Abstract
Objectives: Body weight is one of the factors affecting blood levels of 25-hydroxyvitamin D (25OHD). The aim of this study was to establish whether a vitamin D (vitD) weight-based dosing is more appropriate to a fixed daily dose in patients with inflammatory bowel disease (IBD).
Materials/methods: This was an open label randomised trial. Patients with IBD were assigned to receive oral cholecalciferol at a dose of 28 IU/kg (IU/kg) or 2000 IU per day (IU/day) for 12 weeks during winter months. 25OHD plasma levels and other biochemical parameters were measured at baseline and after supplementation period. The primary outcome measure was 25OHD level after a follow-up period.
Results: A total of 173 patients were analysed. The mean BMI was 25.5 ± 5.1 and initial mean 25OHD level was 62.7 ± 25.5 nmol/l. A similar increase (9.7 ± 26.9 vs 9.8 ± 26.7 nmol/l) in 25OHD levels occurred both in IU/kg and IU/day group. The proportion of subjects with normal and sub-normal levels following the substitution was comparable irrespective of body weight. The change in 25OHD level correlated positively only with the dose of vitD (p < .001) and negatively with the baseline 25OHD level (p < .001). A sustained 25OHD level of 75 nmol/l corresponds with a calculated daily vitD dose of 2034 IU.
Conclusions: Weight-based dosing of vitamin D is not superior to a fixed dose in order to maintain stable 25OHD levels in IBD patients. Cholecalciferol dose of 2,000 IU/day is safe and sufficient during winter period.
1. Introduction
Vitamin D (vitD) is a substance with a number of metabolic and immunomodulatory properties. It affects both innate and adaptive immunity functions. Dysregulation of these immune processes plays a pathophysiological role in the development of inflammatory bowel disease (IBD) [Citation1].
Vitamin D insufficiency is associated with the disease severity and may also adversely affect treatment efficacy [Citation2].
Vitamin D insufficiency is common in IBD cohort [Citation3]. Endogenous sunlight-induced skin synthesis is a major source of vitD. The dietary intake is generally low, averaging only 190 IU/day in Europe [Citation4]. This amount is insufficient to cover the metabolic requirements during winter when the endogenous production is low. A substitution of vitD becomes essential to maintain normal levels. The suggested Recommended Dietary Allowance (RDA) of vitD is 600 IU per day [Citation5,Citation6]. However, some authors have challenged these values, recommending amounts of up to 1500 IU/day [Citation7]. There are currently no guidelines for IBD patients, except for those treated with steroids [Citation8].
The amount of vitD required is influenced by a number of factors such as absorption, gene polymorphisms, age, smoking and sun exposure [Citation9]. 25OHD levels are also affected by body weight. Lower 25OHD concentrations and a smaller increase of 25OHD were recorded in the obese compared with normal body mass index (BMI) cohort [Citation10,Citation11]. Obesity may also have an impact on long-term dynamics of vitD. The decrease of 25OHD level is slower in the obese during winter [Citation12]. However, this relation has not been universally confirmed [Citation13]. It has never been studied in IBD patients before.
We aimed to verify the following hypothesis: a weight-based dosing of vitD is superior to a fixed daily dose in IBD patients. In addition, we seeked to identify the dose required to maintain a normal 25OHD level, along with the factors involved in the efficacy of vitD substitution.
2. Material and methods
2.1. Study design
This was a prospective randomised multicentre study. Patients with Crohn´s disease and ulcerative colitis (age 18–70 years), treated in IBD centres of Bata Regional Hospital in Zlin and University Hospital in Prague, were enrolled from October 2016 to March 2017. The study was approved by the Institutional Review Board of the Bata Regional Hospital. All study participants provided written informed consent. This study is registered at https://clinicaltrials.gov (NCT02958501).
