Abstract
Objectives: Distal cholangiocarcinoma (dCCA) is a malignancy with a dismal prognosis. One of the hallmarks is the presence of a rich desmoplastic stroma believed to contribute to tumor progression and treatment resistance. Secreted protein acidic and rich in cysteine (SPARC) is a matricellular glycoprotein implicated in tumor-stroma interaction with prognostic correlation across several malignancies. The aim of the present study was to evaluate the expression pattern and prognostic significance of SPARC in resected dCCA and paired lymph node metastasis.
Materials and methods: SPARC expression was evaluated in 59 resected dCCA samples and 25 paired lymph node metastases as well as 10 benign bile duct samples using immunohistochemistry. Stromal SPARC expression was scored semi quantitatively. Survival was estimated using the Kaplan–Meier method with associated log-rank test.
Results: SPARC expression was absent in normal bile ducts. In dCCA, peritumoral stromal SPARC was detectable in 47/59 (80%) of samples with 40/59 (68%) classified as high stromal SPARC expression. There was a significantly lower proportion of SPARC positive stroma in paired lymph node metastasis 17/25 (68%) than the corresponding primary tumors 24/25 (96%) (p = .016). Stromal SPARC expression was associated with the presence of lymph node metastasis; high SPARC expression 31/40 (78%) versus low SPARC expression 9/19 (47%) (p = .013). In the present material there was no significant association between stromal SPARC expression and survival.
Conclusions: Stromal SPARC expression occurs frequently in dCCA. Although significantly lower than in primary tumors stromal SPARC is frequently retained in paired lymph node metastasis suggesting a possible role in the metastatic process of dCCA.
Introduction
Cholangiocarcinomas (CCA) is an epithelial tumor originating from the bile ducts. CCA is rare, accounting from approximately 3% of gastrointestinal malignancies, and the estimated overall incidence is 2/100,000 [Citation1,Citation2]. CCA has a silent clinical presentation and an aggressive tumor biology with early metastatic spread, translating to an overall 5-year survival of less than 5% [Citation3]. Currently the only treatment with curative potential is radical resection, however only approximately one third of patients are eligible for resection at diagnosis [Citation4]. Additionally, a majority of patients suffer recurrence after resection [Citation5]. Based on anatomical point of origin CCA is subclassified as intrahepatic (iCCA), perihilar (pCCA) or distal (dCCA). DCCA originates between the ampulla of Vater and the cystic duct insertion [Citation6]. Important intertumoral differences between the CCA subgroups with regards to genetic alterations, microenvironment and molecular biomarkers have been demonstrated [Citation7,Citation8]. One characteristic of CCA biology is the presence of a rich desmoplastic stroma [Citation9]. The stroma is believed to contribute substantially to CCA tumour growth, dissemination and treatment resistance [Citation10,Citation11].
Secreted protein acidic and rich in cysteine (SPARC), also known as osteonectin or BM-40 is a non-structural matricellular protein. SPARC has a pleotropic function and have been shown to impact biological functions such as cellular differentiation, wound healing, extracellular matrix (ECM) remodeling, cell-ECM interactions, bone remodeling and angiogenesis [Citation12–17]. In cancer models, SPARC have been shown to impact ECM-composition, cell adhesion, tumor growth, migration, apoptosis and chemosensitivity [Citation18]. Notably SPARC has been shown to have both tumor promoting and suppressive effects in various pre-clinical and clinical models of different cancers. The mechanisms and influence of SPARC expression is believed to be highly context dependent [Citation19]. Alterations of SPARC expression in both epithelial and stromal compartments, with separate prognostic implications, have been demonstrated in several cancer types [Citation20]. Previously SPARC expression was detected in both epithelium and stroma of iCCA with epithelial positive/stroma negative expression correlated with good prognosis [Citation21]. In two studies evaluating SPARC expression in biliary tract cancer (BTC) cohorts stromal but not epithelial SPARC expression was associated with poor survival [Citation22,Citation23].
The aim of the present study was to investigate the expression pattern of SPARC in normal bile ducts, resected dCCA specimens and paired lymph node metastasis and to evaluate the relationship between SPARC expression and prognosis.
