?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background: Delayed bleeding (DB) occurs in ∼10% after colorectal EMR. Prophylactic clipping (PC) was reported to significantly decrease DB-rate in proximal lesions ≥2 cm.
Objective: Our aim was to determine which predefined variables contribute to using PC in clinical practice.
Methods: We performed an international discrete choice experiment (DCE) among ∼500 endoscopists. Relevant variables for PC use were selected by EMR experts: previous DB, anticoagulants, polyp size, morphology, location, intraprocedural bleeding and visible vessel(s). Respondents answered case scenarios with various variable combinations, each time choosing only one scenario for PC, or the ‘none’ option. Part-worth utilities and importance weights were calculated using HB regression. Subsequently, a predictive model was created to calculate the likelihood of endoscopists choosing PC in any given case.
Results: The survey was completed by 190 EMR endoscopists from 17 countries. In total, 8% would never use PC, whereas 30.9% never chose the ‘none’ option. All variables except polyp type were significant in decision-making for PC (p < .01). The most important factor was anticoagulant use, accounting for 22.5% in decision-making. Polyps <2 cm were considered eligible for PC by 14% in the presence of high-weighing factors such as anticoagulant use. No significant differences were found between high and low-to-moderately experienced endoscopists.
Conclusions: PC after EMR is often considered useful by endoscopists, usually based on risk factors for DB. Anticoagulant use was the most important factor in decision-making for PC, independent of endoscopist experience. Although not considered cost-effective, one in seven endoscopists chose PC for adenomas <2 cm.
Introduction
Endoscopic mucosal resection (EMR) allows resection of large (≥20 mm) colorectal polyps while protecting the underlying submucosa. The most frequent complication after colorectal EMR is delayed bleeding (DB), occurring in approximately 7% after EMR of large polyps, with 2–3% occurring in the distal and 10–12.3% in the proximal colon [Citation1]. Risk factors include polyp size, location in the proximal colon, intraprocedural bleeding (IPB) and restart of antithrombotic medication within seven days [Citation1–3]. Prophylactically closing the resection defect with endoclips is hypothesized to prevent DB. Two recent randomized studies demonstrated that prophylactic clipping (PC) reduces DB after colorectal EMR of large proximal nonpedunculated polyps, and polyps with a substantial DB risk (GSEED-RE score ≥6) [Citation4,Citation5]. However, PC for polyps ≥2 cm was not cost-effective in an economic modelling study [Citation6]. Furthermore, PC has not been effective in reducing the rate of DB after polypectomy of small polyps or polyps ≥1 cm [Citation7,Citation8].
Until the value of PC in preventing DB is more clarified, the clinical implementation of PC remains subject to variation. Guidelines advice against PC for small lesions, but leave room for PC for proximal lesions ≥2 cm [Citation9–11]. Insight in the decision-making for PC in every-day practice could lead to targeted strategies to improve future guideline implementation. The aim of this study is to identify factors that influence the decision-making process by endoscopists to use PC after EMR.
Methods
Study design
We conducted an international discrete choice experiment (DCE) among endoscopists who regularly perform EMR procedures [Citation12]. DCE is a questionnaire method that is commonly used in decision-making/preference studies [Citation13]. Respondents are presented with cases assembled from predefined variables (attributes) with different options (levels) in order to estimate quantifiable preferences. Every respondent is asked to complete a number of tasks in which three cases (concepts) are presented. The objective of each task is to select the case in which they would apply PC, if they could select only one case. By forcing the respondents to make this choice, the group preference for attributes and changes in attribute levels can be measured, also when they are equally (un)important. This allowed us to gain insight in the individual weight of the various factors in decision-making for PC. Additionally, if respondents preferred not to use PC in any case, a ‘none’ option was available. Therefore, this DCE design closely imitated and represented real-world practice.