Exclusion criteria covered conditions affecting vitD metabolism: significant renal, liver or cholestatic disease; malabsorption; prior gastric or bowel surgery (other than a standard ileo-caecal resection); treatment with vitD or its analogues; use of phenytoin; hyperparathyroidism (parathormone >8 pmol/l); hypercalcemia (Ca2+>2.65 mmol/l); malignancy; pregnancy; sarcoidosis; IBD with high disease activity scores: Crohn’s Disease Activity Index (CDAI)>220 or Partial Mayo Clinic Score (pMCS)>6; solar bed exposure; a visit to tropical destination during the observation period; 25OHD level > 120 nmol/l; inability to obtain valid data. The following criteria defined active IBD in subjects that were included in our study: ulcerative colitis with pMCS ≥ 3 or endoscopic Mayo Score ≥ 1; Crohn´s disease with CDAI ≥ 150, endoscopic or imaging evidence of disease activity.
The primary outcome measure was 25OHD level after a follow-up period. The secondary outcome measures included the impact of additional factors on the 25OHD level.
2.2. Measurements
The study was conducted from October to March when the endogenous vitD production is minimal. The following data were obtained at the initial visit: basic demographics; anthropometrics; blood levels of calcium, phosphorus, parathormone, C-reactive protein, alkalic phosphatase (ALP) and 25OHD. Patients were then randomly assigned to one of the intervention groups: the fixed daily dose (group IU/day) or the weight-based dose (group IU/kg). A paired laboratory examination was performed between weeks 13–16.
The substitution by oral cholecalciferol (Vigantol gtt., Merck) at a dose of 2,000 IU/day (IU/day) or 28 IU/kg/day (IU/kg) was chosen according to our previous experience. The total derived vitD weekly dose was split into two doses per week. The serum half-life of 25OHD is several times longer than our dosing interval. There was no reported difference in 25OHD levels between the daily and once weekly administration [Citation14]. We therefore consider this conversion acceptable. The dose and the frequency of vitD use was verified using a questionnaire. Persons with adherence to the recommended dosing regimen in the range of ±10% were considered compliant.
The serum 25OHD concentrations were determined using an immunochemiluminescent method by Abbott Architect and Advia Centaur XP (Siemens Healthcare Diagnostics). The variability of the 25OHD assay was ±4 nmol/l. Both methods are standardised within the VDSP (Vitamin D Standardisation Program). According to the inter-laboratory comparisons, the concordance of the results was acceptable [Citation15].
2.3. Study size and statistical analysis
This was a superiority design study with a margin of 8, test power 0.8 and α of 0.05 with estimated sample size at 73 subjects per arm. We have anticipated a 30% drop-out rate and planned to enroll 92 patients in each arm. The participants were randomised using a software generated stratified permuted block randomisation (block size 8) with stratum 25OHD and body weight.
Continuous variables were analysed using paired t test (Wilcoxon test) or unpaired t test (Mann–Whitney test) according to the data normality. The association between categorical variables was assessed using the Fisher exact test. A univariate and multivariate logistic regression model was applied to measure the association of the baseline characteristics with substitution effectivity. The level of statistical significance was set at 0.05 in all analyses.
3. Results
3.1. Basic characteristics
A total of 184 subjects were randomised and 173 were analysed (). The baseline characteristics are presented in . All biochemical parameters were normal. The mean vitD substitution dose was comparable in both groups (IU/day group: 1936 ± 499 IU/day, IU/kg group: 1854 ± 681 IU/day). 60.3% of subjects were compliant with the substitution regimen. The vitD dose used by the noncompliant subjects was lower only in the IU/kg group (19.6 ± 8.8 vs. 27.5 ± 2.3 IU/kg/day, p < .001).
Table 1. Characteristics of the study groups.