Materials and methods
Study population
We identified all patients that underwent surgery with curative intent for dCCA from March 2000 until September 2015 at the Department of Surgery, Skåne University Hospital from institutional records. The inclusion period was selected based on sample availability and no power calculation was performed. Inclusion criteria were surgery with curative intent and macroscopically curative resection (R0 or R1), histopathologically confirmed dCCA, no history of neoadjuvant treatment, no early (30-day) postoperative mortality and formalin-fixed paraffin-embedded (FFPE) tissue from primary resection available in the archives at the Department of Pathology, Skåne University Hospital.
Patients who underwent pancreaticoduodenectomy with a benign diagnosis not involving the common bile duct were identified from a prospective database of pancreaticobiliary surgery maintained at the Department of Surgery, Skåne University Hospital. Samples included in the study were selected based on good tissue quality without dysplasia or inflammation in the common bile duct upon histopathological evaluation.
Demographic and clinical data were retrospectively collected from medical records. Definition of adjuvant chemotherapy treatment was a minimum of 3 treatment cycles of chemotherapy postoperatively. An experienced gastrointestinal pathologist (A.S) revaluated all samples. The histopathological evaluation was performed in accordance to the WHO classification of tumours of the digestive system, 4th edition [Citation24]. Tumour staging was based on American Joint Committee on Cancer (AJCC) 7th edition [Citation25]. R0 resection was defined as cancer growth ≥1 mm from the resection margin [Citation26].
Patients were monitored for recurrence up to 5 years postoperatively at regular intervals with imaging, clinical examination and tumor markers (CA19-9), consequently event free patients were right censored 5 years postoperatively. Survival status was recorded during September 2019, using the Patient Administrative Support in Skåne (PASIS) database. Disease free survival (DFS) was defined as time from surgery until diagnosis of dCCA recurrence or death from any cause. Overall survival (OS) was defined as time from surgery until death from any cause.
Immunohistochemistry
FFPE specimens were sectioned into 4 µm sections. Samples were stained using an Autostainer Plus (Dako/Agilent, Glostrup, Denmark) using the EnVision + System HRP (DAB)-mouse (K4007; Dako/Agilent, Glostrup, Denmark) in accordance with the manufacturer’s instructions. For deparaffinization, rehydration and antigen retrieval, PT link module (Dako/Agilent, Glostrup, Denmark) was used. Samples were incubated with EnVision FLEX Target Retrieval Solution, low PH (K8005; Dako/Agilent, Glostrup, Denmark) for 20 min at 96 °C. The peroxidase block (Dako/Agilent, Glostrup, Denmark) was used for blocking the endogenous peroxidase activity for 5 min. The sections were then incubated with a mouse monoclonal primary antibody against SPARC clone 15G2 (NCL-O-NECTIN; Leica Biosystem, Newcastle, UK) with a dilution of 1:100 for 2 h in room temperature.This is a well validated antibody that previously have been used to evaluate SPARC expression in carcinomas of the breast [Citation27–29], pancreas [Citation30,Citation31], prostate [Citation32,Citation33] and stomach [Citation34]. Afterwards, the labelled polymer-HRP anti-mouse (Dako/Agilent, Glostrup, Denmark) was used for 30 min, followed by 3,3′-diaminobenzidine (DAB) Chromogen (Dako/Agilent, Glostrup, Denmark) for 10 min. Finally, the slides were counterstained with hematoxylin, dehydrated with graded ethanol solutions and mounted. The antibody was tested against pancreatic cancer sections as a positive control. Omission of primary antibody was used as negative control.
Evaluation of immunoreactivity
An experienced gastrointestinal pathologist (A.S), who was blinded to patient identity and outcome, performed the microscopic evaluation of SPARC expression. Staining was reviewed under light microscopy at a total magnification of 200x. SPARC expression was scored on the full slides without any selection of area examined. The percentage of SPARC positive cells was scored on a scale 0–4 (0–10%, 11–25%, 26–50%, 51–75%, >76%) and the intensity of staining was scored as: 0 (negative), 1 (low), 2 (moderate), 3 (strong). A detection of SPARC staining in above 10% of cells was considered positive expression. For dichotomization we used the cut-off previously described in [Citation35]. High expression was defined as moderate or strong expression in > 25% of the stroma.