Attributes and levels
The following attributes and levels were used: previous episode of DB after polypectomy, continued anticoagulant use (No; anticoagulants including coumarin derivatives, NOACs or heparin; platelet aggregation inhibitors including aspirin, clopidogrel, dipyridamole or a combination of these drugs), polyp size (<2 cm; ≥2 cm), polyp location (proximal; distal), polyp morphology (flat; sessile; mixed type), IPB (yes, treated with coagulation; yes, treated with clip; no) and visible vessels in a dry EMR defect (yes; no). In accordance with DCE design guidelines, these variables were selected from a broad range of known risk factors for DB by an EMR expert panel (EG, PD, LM, PS) [Citation1,Citation2,Citation12]. As the external validity of a DCE depends for a large part on the studied attributes, this was done with special emphasis on relevance for clinical practice. The selected variables were evaluated on relevance and clarity by two independent endoscopists who were representative of the target population (see Supplementary Table 1 for attribute definitions).
Survey design
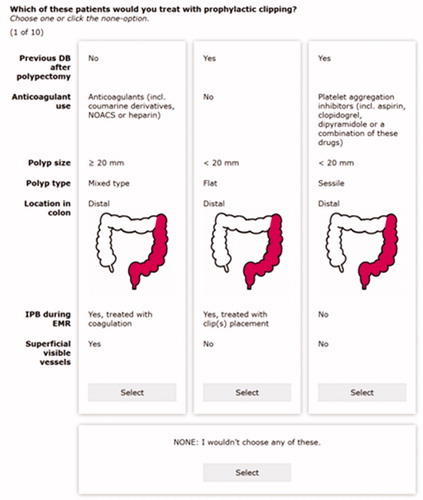
The survey was built in Sawtooth Software’s Lighthouse Studio. We allowed only binary or triple outcome levels for each attribute. This design is relatively balanced and orthogonal, which increases the efficiency of the DCE. shows a screenshot from a random task in the DCE.
In addition to these DCE tasks, recipients were asked to fill out the following individual variables: gender, country, years of experience, estimated total number of EMR’s performed and employment in an academic, peripheral and/or teaching hospital.
A final survey-check was performed by two investigators (EvG, PD), focusing on inconsistencies and comprehensibility.
Study population
The survey was hosted online and a link was sent to 483 gastroenterologists from the Dutch Association for Gastroenterology in November–December 2018. The number of endoscopists performing EMRs among the invitees was unknown, but the invitation clearly explained the target group for the survey. Subsequently, 68 international EMR-specialists were identified by their participation in EMR guideline committees or publications on EMR/PC and were invited to participate. People were invited to forward the survey to other EMR colleagues.
As no medical data were involved in this DCE, medical ethical approval was not required according to Dutch legislation. Nonetheless, all survey responds were anonymous and untraceable to individual participants. Informed consent was given implicitly by filling out the complete questionnaire.
Sample size
The equation for the minimum sample size for conjoint analysis (n) according to Johnson
was used. Our recipients were invited to complete t= 10 tasks with an average a= 3 alternatives (not including the none-concept) and ≤3 levels (c = 3 × 3=9). Based on these parameters, a sample size of 150 can be considered sufficient. Each case in the survey included all seven attributes (a full profile design). As a low response rate was anticipated, the questionnaire was designed to be completed within limited time.
Analysis plan
The primary outcome was endoscopists’ preference for factors in decision-making for PC. The analyses were performed in Lighthouse Studio (Sawtooth Software, North Orem, UT). Hierarchical Bayes regression was used to calculate individual part-worth utility scores per variable (REF-HB). Negative utilities do not trigger gastroenterologists to make a decision, compared to positive utilities that do influence decision-making. Next, importance scores per attribute were derived by subtracting the part-worth utilities for the most and least important level of each attribute. To calculate the likelihood that endoscopists would use PC in any given situation, we used the following model (REF):
where P is the chance that PC would be used by the endoscopists in any given case, and V is the total utility of that case derived from the added part-worth utilities of the variables calculated by a logit analysis. The 95% confidence intervals (CIs) of P were calculated for each case in the model using 95% CI=V ± 1.96ɛ.
