Abstract
Purpose
Clinical trials have demonstrated efficacy of vedolizumab in ulcerative colitis (UC) and Crohn’s disease (CD). Further real-world data is needed to inform clinical practice. The primary outcome was to assess corticosteroid-free and clinical remission after vedolizumab initiation. Secondary outcomes included effect on disease activity scores, biochemical markers, concomitant drug use, endoscopic remission, surgical intervention, hospital admissions and adverse events.
Materials and methods
A multi-centre retrospective observational study was conducted with patients initiated on vedolizumab across seven UK hospitals 1/11/14-30/11/16. Clinical disease activity was assessed using the partial Mayo Scores (pMS) and Harvey Bradshaw Index (HBI). Clinical remission was defined as HBI ≤4 or pMS <2 with a combined stool frequency and rectal bleeding sub score of ≤1. Clinical response was defined as ≥2-point decrease from baseline in pMS and ≥3-point decrease from baseline in HBI.
Results
One hundred ninety-two patients were included in the final analysis. 45% of UC and 10% of CD patients were anti-TNF naive. Over the observation period corticosteroid-free remission rates for UC and CD were 46% and 45%, while clinical remission rates were 52% and 44%, respectively. Time to corticosteroid free remission for UC and CD was 17.6 [IQR: 8.7–29.6] and 14.1 [QR: 6.0–21.7] weeks, respectively. Time to clinical response for UC was 9.4 [IQR: 5.7–15.4] and CD was 9.5 [IQR: 6.1–18.2] weeks. There was a substantial decrease in the concomitant use of immunomodulators and a similar decrease in concomitant corticosteroid use over the study period.
Conclusions
Results in this predominately anti-TNF experienced population mirror other published real-world data, demonstrating good clinical effectiveness and a comparable safety profile.
Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic relapsing inflammatory diseases of the colon and gastrointestinal tract (GI). The estimated prevalence for UC is 250 per 100,000 and 150 per 100,000 for CD [Citation1]. Due to the age of onset and disease duration, IBD medical management incurs a significant financial impact on world-wide healthcare systems [Citation2]. The introduction of anti-tumour necrosis factor (anti-TNF) therapies with an established efficacy has greatly improved options for IBD patients, leading to an improvement in quality of life, reduction in hospitalisation and surgical procedures [Citation3–7]. However, despite the advances in biologic therapy, the rates of primary non-response still remain significant at approximately 30%, and 23%–46% of patients lose response over time, during the maintenance treatment [Citation8–10]. New agents with alternative mechanisms of action have been developed.
Vedolizumab is a humanised monoclonal antibody that binds specifically to the α4β7 integrin, a mediator of gastrointestinal inflammation [Citation11,Citation12]. Binding to α4β7 on memory T-helper lymphocytes inhibits the adhesion to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expressed on gut endothelial cells. The inhibition of this interaction prevents the transmigration of gut-homing memory T cells across the vascular endothelium and the subsequent inflammation [Citation13,Citation14]. The pivotal large-scale GEMINI clinical trials have demonstrated safety and efficacy of vedolizumab in both CD and UC with clinical remission rates of 39% and 42% maintained at 12 months [Citation15,Citation16]. Vedolizumab is effective in the treatment of anti-TNF naive and anti-TNF failure patients and benefits may continue long-term regardless of exposure status [Citation17–19]. However, anti-TNF naive CD patients tend to respond better than those with previous exposure [Citation18,Citation20]. Vedolizumab was granted a Food and Drug Administration (FDA) and European Medicine Agency (EMA) licence for the treatment of moderate to severe UC and CD in 2014. Although clinical trial data is the gold standard on which to base recommendations, results obtained are not always generalisable in clinical practice. Clinical trial patient selection, efficacy outcomes, generalizability and reproducibility vary in comparison to clinical practice. Clinical trials also often lack data that is useful to clinicians and patients, such as time to first clinical remission and response and adverse events in real-world clinical practice. Robust data is required in the real-world setting to help inform practice and the appropriate place of vedolizumab in the IBD treatment algorithm. The recent LOVE-CD study aims to evaluate the efficacy of early versus late use of vedolizumab in CD [Citation21]. While there are phase 3b studies demonstrating the efficacy of vedolizumab (for example, the VERSIFY study [Citation22]), a limited number of real-world studies on vedolizumab have been published to date with only a few based in the UK [Citation23–25].
