio for colorectal cancer of uni- and multivariate analyses (n = 242).
Discussion
In this prospective study, a new quantitative FIT in a previously not investigated symptomatic Swedish population showed a large potential for risk stratification among patients referred for colonoscopy. Very high negative predictive values for colorectal cancer of one FIT at cut-off 10 µg Hb/g means a large proportion (70%) of colonoscopies could potentially be avoided or at least assigned a lower priority, whereas one FIT at cut-off >200 µg Hb/g identified a delimitated group of less than 20% of referred patients and including almost all with colorectal cancer.
We primarily focused our analyses on colorectal cancer as the most urgent diagnosis to be established by colonoscopy. We found a negative likelihood ratio as low as 0.1, signifying that the test shows great capacity to provide clinicians with useful information [Citation13]. For instance, a pre-test probability of 1% and LR (-) of 0.1 means the post-test probability of having colorectal cancer in patients with a negative outcome of the test is 1/1000. Such an ability to rule out disease is valuable in all ages, but not the least for elderly and/or frail patients with severe comorbidity for whom bowel cleansing and a colonoscopy is associated with more practical difficulties and an increased risk of complications [Citation14].
The results of this study are consistent with previous findings for other FITs and our estimates of sensitivity for CRC at cut-off 10 µg Hb/g do not differ from those reported for OC-Sensor and HM-JackArc, both in the range of 90–100% [Citation15]. Studies on single tests of OC-Sensor and HM-JackArc at various cut-offs have shown similarly high values of NPVs as in this study at 99–100% [Citation5–8]. Our findings confirm another recent study showing there is no advantage for diagnostic accuracy of using two versus one FIT [Citation16]. A recent health technology assessment report summarized that faecal immunochemical tests are useful to rule out CRC and triage based on f-Hb concentration may turn up to 75% of colonoscopies in referred patients avoidable, which would be cost-effective compared to no triage [Citation15].
The sensitivity of f-Hb concentration was limited for adenomas, but most AA with a size of 15–20 mm would have been picked up at cut-off 10 µg Hb/g. The overall adenoma detection rate in the study was 16% (38/242), which is lower compared to screening scenarios but consistent with the findings for diagnostic colonoscopies [Citation17–19]. The primary aim of diagnostic colonoscopies is to find or rule out serious disease, and detection of adenoma may be less relevant in some clinical situations. For 6/8 patients with IBD-colitis, first f-Hb concentration was >10 µg Hb/g and this is in accordance with a primary care study reporting the majority of IBD patients are identifiable by FIT [Citation20]. The consequences of diagnostic delay in IBD are far less serious than for CRC, even though it may be associated with an increased risk of surgery [Citation21]. But efforts to avoid false negative results are obviously vital and this may involve a search for any other factor common factor in CRC patients with low f-Hb concentrations. There seems to be an association between anaemia and a negative outcome of any FOBT [Citation22,Citation23], but unfortunately complete data on B-Hb was not available in this study.
The prevalence of CRC in our study was 5% and this is higher than expected for all symptomatic patients referred for routine diagnostic colonoscopies at the participating centres. Only a minor fraction of all eligible patients were included, on average just above one in twenty (6%). The distribution of age, sex and symptoms among patients included indicate they are representative of larger population referred for colonoscopy but yet there must have been some selection biases operating and this is a weakness of the study. The PPVs presented are in other words somewhat overestimated and, on the other hand, the NPVs then slightly underestimated. For instance, PPV of one FIT at cut-off 10 µg Hb/g (19%) was still far above that of a single symptom (10% for rectal bleeding) or any demographic characteristics (12% for age 70–79 years). The NPVs at various cut-offs and of one or two FITs were already 99%. The inclusion rate in our study turned out lower than expected and one unpredictable reason for this was the introduction of standardized cancer pathways for colorectal cancer introduced in Sweden in 2016 [Citation24]. This increased the general workload for endoscopy units, and made it difficult to find enough time to include patients prior to their colonoscopy.