Secondarily, comparative analysis between subgroups was performed in SPSS v. 25 (IBM Corp., Armonk, NY). Skewness of the data was analyzed for each attribute. Student’s t-test for unpaired data was used for the comparative analyses between highly and low-to-moderately experienced EMR endoscopists. High experience was defined as ≥120 EMRs performed in a lifetime [Citation14].
Table 3. Comparison of importance scores per attribute between endoscopists that are highly (>120 EMR procedures lifetime) and moderately experienced in EMR.
Results
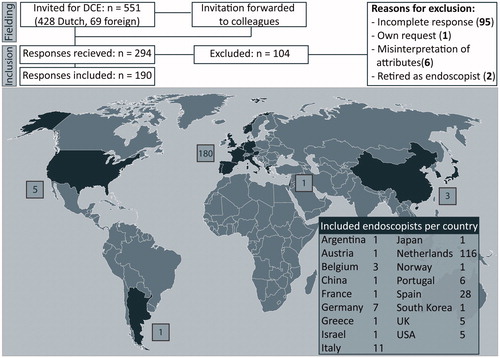
Between November 2018 and March 2019, 294 respondents replied to the DCE. A total of 199 EMR endoscopists fully completed all tasks, yielding a response rate of 36%. Nine respondents were removed from the dataset based on information in the remarks section reflecting misinterpretation of survey instructions.
The majority of these 190 respondents was male and 61% worked in The Netherlands (see ). Baseline characteristics are shown in and were compared between Dutch endoscopists (N = 116) and other nationalities (n = 74) (see Supplementary Table 2). A total of 20.7% of Dutch responders worked in academic hospitals or tertiary referral centers, compared to 83.8% in the other countries.
Table 1. Baseline characteristics of 190 included responders.
Primary outcome
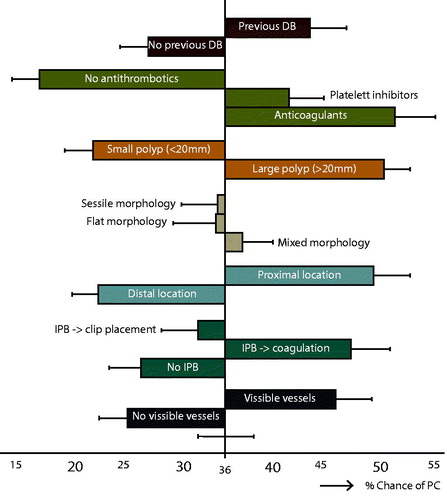
Significant importance scores were found for all attributes except polyp morphology, meaning that these attributes were meaningful to endoscopists in choosing to clip or not. Overall, the ‘none’ option (no PC) was chosen in 27.8% of all questions. Thirteen respondents (6.8%) chose the ‘none’ option in all questions, whereas 59 respondents (31%) never chose this option. Utility scores were moderated for each level of the seven attributes (see Supplementary Table 3). Importance scores were computed for each attribute (see ). Antithrombotic medication, especially anticoagulants such as coumarin derivatives and DOACs, was the most important factor in this study, accounting for 22.5% of decision-making. This means that within our set of attributes and levels, anticoagulants were approximately twice as important as previous DB.
Table 2. Importance scores per attribute.
Prediction model
The simulation model that we created next uses these variables to calculate the likelihood that endoscopists would use PC in any given case (see ). The reference case, in which all variables are unknown, had a 36.2% (95% CI 33.6–38.9) likelihood to be clipped prophylactically. When all variables were set to their minimal-chance levels (no previous DB, no antithrombotics, small polyp size, etc.), this decreased to 2.3% (95% CI 1.8–3.0). On the opposite, after EMR in a patient with only high-chance levels 92.5% (95% CI 90.8–93.9) of the endoscopists would choose PC.
Secondary outcome
There were no significant differences in the importance scores for each attribute between endoscopist with different experience levels (see ).
