Abstract
Objectives: Crohn’s disease and ulcerative colitis are associated with an increased risk to develop anemia, cutaneous diseases, liver diseases, malignancy, osteoporosis, rheumatic diseases, thromboembolism and uveitis. The association between these diseases and microscopic colitis (MC) is not known. The aim of the present systematic review was to examine associations between MC and diseases observed in association with Crohn’s disease and ulcerative colitis.
Material and methods: According to the review protocol, original articles which described the prevalence of abovementioned diseases in relation to MC, were searched for in PubMed, Embase and Web of Science.
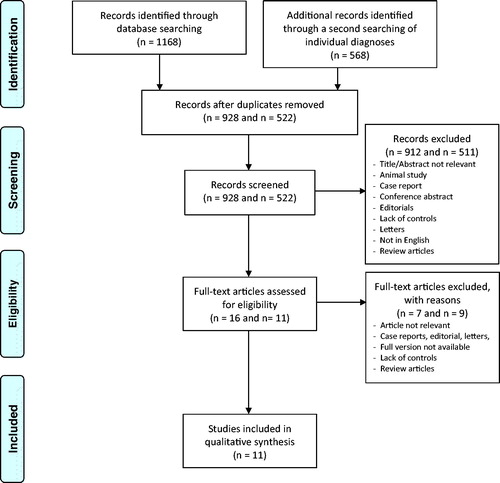
Results: After exclusion of duplicates, 928 articles remained. Based on relevancy of their title, abstract or type of article, 16 articles were ordered in full text and after assessment, nine articles could be included in the review. A second research strategy with individual diseases rendered further two articles. Seven articles covered malignancy/neoplasia, where four showed no association with malignancy and three a reduced association compared with controls. Four articles covering rheumatic diseases showed an association between these diseases and MC. One study showed an association between MC and osteoporosis, whereas one did not. One study showed an association between MC and cutaneous diseases, whereas anemia, eye diseases and thromboembolism showed no associations.
Conclusions: Due to short follow-up time in small studies, with selection bias due to exclusion of former or prevalent malignancy in an older population, no conclusions can be drawn concerning the true association between MC and malignancy. Rheumatic diseases seem to be associated with MC.
Introduction
Microscopic colitis (MC) is a less known disease in the group inflammatory bowel disease (IBD) that is characterized by chronic non-bloody diarrhea, occasionally associated with involuntary weight loss and abdominal cramps [Citation1–3]. MC has an incidence rate of approximately 10/100,000 per year, with a predilection for women and for those ≥60 years of age [Citation1,Citation2]. Both collagenous and lymphocytic colitis have an increased number of intraepithelial lymphocytes, a decreased amount of goblet cells and surface changes which consists of loosely attached cuboidal epithelia. Additionally, in collagenous colitis (CC), the subepithelial collagen layer is thickened (>10 µm) [Citation4]. There is evidence supporting that patients with CC are considered to have a longer and more symptomatic course of their disease than patients with lymphocytic colitis (LC) [Citation5]. The pathophysiology of MC is not completely understood. There is however an association between MC and a certain human leukocyte antigen (HLA) haplotype, namely HLA-DQ2, which is also known to be associated with other autoimmune diseases such as celiac disease, thyroid diseases and type 1 diabetes [Citation6,Citation7].
Despite being classified as an IBD, MC is vastly different in age predilection, symptoms, course of disease and treatment than Crohn’s disease (CD) and ulcerative colitis (UC) [Citation8–10]. UC and CD are often considered as systemic diseases, affecting the whole human body and not only the colorectal area [Citation8,Citation11]. In addition to having an aggressive and often handicapping course of disease, classic IBD is often associated with complications such as colorectal malignancy as well as extra-intestinal malignancy, and several other diseases [Citation12,Citation13].
Since MC is supposed to belong to the IBD group, it is critically important to assess whether the same associated diseases found in classic IBD, are observed in MC as well. The aim of the present systematic review was to examine the association between MC and diseases observed as secondary to, or associated with, classic IBD in comparison with controls.
Material and methods
This review is written according to the PRISMA statement of 2009 (Preferred Reporting Items for Systematic reviews and Meta-Analyses) [Citation14], with the help of the PRISMA E&E (Explanation and Elaboration) document [Citation15]. According to the review protocol, original human articles written in English which described the prevalence of the complications anemia, cutaneous diseases, liver diseases, malignancy, osteoporosis, rheumatic diseases, thromboembolism and uveitis were searched for, because of their association with classic IBD [Citation11–13].