In a multivariate regression model, only FIT remained as a significant covariate. This indicates that once patients have presented with symptoms possibly associated with serious bowel disease, FIT early on is more productive than trying to assess minute details of every specific lower abdominal symptom before deciding on further workup. Likewise, f-Hb has been reported to be more accurate for the detection of CRC in patients referred for colonoscopy than symptom-based referral criteria [Citation25–28]. In this study, FIT discriminated well between CRC and non-CRC even in patients with rectal bleeding. As minor rectal bleeding is both commonly reported and difficult to evaluate in primary care, this observation deserves further attention.
In our study, one out of six patients (57/355) did not provide any faecal sample. Patients may simply have changed their minds or it may be associated with the introduction of standardized clinical cancer pathways. This meant patients with alarm symptoms got an appointment for colonoscopy much more promptly than previously, and, consequently, there was much less time for patients to provide samples or even to be asked for participation in the study. Overall we estimate some half of all patients who were contacted by phone prior to colonoscopy declined participation.
Every tenth of our patients provided faecal samples but never presented for colonoscopy. Nonattendance is a common problem at endoscopy units and a rate of 10% is to be expected [Citation29,Citation30] and adds to the question of optimal use of diagnostic resources. It is a reminder that, even though colonoscopy is unsurpassed for combined diagnostic and interventional purposes, for patients it is also associated with inconvenience, anxiety and pain [Citation31].
In conclusion, this study has confirmed FIT as a promising triage test in symptomatic patients referred for colonoscopy. For endoscopy units struggling with long waiting times and patient safety, it could be used for prioritization purposes. A large Scottish study has recently reported promising results of FIT for risk assessment of significant bowel disease including CRC in primary care [Citation32]. Further studies on how to make the best use of quantitative immunochemical tests in symptomatic patients in various settings should follow.
Acknowledgements
The authors thank Elisabeth Krödel, RN, Eskilstuna, Lars Flood, MD and Anna Jacobsson, RN, Aleris Handen Aleris, and Peter Benno, MD, Hötorget Endoscopy Centre for granting the recruitment of patients, Harriet Liljenbring, Biomedical Scientist, Unilabs Eskilstuna for laboratory assistance.
Disclosure statement
Orion Diagnostica Oy provided sampling devices, a QuikRead go instrument for analysis and sponsored a research nurse at Eskilstuna endoscopy unit. The company was not involved in the design of study, the statistical analyses or the interpretation of data. The authors declare no conflict of interest.
References
- Globocan. Cancer Today. 2018. Available from: https://gco.iarc.fr/today/home
- Hamilton W, Lancashire R, Sharp D, et al. The risk of colorectal cancer with symptoms at different ages and between the sexes: a case-control study. BMC Med. 2009;7:17.
- Adelstein BA, Macaskill P, Chan SF, et al. Most bowel cancer symptoms do not indicate colorectal cancer and polyps: a systematic review. BMC Gastroenterol. 2011;11:65.
- Brenner DR, Ruan Y, Shaw E, et al. Increasing colorectal cancer incidence trends among younger adults in Canada. Prev Med. 2017;105:345–349.
- Godber IM, Todd LM, Fraser CG, et al. Use of a faecal immunochemical test for haemoglobin can aid in the investigation of patients with lower abdominal symptoms. Clin Chem Lab Med. 2016;54:595–602.
- McDonald PJ, Digby J, Innes C, et al. Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis. 2013;15:e151–e159.
- Mowat C, Digby J, Strachan JA, et al. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut. 2016;65:1463–1469.
- Widlak MM, Thomas CL, Thomas MG, et al. Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment Pharmacol Ther. 2017;45:354–363.
- Fraser CG, Rapi S, Rubeca T. RE: A Proposal to Standardize Reporting Units for Fecal Immunochemical Tests for Hemoglobin. J Natl Cancer Inst. 2015;108:djv312.
- Fraser CG, Allison JE, Young GP, et al. Improving the reporting of evaluations of faecal immunochemical tests for haemoglobin: the FITTER standard and checklist. Eur J Cancer Prev. 2015;24:24–26.
- Schisterman EF, Perkins NJ, Liu A, et al. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73–81.
- Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527.
- Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329:168–169.