Feedback from respondents
Thirty-six responders used the remarks-section of the survey. Some responders found it hard to choose one case per task. ‘When there were two cases that I would be equally likely to clip, it was just a flip of the coin’ one respondent wrote.
Twenty responders suggested extra attributes that they consider when choosing PC. These are summarized in .
Table 4. Suggestions for extra attributes left in the remarks-sections of the DCE.
Discussion
The key finding from this DCE is that anticoagulant medication is the most important stimulant for endoscopists to perform PC. Contrary to guidelines, also small polyps ˂2 cm are considered for PC, especially in case of high-weighing risk factors for bleeding (e.g., anticoagulant medication). These findings were independent of the endoscopist’s EMR experience.
Evidence for PC
Although a majority of the EMR endoscopists in this study would use PC in the presence of risk factors for bleeding, PC after colorectal polypectomy or EMR is still controversial. A recent meta-analysis including seven RCTs with heterogeneous populations, could not demonstrate a protective effect of PC [Citation7]. Since then, three RCTs have been published. The first study found that PC did not affect DB-rate after removal of polyps ≥1 cm [Citation8]. In contrast to these findings, an RCT with 615 patients showed PC to be effective in proximal adenomas ≥2 cm with an NNT of 16 [Citation4]. Although a greater bleeding risk was seen with antithrombotic medication, the prophylactic effect of clipping was independent of this. A third RCT with 235 patients showed an effect of PC with an NNT of 14 in patients with an estimated high risk of DB (GSEED-RE score ≥6). However, this was only in case of complete closure, which was achieved in only 57% of patients in the intervention group [Citation5]. As our DCE was conducted before the publication of these RCTs, it shows that EMR specialists were already triggered to clip post-EMR defects with a higher risk of DB, although evidence was still lacking.
Risk factors vs. factors in decision-making
Continued antithrombotic drug use, especially anticoagulants, is known to increase the risk of DB after EMR of both small and large adenomas [Citation15–17]. Therefore, guidelines advise cessation of anticoagulants or switching to bridging therapy prior to polypectomy, with reintroduction of anticoagulant medication within 24–48 h after EMR [Citation17–20]. However, it is still unclear to what extent these strategies are effective in lowering DB risk [Citation21–23]. This may explain why anticoagulant medication was the strongest factor in deciding to perform PC in this study. On the other hand, we also observed a tendency for endoscopists to use PC in patients with continued platelet aggregation inhibitors. This finding was unexpected, because continued aspirin and clopidogrel have not clearly been associated with a higher DB risk [Citation16,Citation24].
Few studies have investigated the correlation between IPB and clinically significant DB, but three studies reported an association [Citation2,Citation25]. This may be largely based on common risk factors. The notion that IPB is a risk factor is supported by this study, as IPB treated by coagulation increased the likelihood of PC. On the other hand, endoscopists were less likely to apply PC when IPB was already treated with hemostatic clips, as they probably considered these clip(s) to have sufficiently treated the most likely source for a DB.
The importance of polyp size and proximal location is consistent with the literature [Citation1,Citation3,Citation26,Citation27]. Both are known risk factors for DB, and PC-studies often focus on high-risk patients with large proximal adenomas.
The presence of submucosal visible vessels also stimulated PC. Recently, Kim et al. concluded that cut vessels and severe coagulation injury are predictive for DB [Citation27]. Intact visible vessels are less clearly associated with DB-rate [Citation28,Citation29], but ≥3 visible vessels in the post-EMR defect have been suggested to also increase the risk of DB [Citation30]. Although prophylactic coagulation of visible vessels does not decrease clinically significant DB after EMR, the effect of clipping is unknown [Citation28,Citation31].
Lastly, previous DB was a significant factor in the decision-making for PC in our respondents. However, there is no evidence to support an association between previous DB and risk of new DB. The importance of previous DB may be confounded by variables that generally stay the same over time (e.g., co-morbidities). Nonetheless, it could also simply reflect they ‘learned from the past’.