Information sources
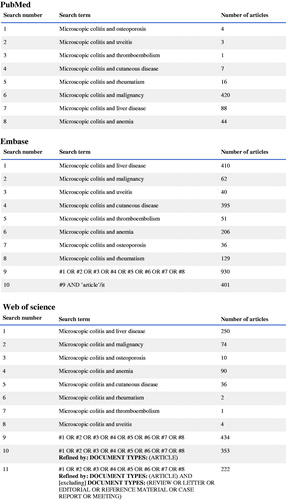
A systematic search strategy was used, with the search terms ‘microscopic colitis and anemia’, ‘microscopic colitis and cutaneous disease’, ‘microscopic colitis and liver disease’, ‘microscopic colitis and malignancy’, ‘microscopic colitis and osteoporosis’, ‘microscopic colitis and rheumatism’, ‘microscopic colitis and thromboembolism’ and ‘microscopic colitis and uveitis’. The studies were identified by searches in three different databases, namely PubMed, Embase and Web of Science, on the 28 November 2019. The same search terms were used in all three databases. The exact search strategy can be found in . After removal of duplicates, the articles were assessed through a first selection based on the title and abstract. Animal studies, case reports, conference abstracts, editorials, letters, reviews, articles not written in English, articles not available in full length and studies which did not include any control group or were of no relevance were excluded (). For an article to be included in this systematic review, they had to be relevant to the scientific question about MC and at least one concomitant disease, considered to be a complication to or associated with IBD. Relevant articles were then read in full text, for evaluation of eligibility.
In a second systematic search strategy performed as described above on the 14 February 2020, individual diseases were used as search terms instead of the group of diseases, to be sure that no disease was overlooked. Thus, the search terms in the second research were ‘microscopic colitis and skin diseases’, ‘microscopic colitis and amyloidosis’, ‘microscopic colitis and erythema nodosum’, ‘microscopic colitis and pyoderma gangrenosum’, ‘microscopic colitis and vitiligo’, ‘microscopic colitis and polyarthritis’, ‘microscopic colitis and sacroiliitis’, ‘microscopic colitis and spondyloarthritis’, ‘microscopic colitis and autoimmune hepatitis’, ‘microscopic colitis and sclerosing cholangitis’, ‘microscopic colitis and episcleritis’, ‘microscopic colitis and iritis’ and ‘microscopic colitis and scleritis’. After screening of titles/abstracts, animal studies, case reports, conference abstracts, editorials, letters, reviews, articles not written in English, articles not available in full length and studies which did not include any control group or were of no relevance were excluded.
The authors conducted the review of titles/abstracts and the full text articles, independently of each other, and compared the results. In case of discordance, the articles were read again and after discussions, consensus were made. All reference lists of included articles were scrutinized, to see whether additional articles could be found.
Quality assessment
One of the authors performed the quality assessment of the articles using the Newcastle-Ottawa Scale for cohort and case-control studies [Citation16]. The scale was constructed to evaluate cohort and case-cohort studies, by questions regarding selection (n = 4), comparability (n = 1) and outcome (n = 3). A maximum of eight stars can be achieved.
Results
The searches resulted in 545 unique citations in PubMed, 401 citations in Embase and 222 citations in Web of Science. After removal of duplicates, 928 articles remained. Of these, 912 articles were removed based on the type of article and study or lack of relevancy of their title and abstract. Sixteen articles were ordered in full text and after assessment, seven were excluded, leaving nine articles to be included in the review. In addition, a secondary search was performed by using more specific disease terms than in the first main search strategy. In total, 54 citations from PubMed, 260 citations from Embase and 254 citations from Web of Science were found. After removals of duplicates, 522 articles remained. Of these, 511 articles were removed based on the type of article and study, lack of relevancy of their title and abstract or because it was already included from the first search. Eleven articles were read in full text and after assessment, nine were excluded, leaving two articles to be included in the review. Thus, altogether, 11 articles were finally included. A summary of the articles included can be found in . No additional articles were included after evaluation of the reference list of the chosen articles. All included studies scored six stars or more on the Newcastle-Ottawa Scale.
Table 1. Study characteristics.