We report the first real-world experience of vedolizumab in the treatment of IBD in the East Midlands. The primary objective was to describe rates of corticosteroid-free remission and clinical remission in patients with IBD after initiation on vedolizumab over the observation period. Secondary objectives were to describe the change in disease activity scores, median time to remission and response, concomitant drug use, endoscopic remission, surgical treatment, IBD-related hospital admissions and adverse events after initiation on vedolizumab.
Materials and methods
Study population and design
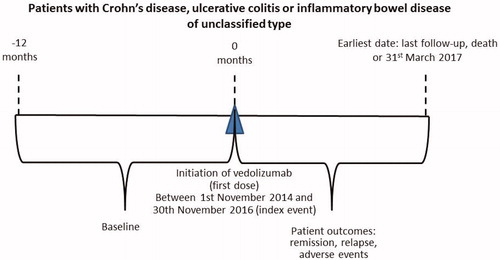
A multicentre retrospective observational study was conducted with all patients initiated on vedolizumab across seven UK hospitals in the East Midlands. The participating centres included both large tertiary sites and smaller district general hospitals (Nottingham University Hospitals NHS Trust, University Hospitals of Derby and Burton NHS Foundation Trust, Sherwood Forest Hospitals NHS Foundation Trust, University Hospitals of Leicester NHS Trust, Chesterfield Royal Hospital NHS Foundation Trust, United Lincolnshire Hospitals NHS Trust and Kettering General Hospital NHS Foundation Trust). The study was designed and conducted according to the requirements of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) [Citation26] and International Society for Pharmacoepidemiology (ISPE) guidance [Citation27]. The study protocol was approved by the Health Research Authority (19/HRA/0008). This study was conducted as part of a regional service improvement project, therefore, NHS REC approval or written informed consent was not required. NHS approval for local conduct of the study and the sharing of anonymised patient data was sought and granted from the Research and Development (R&D) department in each centre. Patients initiated on vedolizumab between 1st November 2014 and 30th November 2016 were followed up until 31st March 2017 ().
Vedolizumab was prescribed as recommended by the manufacturer, induction consisted of intravenous vedolizumab 300 mg at weeks 0, 2 and 6. Maintenance consisted of intravenous vedolizumab 300 mg every 8 weeks until treatment discontinuation or the last follow-up. The dosages of concomitant 5-ASAs, corticosteroids (prednisolone, hydrocortisone, budesonide, beclomethasone) and immunomodulators (azathioprine, mercaptopurine, cyclosporin, methotrexate) were recorded at each clinical visit.
Inclusion and exclusion criteria
Patients aged 18 years or older at initiation of vedolizumab, with a diagnosis of CD, UC or IBD of unclassified type (IBD-U) confirmed by endoscopic, radiological and histological criteria were included [Citation28,Citation29]. IBD-U patients were grouped with CD for the final analysis. Patients whose hospital medical records were unavailable for review or who were enrolled in an interventional clinical trial of an investigational medicinal product during the observation period or who did not receive vedolizumab or were recruited outside the data range for the study were excluded.
Data collection
Data was extracted and anonymised after extensive examination of medical records onto a pre-defined Microsoft Excel spreadsheet. The source data was a combination of clinical electronic and paper records: outpatient letters, prescriptions, day-case visits, laboratory and radiological investigations, endoscopy reports, emergency admissions, radiological and surgical intervention notes. Patient demographics prior to starting vedolizumab and clinical characteristics before and during each clinical visit for the whole treatment duration was recorded. Nottingham University Hospitals was the coordinating site which collated the data and raised any data queries with each participating hospital.
Outcome measures and definitions
Clinical disease activity was assessed at baseline, week 14, 30 and 52 using the Harvey-Bradshaw Index (HBI) and partial Mayo Score (pMS) [Citation30,Citation31]. Sixty-five percent of the disease activity scores were prospectively recorded and thirty-five percent of scores were calculated retrospectively from prospectively collected raw data from medical records. Raw data used for calculating scores was only used if the medical records matched the parameters and clinical evaluations used for the HBI and pMS scores. The primary outcome of this study was to investigate corticosteroid-free clinical remission defined as HBI ≤4 or pMS <2 with a combined stool frequency and rectal bleeding sub score of ≤1 and corticosteroid-free status after initiation of vedolizumab defined as absence of corticosteroid concomitant administration within two weeks either side of clinical scoring date. Secondary outcomes included: time to first clinical remission and clinical response (≥2-point decrease from baseline in pMS and ≥3-point decrease from baseline in HBI), endoscopic remission (absence of ulcers and inflammatory lesions assessed by endoscopy or small bowel magnetic resonance enterography) status at week 14 (±2 weeks), week 30 (±2 weeks) and 52 (±2 weeks) weeks. Other outcome measures included effect on disease activity scores, biochemical markers, concomitant drug use, hospital admissions (admission >24 h) and adverse effects. Patients who failed to show an improvement of symptoms and clinical signs with induction therapy were termed ‘primary non-responders’. Loss of response was defined as when patients lose response to vedolizumab therapy over time.