Based on the abovementioned risk factors, we created a predictive model as a visualization of current clinical practice and as a decision aiding tool. Of course, endoscopists should always consider technical feasibility before starting clip placement. The model shows that the average likelihood of any patient with large lateral spreading lesions to receive PC increases to 92.5% when all high-risk variables are present, and decreases to 2.3% when all variables are set to their minimal-risk levels. So far, this is to be expected. However, we would like to point out two interesting findings from the model. First, we see a surprisingly strong effect of withheld anticoagulants on the likelihood of PC. The smaller, but significant, increase of 5% in case of continued platelet inhibitors is also surprising, as guidelines actually advice the continuation of these drugs based on minimal effect on bleeding risk. Second, although small lesion size reduces the likelihood of PC in our model with roughly 12–15%, this still leaves adenomas <2 cm with a 24% likelihood to be closed with clips, independent of location or antithrombotics use. Small polyps with anticoagulants even have a higher likelihood to be clipped (37.2%) than large polyps without any antithrombotic medication (29.8%). This is not conform current literature and guidelines [Citation9,Citation10]. This finding emphasizes the need for more studies to help convince endoscopists.
Strengths
This study has several strengths. The number of respondents was high, increasing the external validity and generalizability. With 190 complete responses, we well achieved the calculated sample size of 150. Nonetheless, our efficient design was also important to optimize the precision of the estimates [Citation32].
The survey was designed for a target population with preemptive experience in EMR, which was clearly stated in the invitation and instruction. The percentage of invited gastroenterologists that were EMR endoscopists is unknown. Therefore, the response rate of 36% is a rough estimation. The actual response rate, expressed as a percentage of EMR endoscopists that completed the survey, is likely higher, as the majority of non-responders will not have been EMR specialists. The 190 complete responses from 17 countries around the world allow us to draw solid and relevant conclusions.
Our insight in the global application of PC after EMR is further improved by the remarks-section of the survey. This was used by 36 respondents to provide explanations and suggestions in addition to their answers, or relate their experience of filling out the questionnaire.
Limitations
We realize that this study also has some limitations. First, 61% of respondents were Dutch, with a majority working in peripheral or teaching hospitals. Most of the foreign respondents worked in academic or tertiary hospitals. Although in the Netherlands, it is common practice to perform EMRs of large adenomas outside of academic hospitals, we considered EMR experience as a potential confounder. We saw no differences in importance scores between respondents with high vs. low-to-moderate experience. This seems to underline the generalizability of the results.
Second, a selection of the most relevant variables had to be made. In view of the guidelines, we chose risk factors for DB that were paramount to PC efficacy studies over some less studied attributes such as ‘technical feasibility of clip closure’ and ‘cost of clips’. This is not to say that these factors may not play a role, but that they probably operate more on the background of the decision-making process.
Although the variables were carefully selected, the presentation of some attributes in the survey may have benefitted from more detailed explanation of the used definitions. For example, we explained that the cases should be answered from the perspective of everyday clinical practice. In daily practice, people using anticoagulants will generally be adequately bridged before the procedure to decrease the bleeding risk. Nevertheless, some respondents interpreted the variable ‘use of anticoagulants’ in the sense of continued, unbridged use. Although we excluded all respondents of whom we were aware of this misinterpretation from the analysis, this may have caused a slight overestimation of the part-worth utility. Nevertheless, as both bridged and unbridged patients on anticoagulants are to some degree at increased risk for bleeding compared to coagulated patients, we would expect the part-worth utility for this attribute to remain high after correction [Citation22].
Lastly, we performed a subgroup analysis to compare outcomes in endoscopists with low vs. high experience in EMR. Expertise was self-reported by the respondents and are therefore prone to recall bias. We based thresholds for experience on previous research [Citation14,Citation33], and believe the rough division in experience level that we made is clinically relevant. Although we did not find any, various studies report a significant difference in EMR success rates between non-expert and expert endoscopists and advice to refer high-risk EMRs to tertiary centers [Citation9,Citation14]. However, consensus on a clear definition of high expertise is lacking.