Some articles used for this review covered multiple groups of diseases, while some only covered a single group. A majority of the articles [Citation7] covered the topic of malignancy/neoplasia. Rheumatic diseases were covered by four articles, osteoporosis and anemia were covered by two articles each and cutaneous diseases, eye diseases, thromboembolism and uveitis were covered by one article (). One article covered six different topics, namely anemia, cutaneous diseases, eye diseases, malignancy and rheumatic diseases [Citation17]. Not a single article covered the topic of liver diseases. Two articles separated MC into CC and LC [Citation17,Citation18]. Four articles used other patients suffering from functional diarrhea or patients undergoing colonoscopic screening, and not healthy subjects, as controls [Citation21,Citation23,Citation24,Citation27].
Malignancy/neoplasia was covered in seven different articles [Citation17–23]. Of these, four articles showed no statistically significant association between MC and malignancy/neoplasia [Citation17,Citation18,Citation20,Citation23]. Additionally, Levy et al. [Citation23] could not show any association between MC and colonic neoplasia. However, the three other articles showed contradictory evidence. Tontini et al. [Citation21] and Yen et al. [Citation19] showed a significant negative association between MC and colorectal malignancy (odds ratio (OR): 0.22; 95% confidence interval (CI): 0.05–0.97; p = .035, and OR: 0.34; 95% CI: 0.16–0.73; p = .006, respectively). Yen et al. [Citation19] also showed a statistical negative association between MC and colorectal neoplasia (OR: 0.52; 95% CI: 0.39–0.69; p < .0001). Sonnenberg and Genta [Citation22] showed a significant negative association between MC and colonic neoplasia (OR: 0.46; 95% CI: 0.43–0.49 for hyperplastic polyps, OR: 0.24; 95% CI: 0.19–0.30, for serrated adenomas and OR: 0.35; 95% CI: 0.33–0.38 for tubular adenomas).
Rheumatic diseases were covered in four different articles [Citation17,Citation18,Citation20,Citation24]. Two out of four articles had separated their results into CC and LC [Citation17,Citation18]. Roth et al. [Citation20] and Gu et al. [Citation24] concluded that there was a significant association between MC and rheumatoid arthritis (RA) (OR: 7.21; 95% CI: 3.81–13.64 and p = .022, respectively). However, Wickbom et al. [Citation17] showed a significant association between CC and rheumatic diseases (OR: 1.9; 95% CI: 1.0–3.5; p = .042), but the association between LC and rheumatic diseases was not statistically significant. Kao et al. [Citation18] could however show a significant association between CC and RA and Reynaud/CREST syndrome (p < .01 for both). In addition, there was a significant association between LC and RA (p < .01), Reynaud/CREST syndrome (p < .01, fibromyalgia (p < .01), giant cell/temporal arteritis (p < .025) and systemic lupus erythematosus (SLE) (p < .025) [Citation18].
Osteoporosis was covered in two articles, showing conflicting results [Citation25,Citation26]. Lorinczy et al. [Citation25] concluded that there was a significant association between MC and a lower bone mineral density (BMD) compared with the healthy controls (p < .01). However, Wildt et al. [Citation26] could not show a significant association between lower BMD and MC.
Cutaneous diseases were covered in one article, which could show a significant association between CC and cutaneous diseases (OR: 6.0; 95% CI: 1.4–26.0; p = .027), but not between LC and cutaneous diseases [Citation17]. Wickbom et al. [Citation17] also covered the topics of anemia, eye diseases and thromboembolism. However, there were no statistically significant evidence for an association between these different types of diseases and MC [Citation17,Citation27].
Discussion
To the best of our knowledge, this is the first systematic review concerning MC and diseases associated with classic IBD. The results showed that there has been sparse research on this particular topic, and the research that has been done is mostly comprised of studies on smaller patient groups without controls for comparisons or adjustments for confounders and with short follow-up, which might explain contradictory results in different studies. The studies found have focused on an association between MC and several groups of diseases, and not whether there is a causality involved in the pathology.