3.2. Is weight-based dosing superior to a fixed daily dose?
The initial mean 25OHD level was 61.2 ± 26.0 nmol/L (IU/day) and 62.9 ± 24.9 nmol/L (IU/kg). The 25OHD concentration rose by 9.5 ± 26.8 nmol/l (p = .003) in the IU/day, whereas the change in the IU/kg group was not significant (3.3 ± 26.5 nmol/l). There was a significant (p < .012) but similar increase in 25OHD levels (9.7 ± 26.9 vs. 9.8 ± 26.7 nmol/l) during substitution in both arms in the compliant subjects.
The number of subjects with sustained 25OHD level was comparable in both groups (IU/day vs. IU/kg, 12.8% vs. 16.8% respectively). The proportion of subjects with either increased or decreased levels of 25OHD was also similar.
The IU/kg regimen under-dosed slim subjects (1661 IU/day vs. 2009 IU/day, p < .001) and over-dosed the obese (2550 IU/day vs. 1992 IU/day, p < .001) compared to the IU/day regimen. To account for this, we have compared IU/kg and IU/day groups above and below the median of 75 kg. The number of subjects with both increased, decreased or sustained 25OHD levels were similar in both arms, irrespective of body weight (). In addition, the changes in 25OHD level were not statistically significant.
Figure 2. Proportion of subjects with similar change of 25OHD level after substitution (based on weight category).
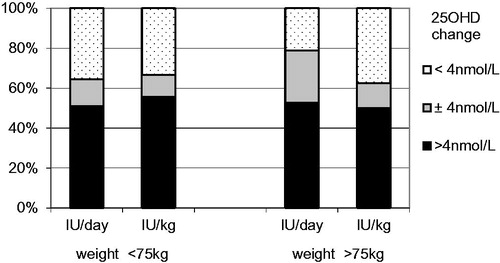
We were unable to show that weight-based dosing of vitD (28 IU/kg) was more effective than 2000 IU of vitD per day. Both dosing regimens were sufficient to prevent a decrease in 25OHD levels during autumn and winter.
3.3. Identifying the required vitD dose
A multifactorial analysis showed an association between the change in 25OHD levels and age (p = .001) and the disease duration (p = .003) only. Strength of these relationships is low (r2 < 0.03). The association with body weight, but not BMI, had only a marginal impact (p = .011, r2=0.05). However, there was a significant association between the change of 25OHD level and the vitD dose (p < .001, r2=0.078) with inverse relationship to the baseline 25OHD level (p < .001, r2=0.35) ().
Table 2. Relationship between 25OH vitamin D level and different variables (multivariate analysis).
In order to estimate the dose required to maintain a satisfactory level of 25OHD, a regression equation was created. Only the relevant variables explaining more than 5% of the total variance (weight, daily dose of vitD, baseline 25OHD level) were included in the analysis.
To establish the dose required for sustained 25OHD level, this equation may be simplified into the following: Daily dose of vitamin D= −357×(9 – 0.15 × initial 25OHD – 0.03 × body weight).
For the average body weight of 75 kg and 25OHD level of 75 nmol/l, this corresponds to the daily vitD dose of 2034 IU.
4. Discussion
On the contrary of expectations, the authors were unable to confirm that a weight-based vitD dosing is superior to a fixed dose.
The association between 25OHD level and obesity, using either BMI or body weight, has been previously described [Citation16–19]. The impact of body weight is seen in efficacy of cholecalciferol substitution. The rise of 25OHD levels is up to 1/3 lower in the obese [Citation11]. In multivariate analysis comprising data from 94 studies, vitD intake per kg of body weight per day was by far the strongest predictor of 25OHD level variation (r2=0.35) [Citation10]. In our multifactorial analysis, both body weight and BMI were of little importance. This could possibly relate to the specific nature of our cohort. The advantage of weight-based dosing might manifest in a cohort with a certain weight span. Different dynamics of 25OHD levels were observed only for the overweight and obese when compared to persons with BMI < 25 [Citation10, Citation18]. This could be the reason we were unable to confirm the higher efficacy of weight-based dosing.