Statistical analysis
Values are given as mean ± standard deviation (SD), or median with interquartile range (IQR). For categorical values absolute numbers and the distribution in percentages on available data are given. Baseline group comparison was performed using unpaired Mann–Whitney U- or t-test when appropriate for continuous variables, and χ2 or Fisher’s exact test when appropriate for categorical variables. The comparison of expression in primary tumors and paired lymph node metastasis was performed using the exact McNemar’s test.
Survival rates were estimated using the Kaplan–Meier method with associated log-rank test. Statistical analysis was performed with Stata MP statistical package version 14.2 (Stata Corporation, College Station, TX, USA). All tests were two-tailed and a p-value of ≤ .05 was considered significant. The reporting was performed in accordance to the REMARK guidelines where applicable [Citation36,Citation37].
The study was approved by the Regional Human Ethics Committee in Lund Sweden (2015/392).
Results
Patient characteristics
In total 64 patients were identified. Five patients were excluded since no archived FFPE-tissues were available and thus a total of 59 patients were included in the study. The cohort consisted of 39 males and 20 females. The mean age was 67 ± 8.1 years (46–85 years). In total 30 (52%) of patients received adjuvant treatment, with the most common regimen being gemcitabine monotherapy in 26 (48%) patients. Patient and tumor characteristics are presented in (). The benign controls consisted of 5 males and 5 females. The mean age was 67 ± 4.5 years (59–73). The histopathological diagnosis in the benign cohort was tubular adenoma (3), serous cystadenoma (2), pancreatic pseudocyst (1), papillary dysplasia, intraductal papillary mucinous neoplasm of the pancreas without invasive carcinoma (1), pyloric/duodenal ulcer (1) and candida infection of the pancreatic duct (1).
Table 1. Clinicopathological data in the distal cholangiocarcinoma cohort (N = 59).
The median follow-up time was 676 (443–1125) days. For DFS at total of 92 years at risk were observed. The median DFS was 1 (0.5–2) years with estimated 1- and 3- year disease-free survival rates of 49% (95% CI 36–61%) and 19% (95% CI 10–29%). For OS, a total of 130 years at risk were observed. The median survival was 1.9 (1.4–2.1) years with estimated 1- and 3-year survival rates of 78% (95% CI 65–87%) and 25% (95% CI 15–37%), respectively.
Distribution of SPARC expression
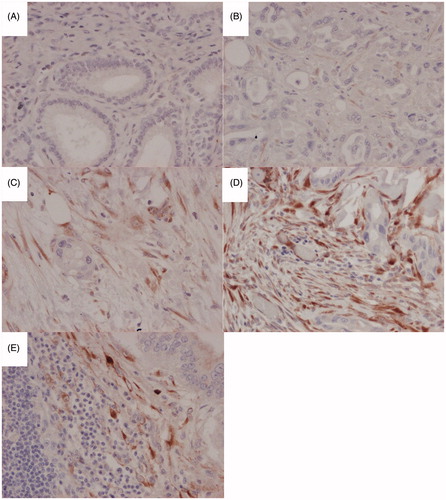
In the 10 normal bile duct specimens stained with the anti-SPARC antibody no immunoreactivity could be discerned. In the dCCA samples abundant peritumoral stromal immunoreactivity was detected. The epithelial cancer component displayed very weak focal immunoreactivity in under 10% cells and as such were not quantified further. The extent of stromal SPARC expression was quantified as 0 in 12 (20%), 1+ in 1 (1.7%), 2+ in 9 (15%), 3+ in 15 (25%) and 4+ in 22 (37%). The intensity of stromal SPARC immunoreactivity was quantified as absent in 12 (20%), weak in 6 (10%), intermediate in 23 (39%) and strong in 18 (31%) (). Positive stromal SPARC expression was detected in 47 (80%) of samples. Stromal SPARC was classified as high in 40 (68%) of samples. Lymph node metastasis was present in 40 patients. Material of sufficient quality to evaluate SPARC expression in paired lymph node metastasis was available from 25 patients. In the paired lymph node metastasis SPARC expression was present and quantified in peritumoral stroma. The intensity of stromal SPARC expression in paired lymph node metastasis was quantified as absent in 8 (32%), weak in 7 (28%), intermediate in 6 (24%) and strong in 4 (16%). Presence of positive stromal SPARC expression decreased from 24/25 (96%) to 17/25 (68%) when comparing primary tumors and paired lymph node metastasis (p = .016).