Statistical analysis
Parametric variables are presented as mean with standard deviations (SD) and non-parametric continuous variables as medians with interquartile ranges [IQR]. Categorical variables are described as frequency with percentages. Time-dependent variables are presented as Kaplan–Meier plots. Wilcoxon signed-rank test was used for analysis of continuous data. A p-value of <.05 was considered as statistically significant. SPSS 23 (IBM, Armonk, NY, USA) software was used for the statistical analysis.
Results
Patient demographics
A total of 206 patients were screened for the study. Fourteen patients were excluded due to not receiving a scheduled first dose of vedolizumab or the initiation date occurred outside the date range for this study. Medical records were available on all patients and none were enrolled into an interventional clinical trial. One hundred ninety-two patients (CD: 99, IBD-U: 6, UC: 87) were treated with vedolizumab. Patient demographics and disease extent are summarised in . One third of patients (29%) had a history of perianal involvement at baseline. Approximately 57% of patients with CD had at least one previous IBD related surgical procedure; 53% of patients had been hospitalised 12 months prior to starting vedolizumab, with a mean rate of hospitalisations during this time of 1.1 (IQR: 0.0–1.0).
Table 1. Patient demographics.
Most patients had prior treatment with corticosteroids (96% and 98% for CD and UC, respectively) and immunomodulators (94% and 93% for CD and UC, respectively). About 90% of CD and 54% of UC patients had previous biologic therapy exposure; 45% and 46% of CD patients failed one or two anti-TNF agents, respectively. For UC, exposure was 41% and 13%, respectively for one or two anti-TNF agents. Median exposure to vedolizumab was 31.0 [IQR 21.3–51.1] weeks for CD and 38.4 [IQR 23.7–61.1] for UC (). The median study observation period for CD and UC was 36.3 weeks [IQR: 22.0–48.1, ≥10 weeks n = 103; ≥26 weeks n = 72; ≥48 weeks n = 30] and 40.4 weeks [IQR: 23.0–56.6 ≥ 10 weeks n = 85; ≥26 weeks n = 61; ≥48 weeks n = 34], respectively. Eleven patients with CD had vedolizumab infusions at week 10 (≥9 and <11). Four of these patients had only received two infusions of vedolizumab prior to their week 10 infusion.
Table 2. Treatment history.
Clinical remission and response
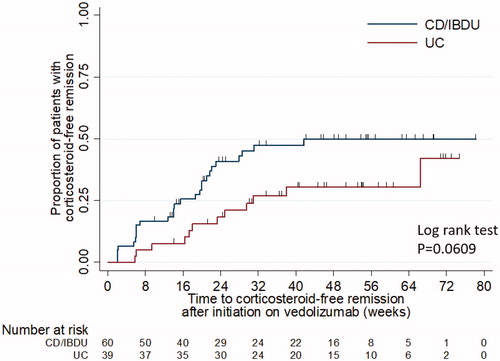
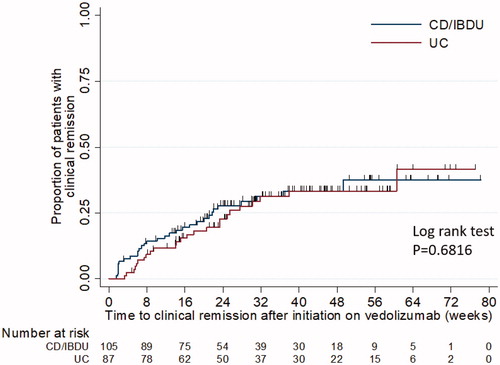
Following initiation on vedolizumab in CD, 27/60 (45%) of patients achieved corticosteroid-free remission with a median time of 14.1 weeks [IQR: 6.0–21.7]. For patients with UC, 39/87(46%) achieved corticosteroid-free remission with median time of 17.6 weeks [IQR: 8.7–29.6], . Clinical remission in CD was observed in 37/70 (44%) of patients (), with a median time of 10.1 weeks [IQR: 3.1–21.0]. For patients with UC, clinical remission was observed in 25/48 (52%) of patients with a median time of 15.1 weeks [IQR: 7.4–24.9]. For patients with CD, 53% achieved clinical response with a median time of 9.5 weeks [IQR: 6.1–18.2]. For patients with UC, 49% achieved clinical response with a median time of 9.4 weeks [IQR: 5.7–15.4]. and summarise clinical remission for all IBD patients and according to prior anti-TNF exposure for UC patients.