Roth et al. [Citation20] and Gu et al. [Citation24] concluded that there was a significant association between MC and RA. When MC was separated into CC and LC, Wickbom et al. [Citation17] showed a statistically significant association between CC and rheumatic diseases, though this was not the case with LC. On the contrary, Kao et al. [Citation18] could show more significant results between LC and different rheumatic diseases, than between CC and these diseases. The sizes of the patient groups were larger in the latter study [Citation18], and this could have an effect on the final results. With this data being available, one can suspect that there is an association between MC and rheumatic diseases. However, while classic IBD is associated with spondyloarthropathies [Citation28], the associations of MC rather seem to be with RA [Citation17,Citation18,Citation20,Citation24]. This difference suggests different etiologies to MC and classic IBD, and the rheumatic diseases observed. Since both proton pump inhibitors and non-steroidal anti-inflammatory drugs (NSAID) are associated with MC [Citation29], and both of these drugs are common in the treatment of rheumatic diseases [Citation28], one must exclude drug use in the etiology of MC in patients who have first developed RA.
Malignancy/neoplasia was a point of focus in seven out of the 11 studies presented in this review. This is not surprising, considering that colorectal malignancy is one of the most well-known complications to classic IBD. The focus of the articles presented are primarily the association between MC and colorectal malignancy/neoplasia, even though there is an increased risk of extra-intestinal malignancy in classic IBD [Citation12,Citation13]. Several studies suggested that patients with MC have a lower risk of being afflicted with these conditions [Citation19,Citation21,Citation22]. There are some theories behind this observation. First, chronic watery diarrhea reduces the transit time in the colon, thus, resulting in less exposure to potentially harmful toxic agents [Citation30]. Second, Yen et al. [Citation19] suggested that an increased amount of intraepithelial lymphocytes in the colonic mucosa recruit δ-gamma T-cells to the colonic mucosa, which are involved in killing cells with a damaged DNA. Contrary to these results, four other articles could not find any association between MC and colorectal malignancy [Citation17,Citation18,Citation20,Citation23], or between MC and any extra-intestinal malignancies [Citation23].
There are however some concerns regarding the methods used in the included studies, mainly with the time frame and with the eligibility criteria for inclusion. The eligibility criteria are most prominent in the studies that cover malignancy. First, the average time frame used in these studies to analyze the risk of malignancy is ranging from about 3 to 8 years [Citation19,Citation23]. This is generally a short follow-up time, since the diseases covered in this review are generally diseases which take a long time to develop [Citation13]. Consequently, the short time frame will make it difficult to determine whether any incidence of concomitant disease should be attributed to MC or simply by chance. Furthermore, most of the studies did only examine the prevalence of malignancy, and did not examine the incidence risk after the diagnosis of MC [Citation17,Citation18,Citation20–22]. Second, in one of the studies, all previous cases of colon malignancy were excluded [Citation22]. Thus, many patients with malignancy were already excluded, which causes a heavy selection bias, and might skew the results to a point where you find a negative association between MC and malignancy. There is, however, a problem with the disease population per se when trying to determine the long-term consequences of MC, since the population is ≥60 years of age at disease development in all included studies. This is contrary to classic IBD, which has a predilection for a younger population [Citation9,Citation10], which gives ample time to study the effects of CD and UC.
There are obvious macroscopic differences in the mucosa between MC and classic IBD, as well as a different range of symptoms. This reflects another kind of inflammation in MC compared with the one in classic IBD, with a less systemic disease in MC than in classic IBD [Citation4,Citation11]. To further differentiate MC from classic IBD, Carmack et al. [Citation31] suggested that MC could be considered a primary disease as well as a secondary disease. Infiltration of lymphocytes is found in the mucosa during many conditions, e.g., celiac disease, viral and bacterial enteritis, drugs and other autoimmune diseases. In these cases, MC should be considered a secondary phenomenon, and not a primary, idiopathic disease [Citation32]. This confounder could explain differences in results between studies, and why LC had more associations with rheumatic diseases treated with several anti-inflammatory drugs [Citation18], which could elicit lymphocyte infiltration in the mucosa [Citation31].
The two articles found covering osteoporosis have opposite conclusions. While Lorinczy et al. [Citation25], with a cohort of 14 patients, stated that there was an association between lower BMD and MC, Wildt et al. [Citation26] could not show the same association in 50 patients. Wildt et al. [Citation26] noted an association between a lower BMD and a longer disease duration and the cumulative amount of budesonide that the patient had been treated with. This could have future implications on treatment of MC. Additionally, in Wildt et al. [Citation26], more of the patients in the MC group were smokers than in the control group (34% vs. 10%). This could affect the outcome, since smoking is an established risk factor for both MC and osteoporosis [Citation33,Citation34]. However, both studies have a low number of patients, and an association between MC and low BMD simply cannot be drawn. Surprisingly, no studies were found covering the topic of liver disease in a MC cohort compared with controls, although primary sclerosing cholangitis (PSC) is strongly associated with classic IBD [Citation35].