Figure 1. Representative immunohistochemical images at 200 x magnification of SPARC expression in the stromal compartment of distal cholangiocarcinoma (A), negative expression, (B) weak expression, (C) moderate expression, (D) strong expression, (E) Strong stromal SPARC expression in a paired lymph node metastasis.
SPARC expression and association with clinicopathological variables and survival
We evaluated the correlation between SPARC expression and clinicopathological variables. High SPARC expression was found to be significantly associated with the presence of lymph node metastasis as compared to low SPARC expression 31/40 (78%) vs 9/19 (47%) (p = .013). The correlation between stromal SPARC expression and clinicopathological factors is presented in . Since the cohort was homogenous with regard to T-stage (96% had stage III tumor) no comparison between different T-stages could be performed. Kaplan–Meier analysis with associated log-rank test revealed no significant difference in either DFS (p = .240) or OS (p = .287) between high and low stromal SPARC ().
Figure 2. Kaplan–Meier curve of disease- free survival (A) and overall survival (B) stratified by SPARC expression.
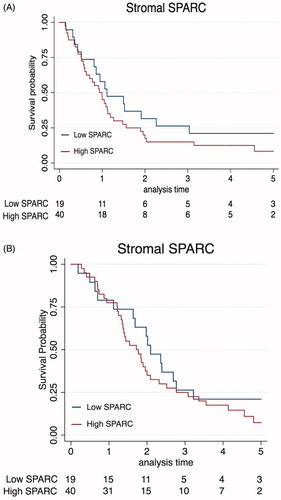
Table 2. Correlation between stromal SPARC expression and clinicopathological factors in distal cholangiocarcinoma.
Discussion
To the best of our knowledge, this is the first study to evaluate SPARC expression in a dCCA-only cohort. We found SPARC to be upregulated in the stroma of a majority of dCCA samples, as compared to absent expression in normal bile ducts. There was a significantly lower proportion of SPARC positive stroma in paired lymph node metastasis as compared to primary tumors, however stromal SPARC expression was frequent also in paired lymph node metastasis. We found stromal SPARC expression to be associated with the presence of lymph node metastasis, however no significant association between stromal SPARC expression and DFS or OS was found.
In addition to stromal SPARC expression cytoplasmic expression in tumor cells has been described in the previous studies of iCCA/BTC. We could only detect focal very weak epithelial immunoreactivity in <10% of cancer cells in the present study. In addition to difference in tumor biology possible explanations for this discrepancy might be differences in SPARC-antibody specificity, however, the antibody used in our study have detected cytoplasmic SPARC expression epithelial tumor cells in several other cancers [Citation27–30,Citation33]. Predominantly stromal SPARC expression has been found in previous studies of different cancers [Citation38,Citation39].
To be best of our knowledge this is the first study to compare the expression of SPARC in primary tumor and paired lymph node metastasis in any CCA subgroup. Although significantly lower than in primary tumors the presence of stromal SPARC in 68% of paired lymph node metastasis suggests that SPARC might be involved in the metastatic process of dCCA. Similar findings have previously been found in pancreatic cancer [Citation31]. The significant association between stromal SPARC-expression in primary tumors and the presence of lymph node metastasis in the present study also supports such a hypothesis.
Stromal SPARC expression has been found to be negatively associated with prognosis after resection in iCCA and BTC [Citation21–23]. In the present study we could not detect a negative prognostic association between stromal SPARC and survival. Admittedly the small sample size makes our study underpowered to detect anything other than a large effect. Reasons for the discrepancy in prognostic association between studies could be the different proportion of samples classified as high stromal SPARC expression. Toyota et al., which used a similar cut-off as in the present study classified 31% in the dCCA subgroup as SPARC high as compared to 68% in our study [Citation22]. Nakashima et al., which also used a similar cut-off to denote positive expression as in our study classified 58% of the samples in the eCCA group as positive as compared to 80% in our study [Citation23]. In the study of the iCCA-cohortwhere moderate to strong intensity in >10% of cells were denoted as high, 32% of samples were classified as stromal SPARC high [Citation21]. Reasons for the higher proportion samples with high stromal SPARC expression in our study could be differences in tumor biology, cut-offutilized, antibody-used and patient selection.