Figure 4. Kaplan-Meier plot of time to corticosteroid-free remission according to exposure to anti-TNF therapy for UC cohort only.
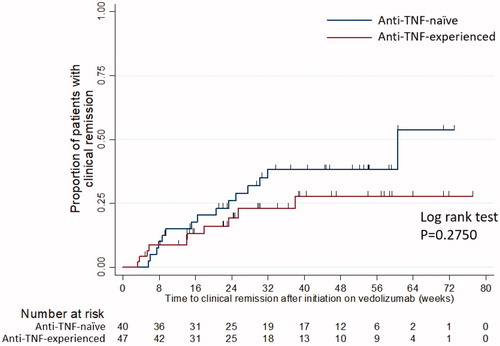
Table 3. Rates of remission and response to vedolizumab achieved during observation period.
Disease activity
Clinical disease activity scores for UC reduced from baseline to: 14 weeks pMS 5 (IQR: 3–6) vs. 3 (IQR: 1–5) p = .025; 30 weeks pMS 5 (IQR: 3–6) vs. 2(IQR: 1–5.5) p = .032; 52 weeks 5 [IQR: 3–6] vs. pMS 2 [IQR: 0–5] p = .27. For CD, disease activity scores decreased from baseline to: 14 weeks HBI 7 (IQR: 5–11) vs. 5 (IQR: 3–7.5) p = .059; 30 weeks HBI 7 (IQR: 5–11) vs. 6.5 (IQR: 2.5–8) p = .61; 52 weeks HBI 7 (IQR: 5–11) vs. 7 (IQR: 7–7) p = .78. Endoscopic remission (endoscopic examination or MRI) was achieved in 53% (25/47), 45% (15/33) and 50% (8/16) at 14, 30 and 52 weeks after initiation of vedolizumab, respectively. Endoscopically measured remission rates (based on SES-CD or endoscopic Mayo [UC] score) were 88% (21/24), 78% (14/18) and 50% (5/10) at week 14, 30 and 52 weeks, respectively. C-reactive protein for UC decreased from baseline by 52 weeks: 29.1 mg/L vs. 13.4 mg/L and decreased from baseline for CD at 52 weeks: 17.7 mg/L vs. 3.8 mg/L. Faecal calprotectin decreased by 14 weeks for UC: 1145 µg/g vs. 664 µg/g and CD 361 µg/g vs. 270 µg/g.
Continuation of vedolizumab
At the end of the observation period, 63% of CD and 70% of UC patients remained on vedolizumab. A total of 65 patients discontinued vedolizumab therapy. The most common reasons for discontinuing vedolizumab in patients with CD/IBDU were primary non-response (28/36 [78%]) and intolerance (5/36 [14%]). The most common reasons in patients with UC were primary non-response (20/24 [83%]) and loss of response (3/24 [13%]).
Concomitant medication
For patients with CD, the number of patients with concomitant medication use decreased over the time of the study. At week 0, 27%, 26% and 23% of patients were co-prescribed corticosteroids, immunomodulators and aminosalicylates, respectively. By week 52, these proportions had decreased to 10%, 7% and 17%. A similar pattern was seen in patients with UC; at week 0, 48%, 41% and 67% of patients were taking corticosteroids, immunomodulators and aminosalicylates, respectively and by week 52 this had decreased to 15%, 18% and 41%. In patients who remained on concomitant therapy there was a reduction in the median dose from baseline to week 52. Budesonide median dose reduced from 9 mg (IQR: 6.0–9.0) at baseline to 2.5 mg (IQR: 2.0–3.0) at 52 weeks. Prednisolone median dose reduced from 30 mg (IQR: 27.5–40.0) at baseline to 20 mg (IQR: 10.0–25.0) at 52 weeks.