Conclusions
PC after EMR is commonly considered useful by endoscopists, usually based on known risk factors for DB. Anticoagulant use was the most important factor in decision-making for PC, independent of endoscopist experience. Although not considered (cost-)effective, one in seven EMR endoscopists also use PC for adenomas <2 cm in the presence of high-weighing risk factors. These results can be used to guide future research and selective implementation of PC.
Author contributions
AT designed and performed the study, collected and analysed the data and wrote the paper. PD and EvG contributed to the design of the study, interpretation of the data and writing the paper. YP contributed to the analysis and interpretation of the data and reviewed the manuscript. LM and PS contributed to the design of the study and writing the paper. RMS contributed to the design of the study and reviewed the manuscript. All authors gave final approval of this version to be published.
Supplemental Material
Download PDF (60.6 KB)Disclosure statement
EvG currently receives clips as material research support from Olympus (Japan) for the CLIPPER trial on prophylactic clipping after EMR, and receives research support from MTW (Germany). PS receives research support from Pentax (Japan) and is on the advisory board of Boston Scientific (USA). LM is a consultant for Boston Scientific (USA). AT’s PhD position is financed by a research grant from the Dutch Digestive Foundation (MLDS). All other authors have nothing to declare.
Data availability statement
The data supporting the results and analyses presented in this paper are available via the corresponding author at reasonable request.
Additional information
Funding
References
- Metz AJ, Bourke MJ, Moss A, et al. Factors that predict bleeding following endoscopic mucosal resection of large colonic lesions. Endoscopy. 2011;43(6):506–511.
- Burgess NG, Metz AJ, Williams SJ, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol. 2014;12(4):651–661.e1-3.
- Bahin FF, Rasouli KN, Byth K, et al. Prediction of clinically significant bleeding following wide-field endoscopic resection of large sessile and laterally spreading colorectal lesions: a clinical risk score. Am J Gastroenterol. 2016;111(8):1115–1122.
- Pohl H, Grimm IS, Moyer MT, et al. Clip closure prevents bleeding after endoscopic resection of large colon polyps in a randomized trial. Gastroenterology. 2019;157(4):977–984.e3.
- Albeniz E, Alvarez MA, Espinos JC, et al. Clip closure after resection of large colorectal lesions with substantial risk of bleeding. Gastroenterology. 2019;157(5):1213–1221.e4.
- Bahin FF, Rasouli KN, Williams SJ, et al. Prophylactic clipping for the prevention of bleeding following wide-field endoscopic mucosal resection of laterally spreading colorectal lesions: an economic modeling study. Endoscopy. 2016;48(8):754–761.
- Forbes N, Frehlich L, James MT, et al. Routine prophylactic endoscopic clipping is not efficacious in the prevention of delayed post-polypectomy bleeding: a systematic review and meta-analysis of randomized controlled trials. J Can Assoc Gastroenterol. 2019;2(3):105–117.
- Feagins LA, Smith AD, Kim D, et al. Efficacy of prophylactic hemoclips in prevention of delayed post-polypectomy bleeding in patients with large colonic polyps. Gastroenterology. 2019;157(4):967–976.e1.
- Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49(3):270–297.
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27(4):417–434.
- Dutch Association of Gastroenterologists (NVMDL). [Dutch Guideline for Endoscopic Polypectomy in the colon] Nederlandse Richtlijn Endoscopische Poliepectomie van het colon [Guideline in Dutch]; 2019. Available from: https://www.mdl.nl/sites/www.mdl.nl/files/richlijnen/Nederlandse%20Richtlijn%20Endoscopische%20poliepectomie-definitef_0.pdf
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health-a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413.
- Clark MD, Determann D, Petrou S, et al. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32(9):883–902.
- Bhurwal A, Bartel MJ, Heckman MG, et al. Endoscopic mucosal resection: learning curve for large nonpolypoid colorectal neoplasia. Gastrointest Endosc. 2016;84(6):959–968.e7.