SPARC has been proposed as a treatment predictive biomarker for the response of gemcitabine [Citation40] and nab-paclitaxel [Citation41]. Toyota et al., as well as Nakashima et al. found the negative prognostic impact of SPARC expression to be increased in patients treated with adjuvant gemcitabine, suggesting treatment resistance [Citation22,Citation23]. The low sample size and the non-randomised treatment in our current cohort makes a treatment interaction analysis uncertain, however exploratory subgroup analysis did not reveal any significant interaction (data not shown).
There are limitations with the current study such as the retrospective design. Additionally, the sample size is low, in particular regarding data on expression in paired lymph node metastasis. A strength of the current study is the strict inclusion criteria and systematic reevaluation of samples ensuring a cohort consisting of only dCCA samples.
In conclusion, we found stromal SPARC expression to be frequent in dCCA but absent in benign bile ducts. Stromal SPARC expression was associated with the presence of lymph node metastasis but not significantly associated with prognosis. There was a significantly lower proportion of SPARC positive stroma in paired lymph node metastasis as compared to primary tumors, however stromal SPARC expression was frequently present in paired lymph node metastasis. Further research is warranted to understand the functional role of SPARC in dCCA pathogenesis and its role during metastatic development.
Author contributions
JB, RA and BA conceived and designed the study. KSH performed immunohistochemical staining. AS performed clinicopathological evaluation and evaluation of immunohistochemical staining. JB, JN, BA performed the statistical analysis. JB wrote the manuscript. JB, BA, JN analysed and interpreted the data and revised the manuscript. RA, KSH, AS performed critical revision of the manuscript. All authors approved the final version for publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Kirstein MM, Vogel A. Epidemiology and risk factors of cholangiocarcinoma. Visc Med. 2016;32(6):395–400.
- Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29(2):221–232.
- Mosconi S, Beretta GD, Labianca R, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2009;69(3):259–270.
- Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–1669.
- Khan AS, Dageforde LA. Cholangiocarcinoma. Surg Clin North Am. 2019;99(2):315–335.
- Kendall T, Verheij J, Gaudio E, et al. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):7–18.
- Bragazzi MC, Ridola L, Safarikia S, et al. New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Ann Gastroenterol. 2018;31(1):42–55.
- Byrling J, Andersson B, Marko-Varga G, et al. Cholangiocarcinoma-current classification and challenges towards personalised medicine. Scand J Gastroenterol. 2016;51(6):641–643.
- Sirica AE, Gores GJ. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology. 2014;59(6):2397–2402.
- Brivio S, Cadamuro M, Strazzabosco M, et al. Tumor reactive stroma in cholangiocarcinoma: the fuel behind cancer aggressiveness. World J Hepatol. 2017;9(9):455–468.
- Cadamuro M, Stecca T, Brivio S, et al. The deleterious interplay between tumor epithelia and stroma in cholangiocarcinoma. Biochim Biophys Acta. 2018;1864(4 Pt B):1435–1443.
- Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107(9):1049–1054.
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19(8):815–827.
- Rivera LB, Bradshaw AD, Brekken RA. The regulatory function of SPARC in vascular biology. Cell Mol Life Sci. 2011;68(19):3165–3173.
- Delany AM, Hankenson KD. Thrombospondin-2 and SPARC/osteonectin are critical regulators of bone remodeling. J Cell Commun Signal. 2009;3(3-4):227–238.
- Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009;3(3–4):239–246.
- Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol. 2012;44(3):480–488.
- Nagaraju GP, Dontula R, El-Rayes BF, et al. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis. 2014;35(5):967–973.
- Arnold SA, Brekken RA. SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal. 2009;3(3–4):255–273.
- Ma Y, Chen H, Ma H, et al. Prognostic role of secreted protein acidic and rich in cysteine in patients with solid tumors. Saudi Med J. 2019;40(8):755–765.