Hospital admissions and adverse events
Patients (53%) had at least one hospital admission in the 12 months preceding vedolizumab commencement. This reduced to 30% during the observation period. The mean rate of IBD-related hospital admissions prior to vedolizumab initiation per patient per year for CD and UC was 1.0 (IQR 0–1.0) and 0.9 (IQR 0–2.0), respectively. Following vedolizumab initiation, median rate of hospitalisation for CD and UC were 1.0 (IQR 0–1.0) and 1.0 (IQR 0–1.0), respectively. Twenty-six patients had IBD related surgery (12 patients with CD and 14 patients with UC) during the observation period after initiation of vedolizumab. Four of these patients had two surgical procedures. Overall, 12 adverse events were recorded in patients receiving vedolizumab during the study observation period. The most common was intolerance of vedolizumab (n = 5) (). About 9% of patients were admitted with infections, with respiratory tract infections being the most common. Three patients were diagnosed with a malignancy during the observation period. One patient was diagnosed with basal cell carcinoma and treated with surgery. They received 14 doses of vedolizumab which was later stopped due to loss of response. Another patient who received four doses of vedolizumab (which was later stopped due to non-response) was diagnosed with acute myeloid leukaemia and was started on chemotherapy. The final patient underwent a prostatectomy for prostate cancer. The vedolizumab was continued for more than 12 months by the end of the observation period.
Table 4. Distribution of adverse events in patients receiving vedolizumab.
Discussion
Following initiation on vedolizumab, clinical remission in patients with CD in this study was observed in 44% of patients with a median time of 10.1 weeks [IQR: 3.1–21.0]. For patients with UC, clinical remission was observed in 52% of patients with a median time of 15.1 weeks [IQR: 7.4–24.9]. In CD, 45% of patients achieved corticosteroid-free remission with a median time of 14.1 weeks [IQR: 6.0–21.7]. For patients with UC, 46% achieved corticosteroid-free remission with median time of 17.6 weeks [IQR: 8.7–29.6]. Despite positive results from the GEMINI licencing trials [Citation15,Citation16], real-world data is needed to describe the effectiveness of new therapeutic agents in routine clinical practice. The majority of patients in clinical practice do not fit the tight descriptors typical of pivotal licencing trials. Findings from this multi-centre cohort of patients with IBD demonstrate clinical effectiveness that is comparable to results from randomised controlled trials (RCTs) [Citation15,Citation16] and other studies [Citation23,Citation25,Citation32–36]. Data from this study are generalizable to the rest of the UK due to the inclusion of both small district general hospitals and tertiary centres. Often real-world studies are hampered by incomplete datasets with drug persistence often being the primary outcome to determine long-term effectiveness. This study uses clinically measured disease scores as the tool of choice, making our findings more reflective of patient response to therapy.
Patient characteristics in this cohort, in particular median age and gender distribution are typical for IBD in the UK. Patients with CD were predominately anti-TNF experienced with a median disease duration of over 12 years. This reflects UK guidance from the National Institute of Health and Care Excellence (NICE) for vedolizumab which recommends use after an anti-TNF agent or when one is contraindicated. Whereas in UC, vedolizumab is recommended for use in biologic naive patients when conventional therapy has failed [Citation37,Citation38]. In line with this UK guidance, the rates of corticosteroid and immunomodulatory drug use prior to initiation of vedolizumab were similar to previous real-world studies [Citation32,Citation33,Citation39].
Drug persistence for vedolizumab was 70% and 63% respectively for UC and CD at the end of the study period, similar to previous studies. Ylisaukko-Oja et al., a Finnish cohort of 247 patients reported persistence rates of 66% and 75% for UC and CD, respectively at 6 months initiation [Citation40]. Stallmach et al., a prospective study reported rates of 68% and 55% for UC and CD, respectively at week 30 [Citation41]. Corticosteroid free remission, clinical remission and clinical responses were approximately 50% in this cohort. The GEMINI study reported clinical remission rates of 39% and 42% at 12 months for CD and UC, respectively [Citation15,Citation16] and the recent VARSITY study in UC patients reported clinical remission rates of 31.3% for vedolizumab patients at 12 months [Citation42]. Real-world studies have reported clinical remission rates varying between 21% and 76%, measured at various time-points up to 54 weeks, for both CD and UC [Citation43].