Another important aspect of the weight-based dosing is the size of the dose used. The difference between our groups with a fixed dose and a weight-based dose is approximately 500 IU/day, roughly about 25% of the daily dose. This amount is close to the limit of a possible influence on 25OHD levels [Citation19]. The authors believe these facts could explain the insignificant impact of body weight in our cohort.
Effective substitution in IBD patients may be influenced by certain disease specific factors. Terminal ileal resection has been linked to poor vitD absorption. Farraye et al. studied the dynamics of 25OHD levels following oral vitD administration in patients with both ileal form of CD or ileal resection. 25OHD concentration had no relationship to a particular resected bowel segment [Citation20]. The resorption was proportionate to the length of the bowel resection. Nevertheless, small bowel resections of up to 100 cm (i.e. significantly more than a standard ileo-caecal resection), had no real impact on the efficacy of oral substitution. Other associations such as age and the disease duration did reach statistical significance, however, their contribution to overall variability was minimal.
Groningen et al. found that the required dose of cholecalciferol depends on both the body weight and the baseline 25OHD level [Citation21]. This baseline 25OHD level had the largest and inverse impact on the change of 25OHD in our study. This finding is not unique [Citation22,Citation23]. It is independent of the mode of vitD administration [Citation19,Citation24]. It is therefore a vitD metabolism issue, rather than that of vitD absorption. 25-hydroxylase enzyme isn’t feedback regulated. Following the administration of cholecalciferol, 25OHD level rapidly increases in a linear fashion only up to the serum concentration of 80–100 nmol/l. From this point on, the rise is much slower given the saturated enzymatic capacity of 25-hydroxylase [Citation19].
The authors found the required vitD dose to be about three times that of the recommended amount. This could be explained by the above-mentioned non-linear relationship between the cholecalciferol dose and 25OHD level. The aim of substitution is to maintain normal levels of vitD, i.e. >75 nmol/l 25OHD. If we accept this target value, then the calculated dose for the subject would reach 2034 IU/day. This is in line with critical views on the IOM recommendations [Citation25,Citation26].
One of the reasons of vitD under-dosing is a toxicity concern. Based on the findings in this study, along with the published literature, these concerns are not justified. At a dose of around 2000 IU/day, the calcium and phosphorus levels did not change during the follow-up period. The highest 25OHD value after substitution did not exceed 147 nmol/l. This is far from the reported potentially toxic concentration over 600 nmol/l [Citation27].
4.1. Conclusions
There is no difference between weight-based and fixed dose vitD supplementation in maintaining steady 25OHD plasma levels in IBD patients. Cholecalciferol dose of 2,000 IU/day is safe and sufficient during winter period. It is advisable to actively monitor vitD supplementation as the compliance may be suboptimal.
4.2. Study limitations
The actual vitD dose was based on the patient questionnaire alone and it could not be further verified.
25OHD levels can only be measured after reaching its steady state. This takes at least 4-5 half-lives (15–21 days) [Citation28,Citation29]. The supplementation period of three months might be considered short, however, it has more than accounted for 25OHD levels reaching the steady state. It has also allowed to recruit sufficient numbers of test subjects within the relatively short winter season.
Author contributions
1. Study Design: Vladimir Kojecky, Jan Matous, Bohuslav Kianicka
2. Data Collection: Vladimir Kojecky, Jan Matous, Zdena Zadorova
3. Statistical Analysis: Martina Hlostova, Michal Uher
4. Data Interpretation: Vladimir Kojecky, Martina Hlostova
5. Manuscript Preparation: Vladimir Kojecky, Jan Kubovy, Petr Dite
6. Literature Search: Vladimir Kojecky, Bohuslav Kianicka
Disclosure statement
The authors report no conflict of interest.
References
- Cantorna MT, Zhu Y, Froicu M, et al. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80(6 Suppl):1717S–1720S.