- Cheng CT, Chu YY, Yeh CN, Huang SC, et al. Peritumoral SPARC expression and patient outcome with resectable intrahepatic cholangiocarcinoma. Onco Targets Ther. 2015;8:1899–1907.
- Toyota K, Murakami Y, Kondo N, et al. Impact of secreted protein acidic and rich in cysteine (SPARC) expression on prognosis after surgical resection for biliary carcinoma. J Gastrointest Surg. 2017;21(6):990–999.
- Nakashima S, Kobayashi S, Sakai D, et al. Prognostic impact of tumoral and/or peri-tumoral stromal SPARC expressions after surgery in patients with biliary tract cancer. J Surg Oncol. 2014;110(8):1016–1022.
- Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. 4th ed. Lyon: World Health Organization; International Agency for Research on Cancer; 2019.
- Edge S, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York (NY): Springer Verlag; 2010.
- Markov P, Satoi S, Kon M. Redefining the R1 resection in patients with pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. 2016;23(9):523–532.
- McCart Reed AE, Kutasovic JR, Vargas AC, et al. An epithelial to mesenchymal transition programme does not usually drive the phenotype of invasive lobular carcinomas. J Pathol. 2016;238(4):489–494.
- Lindner JL, Loibl S, Denkert C, et al. Expression of secreted protein acidic and rich in cysteine (SPARC) in breast cancer and response to neoadjuvant chemotherapy. Ann Oncol. 2015;26(1):95–100.
- Schneeweiss A, Seitz J, Smetanay K, et al. Efficacy of nab-paclitaxel does not seem to be associated with SPARC expression in metastatic breast cancer. Anticancer Res. 2014;34(11):6609–6615.
- Sinn M, Sinn BV, Striefler JK, et al. SPARC expression in resected pancreatic cancer patients treated with gemcitabine: results from the CONKO-001 study. Ann Oncol. 2014;25(5):1025–1032.
- Gundewar C, Sasor A, Hilmersson KS, et al. The role of SPARC expression in pancreatic cancer progression and patient survival. Scand J Gastroenterol. 2015;50(9):1170–1174.
- Sung S-Y, Chang J-L, Chen K-C, et al. Co-targeting prostate cancer epithelium and bone stroma by human osteonectin-promoter-mediated suicide gene therapy effectively inhibits androgen-independent prostate cancer growth. PLOS One. 2016;11(4):e0153350–e.
- Thomas S, Waterman P, Chen S, et al. Development of secreted protein and acidic and rich in cysteine (SPARC) targeted nanoparticles for the prognostic molecular imaging of metastatic prostate cancer. J Nanomed Nanotechnol. 2011;2(112):2157–7439.
- Wang L, Yang M, Shan L, et al. The role of SPARC protein expression in the progress of gastric cancer. Pathol Oncol Res. 2012;18(3):697–702.
- Ormanns S, Haas M, Baechmann S, et al. Impact of SPARC expression on outcome in patients with advanced pancreatic cancer not receiving nab-paclitaxel: a pooled analysis from prospective clinical and translational trials. Br J Cancer. 2016;115(12):1520–1529.
- Altman DG, McShane LM, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLOS Med. 2012;9(5):e1001216.
- McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):387–391.
- Nakajima M, Yoshino S, Kanekiyo S, et al. High secreted protein acidic and rich in cysteine expression in peritumoral fibroblasts predicts better prognosis in patients with resectable gastric cancer. Oncol Lett. 2018;15(1):803–812.
- Bloomston M, Ellison EC, Muscarella P, et al. Stromal osteonectin overexpression is associated with poor outcome in patients with ampullary cancer. Ann Surg Oncol. 2006;14(1):211–217.
- Liang C, Shi S, Meng Q, et al. Do anti-stroma therapies improve extrinsic resistance to increase the efficacy of gemcitabine in pancreatic cancer? Cell Mol Life Sci. 2018;75(6):1001–1012.
- Giordano G, Pancione M, Olivieri N, et al. Nano albumin bound-paclitaxel in pancreatic cancer: Current evidences and future directions. World J Gastroenterol. 2017;23(32):5875–5886.