In this study, UC response rates were lower than those in CD. This finding is unique and had not been observed in previous real world and licencing trials. Forty-eight percent of UC patients were on concomitant corticosteroid therapy at the start of vedolizumab therapy as opposed to 27% in CD. Moreover, baseline disease activity in UC could be considered higher with a median pMS of 5 and a faecal calprotectin of 1145 µg/g as compared to a median HBI of 7 and a faecal calprotectin of 361 µg/g. These variables might indicate differential disease severity at baseline in these two diseases hence possibly explaining the observed efficacy in CD as compared to UC. Corticosteroid use in this study was consistent with previous studies which report rates between 29% and 84% [Citation19,Citation33,Citation34,Citation44,Citation45]. Inflammatory burden of disease measured by CRP and FCP reduced following treatment initiation as expected.
The safety profile during follow-up was comparable to previous studies with rates of infections ranging between 1.7% and 12.6% [Citation24,Citation46,Citation47]. However, the sample size and follow-up period were too small to detect significant safety signals.
Over the observation period approximately half of patients with available endoscopic images or small bowel magnetic resonance enterography demonstrated endoscopic remission. These data suggest endoscopic remission with vedolizumab in both CD and UC though the number of patients undergoing these repeated objective investigations are relatively small (29 in CD and 18 in UC). The GEMINI 1 study reported mucosal healing rates in UC of 51.6% at 52 weeks [Citation16]. Dulai et al., a real-world study reported mucosal healing cumulative rates in CD of 63% by 52 weeks of therapy [Citation45]. Over time, a significant number of patients were able to de-escalate corticosteroid and immunomodulator use. Previous trials have demonstrated superiority of combination therapy (biologics with an immunomodulatory agent) over monotherapy [Citation48,Citation49]. Benefit with vedolizumab has not been extensively researched, however, post hoc analysis from GEMINI trials did not suggest additional benefit [Citation50]. Allegretti et al., a multi-centre retrospective study of 136 patients conducted in the US described higher clinical responses for CD but not UC patients with combination therapy [Citation51].
This was the first study to describe the reduction in concomitant medication with vedolizumab therapy in a large cohort of patients. Previous studies have described frequency but not the actual dosage change with vedolizumab. This study also describes ‘time-to’ data in a real-world setting, with results comparable to those reported in the GEMINI and VARSITY studies [Citation15,Citation16,Citation42].
A major limiting factor for this study is the retrospective nature of the design with significant potential for missing data and short follow-up periods for some individuals. The variability of real-world clinical follow-up might have led to selection bias for clinical outcomes. The median time to analysis was defined by time seen in the clinic after induction therapy as reflects real-world practice. Although the majority of disease activity scores were recorded prospectively, some were derived retrospectively from medical records which may limit the accuracy of this data, an example of this is higher values of steroid-free remission seen than complete remission (baseline data was not always available to calculate complete response, whereas steroid-free remission is an absolute value at a specific timepoint). Endoscopic evaluation was also not uniformly available. When available it tended to be performed on individuals with a greater severity of disease. Mucosal healing observations should, therefore, be interpreted with caution as this was only assessed in a quarter of the cohort. Comparison with previous studies cannot accurately be made due to the differences in sample size, study region, clinical practice, patients and study outcome measures. Disease activity scores used to define remission and response vary amongst studies making interpretation and comparison with other cohorts problematic. In this study, a combination of disease activity scores and persistence data were used. Shelton et al. reported simple clinical colitis activity index (SCCAI); Lenti et al. reported physician global assessments and Kopylov et al. used a combination [Citation24,Citation34,Citation35]. Although our patient numbers are comparable to real-world studies, the sample size is severely limited when compared to RCTs. The majority of our patients had previous exposure to anti-TNF. This may be due to the fact that this study was undertaken shortly after NICE approval of vedolizumab in the UK and hence a large number of TNF-refractory patients were switched to vedolizumab. This might indicate the relatively more refractory nature of this cohort when compared to published trials. Another limitation is that the interpretation of adverse event rates is challenging due to the retrospective design leaving the study open to recall bias in the collection of data alongside the lack of a control group. Moreover, another potential limitation was the lack of central reading for endoscopy reports or radiological imaging.
In this real-world vedolizumab cohort study, efficacy data for both UC and CD mirror previous RCTs and real-world data. A significant proportion of patients remained on vedolizumab by the end of the observation period. Our data demonstrated improved disease control, corticosteroid sparing and a decline in concomitant drug use with a comparable safety profile to phase III randomised controlled trials. In a cohort of complex and disease refractory IBD patients, vedolizumab is effective in a UK real-world setting.