- Laanani M, Coste J, Blotiere PO, et al. Patient, procedure, and endoscopist risk factors for perforation, bleeding, and splenic injury after colonoscopies. Clin Gastroenterol Hepatol. 2019;17:719–727.e13.
- Westwood M, Corro Ramos I, Lang S, et al. Faecal immunochemical tests to triage patients with lower abdominal symptoms for suspected colorectal cancer referrals in primary care: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2017;21:1–234.
- Turvill J, Mellen S, Jeffery L, et al. Diagnostic accuracy of one or two faecal haemoglobin and calprotectin measurements in patients with suspected colorectal cancer. Scand J Gastroenterol. 2018;53:1526–1534.
- Boroff ES, Disbrow M, Crowell MD, et al. Adenoma and polyp detection rates in colonoscopy according to indication. Gastroenterol Res Pract. 2017;2017:7207595.
- Rex DK, Ponugoti PL. Calculating the adenoma detection rate in screening colonoscopies only: is it necessary? Can it be gamed? Endoscopy. 2017;49:1069–1074.
- Yang PF, Wong SW. Adenoma detection rate in colonoscopy: is indication a predictor? Surg Laparosc Endosc Percutan Tech. 2016;26:156–161.
- Hogberg C, Karling P, Rutegard J, et al. Diagnosing colorectal cancer and inflammatory bowel disease in primary care: the usefulness of tests for faecal haemoglobin, faecal calprotectin, anaemia and iron deficiency. A prospective study. Scand J Gastroenterol. 2017;52:69–75.
- Lee DW, Koo JS, Choe JW, et al. Diagnostic delay in inflammatory bowel disease increases the risk of intestinal surgery. World J Gastroenterol. 2017;23:6474–6481.
- Gillberg A, Ericsson E, Granstrom F, et al. A population-based audit of the clinical use of faecal occult blood testing in primary care for colorectal cancer. Colorectal Dis. 2012;14:e539–46.
- Hogberg C, Karling P, Rutegard J, et al. Immunochemical faecal occult blood tests in primary care and the risk of delay in the diagnosis of colorectal cancer. Scand J Prim Health Care. 2013;31:209–214.
- Wilkens J, Thulesius H, Schmidt I, et al. The 2015 National Cancer Program in Sweden: Introducing standardized care pathways in a decentralized system. Health Policy. 2016;120:1378–1382.
- Cubiella J, Salve M, Diaz-Ondina M, et al. Diagnostic accuracy of the faecal immunochemical test for colorectal cancer in symptomatic patients: comparison with NICE and SIGN referral criteria. Colorectal Dis. 2014;16:O273–282.
- Digby J, Steele RJ, Strachan JA, et al. Do other variables add value to assessment of the risk of colorectal disease using faecal immunochemical tests for haemoglobin? Ann Clin Biochem. 2019;56:472–479.
- Herrero JM, Vega P, Salve M, et al. Symptom or faecal immunochemical test based referral criteria for colorectal cancer detection in symptomatic patients: a diagnostic tests study. BMC Gastroenterol. 2018;18:155.
- Quyn AJ, Steele RJ, Digby J, et al. Application of NICE guideline NG12 to the initial assessment of patients with lower gastrointestinal symptoms: not FIT for purpose? Ann Clin Biochem. 2018;55:69–76.
- Adams LA, Pawlik J, Forbes GM. Nonattendance at outpatient endoscopy. Endoscopy. 2004;36:402–404.
- Chopra D, Hookey LC. Comorbid illness, bowel preparation, and logistical constraints are key reasons for outpatient colonoscopy nonattendance. Can J Gastroenterol Hepatol. 2016;2016:1–6.
- Shafer LA, Walker JR, Waldman C, et al. Factors associated with anxiety about colonoscopy: the preparation, the procedure, and the anticipated findings. Dig Dis Sci. 2018;63:610–618.
- Mowat C, Digby J, Strachan JA, et al. Impact of introducing a faecal immunochemical test (FIT) for haemoglobin into primary care on the outcome of patients with new bowel symptoms: a prospective cohort study. BMJ Open Gastroenterol. 2019;6:e000293.