- Sawhney MS, Salfiti N, Nelson DB, et al. Risk factors for severe delayed postpolypectomy bleeding. Endoscopy. 2008;40(2):115–119.
- Shalman D, Gerson LB. Systematic review with meta-analysis: the risk of gastrointestinal haemorrhage post-polypectomy in patients receiving anti-platelet, anti-coagulant and/or thienopyridine medications. Aliment Pharmacol Ther. 2015;42(8):949–956.
- Acosta RD, Abraham NS, Chandrasekhara V, et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83(1):3–16.
- Veitch AM, Vanbiervliet G, Gershlick AH, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut. 2016;65(3):374–389.
- Chan FKL, Goh KL, Reddy N, et al. Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut. 2018;67(3):405–417.
- Dutch Association of Gastroenterolgists (NVMDL). [Dutch Guideline for the management of antithrombotic therapy around endoscopic procedures] Nederlandse Richtlijn Beleid antitrombotische therapie rondom endoscopische procedures [guideline in Dutch]; 2016. Available from: https://www.mdl.nl/sites/www.mdl.nl/files/richlijnen/Richtlijn_antitrombotische_therapie_final_mei_2016.pdf
- Yu JX, Oliver M, Lin J, et al. Patients prescribed direct-acting oral anticoagulants have low risk of postpolypectomy complications. Clin Gastroenterol Hepatol. 2019;17(10):2000–2007.e3.
- Beppu K, Osada T, Sakamoto N, et al. Optimal timing for resuming antithrombotic agents and risk factors for delayed bleeding after endoscopic resection of colorectal tumors. Gastroenterol Res Pract. 2014;2014:825179.
- Ishigami H, Arai M, Matsumura T, et al. Heparin-bridging therapy is associated with a high risk of post-polypectomy bleeding regardless of polyp size. Dig Endosc. 2017;29(1):65–72.
- Feagins LA, Uddin FS, Davila RE, et al. The rate of post-polypectomy bleeding for patients on uninterrupted clopidogrel therapy during elective colonoscopy is acceptably low. Dig Dis Sci. 2011;56(9):2631–2638.
- Zhang Q, An S, Chen Z, et al. Assessment of risk factors for delayed colonic post-polypectomy hemorrhage: a study of 15553 polypectomies from 2005 to 2013. PLoS One. 2014;9(10):e108290.
- Albeniz E, Fraile M, Ibanez B, et al. A scoring system to determine risk of delayed bleeding after endoscopic mucosal resection of large colorectal lesions. Clin Gastroenterol Hepatol. 2016;14(8):1140–1147.
- Kim GU, Seo M, Song EM, et al. Association between the ulcer status and the risk of delayed bleeding after the endoscopic mucosal resection of colon. J Gastroenterol Hepatol. 2017;32(11):1846–1851.
- Bahin FF, Naidoo M, Williams SJ, et al. Prophylactic endoscopic coagulation to prevent bleeding after wide-field endoscopic mucosal resection of large sessile colon polyps. Clin Gastroenterol Hepatol. 2015;13(4):724–730.e1-2.
- Elliott TR, Tsiamoulos ZP, Thomas-Gibson S, et al. Factors associated with delayed bleeding after resection of large nonpedunculated colorectal polyps. Endoscopy. 2018;50(8):790–799.
- Desomer L, Tate DJ, Bahin FF, et al. A systematic description of the post-EMR defect to identify risk factors for clinically significant post-EMR bleeding in the colon. Gastrointest Endosc. 2019;89(3):614–624.
- Lee HS, Jeon SW, Kwon YH, et al. Prophylactic endoscopic coagulation to prevent delayed postendoscopic mucosal resection bleeding in the colorectum: a prospective randomized controlled trial (with videos). Gastrointest Endosc. 2019;90(5):813–822.
- Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13.
- Chawla S, Qayed E. Learning curve for EMR of large nonpolypoid colorectal neoplasia: an alternative analysis method using longitudinal models. Gastrointest Endosc. 2017;85(6):1309–1310.