Ethical approval
The Health Research Authority does not consider regional service improvement projects or post marketing surveillance research and therefore NHS REC approval was not required for this study.
Author contributions
Guarantor of the article: J.R.W. Conception and design of the study: J.R.W and G.W.M. Data collection: J.R.W, S.D, R.J.M.I, S.F, M.A.A, R.R, R.F, E.T, M.J, D.E, E.A, A.N, M.A, A.S, G.W.M. Analysis and interpretation of data: pH Associates Ltd, J.R.W, G.W.M. Drafting or revision of the manuscript: J.R.W, S.D, R.J.M.I, S.F, M.A.A, R.R, R.F, E.T, M.J, D.E, E.A, A.N, M.A, A.S, N.H, S.M, G.W.M. All the authors approved the final version of the manuscript.
Acknowledgments
Thomas Chu was involved in the conception and design of the study. The abstract of this article was presented as a part of conference proceeding at the European Crohn’s and Colitis Organisation annual meeting (Copenhagen, Denmark, March 2019) and the British Society of Gastroenterology annual meeting (Glasgow, UK, June 2019). Abstracts can be accessed through https://academic.oup.com/ecco-jcc/article-abstract/13/Supplement_1/S454/5300265?redirectedFrom=fulltext and https://gut.bmj.com/content/68/Suppl_2/A102.1. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Disclosure statement
N.H and S.M are employees of Takeda UK Ltd., and were both involved in the interpretation of the data and manuscript development. No other conflicts of interest to report for any authors.
Additional information
Funding
References
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017; 390(10114):2769–2778.
- Baumgart DC, Le Claire M. The expenditures for academic inpatient care of inflammatory bowel disease patients are almost double compared with average academic gastroenterology and hepatology cases and not fully recovered by diagnosis-related group (DRG) proceeds. PLoS One 2016;11(1):e0147364.
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359(9317):1541–1549.
- Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142(2):257.
- Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4(4):355–366.
- Scott FI, Johnson FR, Bewtra M, et al. Improved quality of life with anti-TNF therapy compared with continued corticosteroid utilization in Crohn's disease. Inflamm Bowel Dis. 2019;25(5):925–936.
- Ma C, Moran GW, Benchimol EI, et al. Surgical rates for Crohn's disease are decreasing: a Population-based time trend analysis and validation study. Am J Gastroenterol. 2017;112(12):1840–1848.
- Gisbert JP, Marín AC, McNicholl AG, et al. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41(7):613–623.
- Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7:e135.
- Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341–353.
- Krupka N, Baumgart DC. Designing biologic selectivity for inflammatory bowel disease-role of vedolizumab. Drug Des Devel Ther. 2015;9:147–154.
- Cherry LN, Yunker NS, Lambert ER, et al. Vedolizumab: an α4β7 integrin antagonist for ulcerative colitis and Crohn's disease. Ther Adv Chronic Dis. 2015;6(5):224–233.
- Petkau JM, Eksteen B. Selective biologics for ulcerative colitis and Crohn's disease - clinical utility of vedolizumab. Biologics 2016;10:33–52.
- Lobaton T, Vermeire G, Van Assche G, et al. Review article: anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39(6):579–594.
- Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369(8):711–721.
- Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
- Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2017;15(2):229–239.e5.
- Vermeire S, Loftus EV, Colombel J-F, et al. Long-term efficacy of vedolizumab for Crohn's disease. J Crohns Colitis 2017;11(4):412–424.
- De Vos M, Dhooghe B, Vermeire S, et al., Belgian Inflammatory Bowel Disease Research and Development (BIRD). Efficacy of vedolizumab for induction of clinical response and remission in patients with moderate to severe inflammatory bowel disease who failed at least two TNF antagonists. United European Gastroenterol J. 2018;6(3):439–445.
- Sands BE, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease in patients naive to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. 2017;23(1):97–106.
- Berends S, Löwenberg M, Baert F, et al. DOP046 Higher serum concentrations of vedolizumab are associated with superior endoscopic outcomes in Crohn’s disease: data from the LOVE-CD trial. ECCO. 2018;12(Supp 1):S063–S063.
- Danese S, Sandborn WJ, Colombel J-F, et al. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn's disease. Gastroenterology 2019;157(4):1007–1018.e7.
- Samaan MA, Pavlidis P, Johnston E, et al. Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol. 2017;8(3):196–202.