The quality of the included studies was generally low, due to small patient groups and issues with selection bias, mentioned above. Furthermore, in included studies, controls were matched for age and gender [Citation17,Citation18,Citation21,Citation24–27], and in the case-cohort studies, calculations were adjusted for age and sex [Citation19,Citation22]. However, only two studies adjusted for smoking, alcohol intake and BMI [Citation20,Citation23]. This may affect the conclusions, since several lifestyle habits are associated with MC [Citation3,Citation32–34]. No study adjusted for drug consumption. Several articles excluded did not use any control group [Citation36,Citation37], which makes it difficult to draw any conclusions. Also in the included articles, some of the control groups consisted of other patients with functional diarrhea [Citation21,Citation24,Citation27], which may affect the outcomes of the study.
There are some limitations to this systematic review. First, variations of the search terms used could have been searched for. This was not done due to the relatively large scope of different topics that this systematic review was supposed to cover. Second, there was no attempt of a meta-analysis of the results presented, due to the low number of articles and different design and control cohorts used in the articles. Third, since all studies were cross-sectional or retrospective, it was not possible to determine any causality or whether MC was the primary disease, or was developed after the onset of the other diseases.
In conclusion, it is hard to draw any firm conclusions from the evidence presented in this systematic review, because of the lack of data and conflicting results. With the data available, rheumatic diseases were the only diseases where an association with MC can be suspected. Three studies presented a negative association between MC and colorectal malignancy, while four studies could not find any association between MC and malignancy at all. Based on the data presented, no true conclusions can be drawn concerning the association between MC and complications often seen in classic IBD. This topic needs more research, with bigger sample sizes, longer follow-up time and statistical models adjusting for confounders concerning drug consumption and lifestyle habits such as alcohol consumption, BMI, smoking and sun exposure.
| Abbreviations | ||
| BMD | = | bone mineral density |
| CC | = | collagenous colitis |
| cuSCC | = | cutaneous squamous cell carcinoma |
| CD | = | Crohn’s disease |
| CI | = | confidence interval |
| HLA | = | human leukocyte antigen |
| IBD | = | inflammatory bowel disease |
| LC | = | lymphocytic colitis |
| MC | = | microscopic colitis |
| OR | = | Odds ratio |
| PRISMA | = | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| UC | = | Ulcerative colitis |
Acknowledgements
The authors thank Maria Björklund, Library of Lund University, for valuable help with searching and search strategies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nguyen GC, Smalley WE, Vege SS, et al. American gastroenterological association institute guideline on the medical management of microscopic colitis. Gastroenterology. 2016;150:242–246.
- Vigren L, Olesen M, Benoni C, et al. An epidemiological study of collagenous colitis in southern Sweden from 2001–2010. World J Gastroenterol. 2012;18:2821–2826.
- Munch A, Aust D, Bohr J, et al. Microscopic colitis: current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis. 2012;6:932–945.
- Langner C, Aust D, Ensari A, et al.; The Working Group of Digestive Diseases of the European Society of Pathology (ESP) and the European Microscopic Colitis Group (EMCG). Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology. 2015;66:613–626.
- Rasmussen MA, Munck LK. Systematic review: are lymphocytic colitis and collagenous colitis two subtypes of the same disease – microscopic colitis? Aliment Pharmacol Ther. 2012;36:79–90.
- Fernández-Bañares F, Esteve M, Farré C, et al. Predisposing HLA-DQ2 and HLA-DQ8 haplotypes of coeliac disease and associated enteropathy in microscopic colitis. Eur J Gastroenterol Hepatol. 2005;17:1333–1338.
- Westerlind H, Mellander MR, Bresso F, et al. Dense genotyping of immune-related loci identifies HLA variants associated with increased risk of collagenous colitis. Gut. 2017;66:421–428.
- Long MD, Hutfless S, Kappelman MD, et al. Challenges in designing a national surveillance program for inflammatory bowel disease in the United States. Inflamm Bowel Dis. 2014;20:398–415.
- Wolters FL, Russel MG, Sijbrandij J, et al. Crohn’s disease: increased mortality 10 years after diagnosis in a Europe-wide population based cohort. Gut. 2006;55:510–518.