- Zator ZA, Cantu SM, Konijeti GG, et al. Pretreatment 25-hydroxyvitamin D levels and durability of anti-tumor necrosis factor-α therapy in inflammatory bowel diseases. JPEN J Parent Enteral Nutr. 2014;38(3):385–391.
- Narula N, Marshall JK. Management of inflammatory bowel disease with vitamin D: beyond bone health. J Crohns Colitis. 2012;6(4):397–404.
- Gose M, Krems C, Heuer T, et al. Trends in food consumption and nutrient intake in Germany between 2006 and 2012: results of the German National Nutrition Monitoring (NEMONIT). Br J Nutr. 2016;115(8):1498–1507.
- Institute of Medicine (US). Committee to review dietary reference intakes for vitamin D and calcium. Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press (US); 2011.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for vitamin D. EFSA J. 2016;10: 4.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Endocrine Society, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930.
- Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, European Crohn’s and Colitis Organisation [ECCO], et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670.
- Hossein-Nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88(7):720–755.
- Zittermann A, Ernst JB, Gummert JF, et al. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur J Nutr. 2014;53(2):367–374.
- Ekwaru JP, Zwicker JD, Holick MF, et al. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE. 2014;9(11):e111265.
- Forsythe LK, Livingstone MBE, Barnes MS, et al. Effect of adiposity on vitamin D status and the 25-hydroxycholecalciferol response to supplementation in healthy young and older Irish adults. Br J Nutr. 2012;107(1):126–134.
- Gallagher JC, Sai A, Templin T, et al. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156(6):425–437.
- Heaney RP, Armas LA, Shary JR, et al. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87(6):1738–1742.
- Freeman J, Wilson K, Spears R, et al. Performance evaluation of four 25-hydroxyvitamin D assays to measure 25-hydroxyvitamin D2. Clin Biochem. 2015;48(16-17):1097–1104.
- Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196–1199.
- Jungert A, Roth HJ, Neuhäuser-Berthold M. Serum 25-hydroxyvitamin D3 and body composition in an elderly cohort from Germany: a cross-sectional study. Nutr Metab (Lond). 2012;9(1):42.
- Camozzi V, Frigo AC, Zaninotto M, et al. 25-Hydroxycholecalciferol response to single oral cholecalciferol loading in the normal weight, overweight, and obese. Osteoporos Int. 2016;27(8):2593–2602.
- Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77(1):204–210.
- Farraye FA, Nimitphong H, Stucchi A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn's disease. Inflamm Bowel Dis. 2011;17(10):2116–2121.
- van Groningen L, Opdenoordt S, van Sorge A, et al. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur J Endocrinol. 2010;162(4):805–811.
- Mazahery H, von Hurst P. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients. 2015;7(7):5111–5142.
- Ng K, Scott JB, Drake BF, et al. Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am J Clin Nutr. 2014;99(3):587–598.
- Mawer EB, Berry JL, Sommer-Tsilenis E, Beykirch W, et al. Ultraviolet irradiation increases serum 1,25-dihydroxyvitamin D in vitamin-D-replete adults. Miner Electrolyte Metab. 1984;10(2):117–121.
- Singh G, Bonham AJ. A predictive equation to guide vitamin D replacement dose in patients. J Am Board Fam Med. 2014;27(4):495–509.
- Cashman KD, Dowling KG, Škrabáková Z, et al. Vitamin D deficiency in Europe: pandemic. Am J Clin Nutr. 2016;103(4):1033–1044.
- Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, et al. Benefit–risk assessment of vitamin D supplementation. Osteoporos Int. 2010;21(7):1121–1132.
- Feldman D, Pike JW, Bouillon R, et al. Volume 1: Biochemistry, physiology and diagnostics. 4th ed. New York: Academic Press; December 2017, p. 649.
- Vaes AMM, Tieland M, de Regt MF, et al. Dose–response effects of supplementation with calcifediol on serum 25-hydroxyvitamin D status and its metabolites: a randomized controlled trial in older adults. Clin Nutr. 2018;37(3):808–814.