- Lenti MV, Levison S, Eliadou E, et al. A real-world, long-term experience on effectiveness and safety of vedolizumab in adult patients with inflammatory bowel disease: the Cross Pennine study. Dig Liver Dis. 2018;50(12):1299–1304.
- Plevris N, Chuah CS, Allen RM, et al. Real-world effectiveness and safety of vedolizumab for the treatment of inflammatory bowel disease: the Scottish Vedolizumab Cohort. J Crohns Colitis 2019;13(9):1111–1120.
- European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. [cited 2020 Jul 3]. Available from: www.encepp.eu.
- Public Policy Committee, International Society for Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf 2016;25:2–10. DOI:10.1002/pds.3891
- Magro F, Gionchetti P, Eliakim R, et al., European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11(6):649–670.
- Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: Diagnosis and medical management. J Crohns Colitis 2017;11(1):3–25.
- Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980;1(8167):514.
- Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14(12):1660–1666.
- Amiot A, Serrero M, Peyrin-Biroulet L, et al., OBSERV-IBD study group and the GETAID. One-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther. 2017;46(3):310–321.
- Baumgart DC, Bokemeyer B, Drabik A, et al., Vedolizumab Germany Consortium. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice-a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43(10):1090–1102.
- Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis. 2015;21(12):2879–2885.
- Kopylov U, Ron Y, Avni-Biron I, et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease-the Israeli real-world experience. Inflamm Bowel Dis. 2017;23(3):404–408.
- Engel T, Ungar B, Yung DE, et al. Vedolizumab in IBD-lessons from real-world experience; a systematic review and pooled analysis. J Crohns Colitis 2018;12(2):245–257.
- Vedolizumab for treating moderately to severely active Crohn’s disease after prior therapy: NICE technology appraisal guidance [TA352]. 2015. [cited 2020 Jul 3]. Available from: http://www.nice.org.uk/guidance/ta352.
- Vedolizumab for treating moderately to severely active ulcerative colitis: NICE technology appraisal guidance [TA342]. 2015. [cited 2020 Jul 3]. Available from: http://www.nice.org.uk/guidance/ta342.
- Cummings F, Gaya DR, Levison S, et al. A retrospective observational study of early experiences of vedolizumab treatment for inflammatory bowel disease in the UK: the REVIVE study. Medicine (Baltimore), 2019;98(9):e14681.
- Ylisaukko-Oja T, Aaltonen J, Nuutinen H, et al. High treatment persistence rate and significant endoscopic healing among real-life patients treated with vedolizumab - a Finnish Nationwide Inflammatory Bowel Disease Cohort Study (FINVEDO). Scand J Gastroenterol. 2018;53(2):158–167.
- Stallmach A, Langbein C, Atreya R, et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease – a prospective multicenter observational study. Aliment Pharmacol Ther. 2016;44(11–12):1199–1212.
- Schreiber S, Peyrin-Biroulet L, Loftus EV, et al. OP34 VARSITY: a double-blind, double-dummy, randomised, controlled trial of vedolizumab versus adalimumab in patients with active ulcerative colitis. J Crohns Colitis 2019;13(Suppl 1):S612–S613.
- Shim HH, Chan PW, Chuah SW, et al. A review of vedolizumab and ustekinumab for the treatment of inflammatory bowel diseases. JGH Open 2018;2(5):223–234.
- Amiot A, Grimaud J-C, Peyrin-Biroulet L, et al., Groupe d'Etude Therapeutique des Affections Inflammatoires du tube Digestif. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14(11):1593–1601.e2.
- Dulai PS, Singh S, Jiang X, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn's disease: results from the US VICTORY Consortium. Am J Gastroenterol. 2016;111(8):1147–1155.
- Kotze PG, Ma C, Almutairdi A, et al. Real-world clinical, endoscopic and radiographic efficacy of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48(6):626–637.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46(1):3–15.
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362(15):1383–1395.
- Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146(2):392–400.e3.
- Colombel J-F, Loftus EV, Siegel CA, et al. Sa1270 efficacy of vedolizumab with concomitant corticosteroid or immunomodulator use in patients with Crohn’s disease from GEMINI 2. Gastroenterology 2015;148(4):S-277.
- Allegretti JR, Barnes EL, Stevens B, et al. Predictors of clinical response and remission at 1 year among a multicenter cohort of patients with inflammatory bowel disease treated with vedolizumab. Dig Dis Sci. 2017;62(6):1590–1596.