- Hoie O, Schouten LJ, Wolters FL, et al.; On behalf of the European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD). Ulcerative colitis: no rise in mortality in a European-wide population based cohort 10 years after diagnosis. Gut. 2007;56:497–503.
- Karlinger K, Györke T, Makö E, et al. The epidemiology and the pathogenesis of inflammatory bowel disease. Eur J Radiol. 2000;35:154–167.
- Pedersen N, Duricova D, Elkjaer M, et al. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol. 2010;105:1480–1487.
- Kappelman MD, Farkas DK, Long MD, et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014;12:265–273.e1.
- Moher D, Liberati A, Tetzlaff J, et al.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. [cited 2019 Dec 20]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Wickbom A, Nyhlin N, Montgomery SM, et al. Family history, comorbidity, smoking and other risk factors in microscopic colitis: a case-control study. Eur J Gastroenterol Hepatol. 2017;29:587–594.
- Kao KT, Pedraza BA, McClune AC, et al. Microscopic colitis: a large retrospective analysis from a health maintenance organization experience. World J Gastroenterol. 2009;15:3122–3127.
- Yen EF, Pokhrel B, Bianchi LK, et al. Decreased colorectal cancer and adenoma risk in patients with microscopic colitis. Dig Dis Sci. 2012;57:161–169.
- Roth B, Manjer J, Ohlsson B. Microscopic colitis is associated with several concomitant diseases. Drug Target Insights. 2013;7:19–25.
- Tontini GE, Pastorelli L, Spina L, et al. Microscopic colitis and colorectal neoplastic lesion rate in chronic nonbloody diarrhea: a prospective, multicenter study. Inflamm Bowel Dis. 2014;20:882–891.
- Sonnenberg A, Genta RM. Low prevalence of colon polyps in chronic inflammatory conditions of the colon. Am J Gastroenterol. 2015;110:1056–1061.
- Levy A, Borren NZ, Maxner B, et al. Cancer risk in microscopic colitis: a retrospective cohort study. BMC Gastroenterol. 2019;19:1.
- Gu HX, Zhi FC, Huang Y, Li AM, et al. Microscopic colitis in patients with chronic diarrhea and normal colonoscopic findings in Southern China. Int J Colorectal Dis. 2012;27:1167–1173.
- Lorinczy K, Lakatos G, Mullner K, et al. Low bone mass in microscopic colitis. BMC Gastroenterol. 2011;11:58.
- Wildt S, Munck LK, Becker S, et al. Risk of osteoporosis in microscopic colitis. Postgrad Med. 2018;130:348–354.
- Guagnozzi D, Lucendo AJ, Angueira-Lapeña T, et al. Prevalence and incidence of microscopic colitis in patients with diarrhoea of unknown aetiology in a region in central Spain. Dig Liver Dis. 2012;44:384–388.
- Fragoulis GE, Liava C, Daoussis D, et al. Inflammatory bowel diseases and spondyloarthropathies: from pathogenesis to treatment. World J Gastroenterol. 2019;25:2162–2176.
- Masclee GM, Coloma PM, Kuipers EJ, et al. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal antiinflammatory drugs. Am J Gastroenterol. 2015;110:749–759.
- Kobayashi J. Effect of diet and gut environment on the gastrointestinal formation of N-nitroso compounds: a review. Nitric Oxide. 2018;73:66–73.
- Carmack SW, Lash RH, Gulizia JM, et al. Lymphocytic disorders of the gastrointestinal tract: a review for the practicing pathologist. Adv Anat Pathol. 2009;16:290–306.
- Ohlsson B. New insights and challenges in microscopic colitis. Therap Adv Gastroenterol. 2015;8:37–47.
- Vigren L, Sjoberg K, Benoni C, et al. Is smoking a risk factor for collagenous colitis? Scand J Gastroenterol. 2011;46:1334–1339.
- Munch A, Tysk C, Bohr J, et al. Smoking status influences clinical outcome in collagenous colitis. J Crohns Colitis. 2016;10:449–454.
- de Vries AB, Janse M, Blokzijl H, et al. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956–1971.
- Vigren L, Tysk C, Strom M, et al. Celiac disease and other autoimmune diseases in patients with collagenous colitis. Scand J Gastroenterol. 2013;48:944–950.
- Larsson JK, Dabos KJ, Hoglund P, et al. Cancer risk in collagenous colitis. J Clin Med. 2019;8:1942.