Abstract
Objective
To examine the role of eosinophils in the pre-diagnostic phase of inflammatory bowel disease (IBD), we studied the influence of genetic and shared environmental risk factors in a twin cohort of IBD.
Material and methods
We analysed eosinophil derived neurotoxin (EDN) and eosinophil cationic protein (ECP) in faecal samples from twin pairs with Crohn’s disease (n = 37) or ulcerative colitis (n = 21) and from external healthy controls (n = 44). Eosinophils stained with eosinophil peroxidase (EPO) were quantified in rectal biopsies. Ratios with 95% confidence intervals were calculated.
Results
Twins with Crohn’ disease displayed higher levels of EDN (Ratio = 2.98, 1.65–5.37) and ECP (Ratio 1.83, 1.24–2.70) than their healthy siblings. Levels did not differ between healthy twin-siblings and external controls (EDN, Ratio = 1.52, 0.79–2.94 and ECP, Ratio = 0.93, 0.56–1.54). Higher levels of EDN (Ratio = 2.43, 1.13–5.24) and ECP (Ratio = 1.53, 0.92–2.53) were observed among twins with ulcerative colitis vs their healthy siblings. Levels did not differ between healthy twin-siblings and external controls (EDN, Ratio = 1.08, 0.51–2.25 and ECP, Ratio = 1.29, 0.74–2.26). Using intra-class correlation coefficient (ICC), we found no agreement in levels of EDN or ECP in discordant pairs, except for ECP in monozygotic Crohn’s disease pairs (ICC = 0.63). In contrast, agreement was observed in monozygotic pairs concordant for Crohn’s disease (EDN, ICC = 0.67 and ECP, ICC = 0.66). The number of eosinophils in rectum was increased in twins with ulcerative colitis vs their healthy sibling (Ratio = 2.22, 1.50–3.27).
Conclusions
Activation of eosinophils in IBD seems to be a consequence of inflammation rather than an effect of genetic and shared environmental risk factors alone.
Introduction
Inflammatory bowel disease (IBD), encompassing the main subtypes Crohn’s disease and ulcerative colitis, is an immune-mediated disease, characterized by a chronic inflammation of the gastrointestinal tract. The precise pathophysiology of IBD is yet to be defined, but based on the currently accepted hypothesis the inflammation arises due to an aberrant immune response to gut microbiota in genetically susceptible individuals. Exposure to environmental risk factors seems to initiate and propagate dysregulation of the innate and adaptive immune pathways. Upon mucosal barrier injury and inflammation, monocytes, mast cells, neutrophil- and eosinophil granulocytes are recruited and accumulated in the intestinal mucosa, whereby the inflammation is maintained by the adaptive immune cells [Citation1,Citation2].
The role of neutrophils in the inflammatory process is well studied and their infiltration of the intestinal mucosa is seen as a hallmark of active disease. More recently, also eosinophils have been associated with IBD. An increasing amount of data indicate that these multi-functional cells may impact on both innate and adaptive immunity by expressing various types of receptors, releasing bioactive mediators, and by activating T-cells through antigen presentation [Citation3]. In addition, eosinophils seem to participate in tissue repair by stimulating fibroblasts [Citation4,Citation5]. Similarly, to the well-recognized release of calprotectin from activated neutrophils, the eosinophils may release various cytotoxic granule proteins such as eosinophil cationic protein (ECP), eosinophil peroxidase (EPO) and eosinophil-derived neurotoxin (EDN) upon activation [Citation6]. The ability to activate both immune cells and fibroblast indicates a dual role for eosinophils in IBD, potentially contributing to both chronic inflammation, tissue remodelling and repair [Citation7]. Previous data indicate a potential difference between Crohn’s disease and ulcerative colitis with respect to the dual role for eosinophils. This difference may reflect the distinct cytokine milieus in the two diseases, as eosinophil-activating Th2-cytokines are much more prominent in ulcerative colitis than in Crohn’s disease [Citation1,Citation8].
Intriguingly, the onset of clinical manifest IBD seems to be preceded by a pre-clinical phase, characterized by immunological changes [Citation9]. Experiences from other chronic immune-mediated diseases indicate that exposure to genetic and environmental risk factors represents the initial key drivers that lead to the initiation and propagation of a dysregulated immune response and ultimately clinical manifest IBD. Studies of healthy first-degree relatives of patients with IBD have reported that a subset of these relatives express increased levels of various markers of innate and adaptive immunity. More specifically, we previously demonstrated that healthy twin siblings in twin pairs discordant for IBD display a dysfunctional intestinal barrier [Citation10], with a subclinical mucosal inflammation and increased neutrophil activity, as reflected by elevated mucosal levels of nuclear factor kappaB (NFκB) and myeloperoxidase (MPO) as well as faecal levels of calprotectin [Citation11]. Whether the eosinophil granulocytes are activated as part of this early subclinical immune response, or if these cells become activated as a consequence of disease is unknown. Similarly, the relative contribution of genetic and environmental risk factors to activation of eosinophils in IBD is yet to be explored. Twin studies could be of value in this respect. Monozygotic twins are genetically identical and share environmental factors during childhood and adolescence while dizygotic twins share environment but only half of the genes are common.
Therefore, to assess whether activation of eosinophils is a primary defect or a consequence of inflammation, we performed a twin study, and examined the concentration of EDN and ECP in faecal samples and also the number of activated eosinophils in mucosal biopsies. In addition, we aimed to evaluate the influence of genetics and shared environmental exposure on eosinophilic granulocytes.
Materials and methods
Twin cohort
The twins included were identified from a previously described national population-based cohort of twins with IBD in Sweden. In short, the unique personal identification number, issued to all Swedish residents [Citation12], was used to link records from the Twin Registry [Citation13] and the National Patient Register. All twins who had been admitted to hospital with a diagnosis of IBD and their siblings were identified. Both twins in each pair were asked to respond to a questionnaire regarding general gastrointestinal symptoms. The medical notes were scrutinized, after written consent from each twin, for verification of diagnosis of IBD and for determination of disease phenotype, according to the Montreal Classification [Citation14]. Zygosity was determined through questionnaire data about intra-pair similarities in childhood, being of opposite sex, or DNA analyses by the Twin Registry. The questionnaire has been validated previously and has an estimated accuracy of >98% when compared with DNA analyses [Citation15].
Twins of the same sex were asked to provide a faecal sample for measurement of EDN and ECP concentrations and those who were living in the middle part of Sweden were also invited to undergo a colonoscopy. Previous extensive IBD-related surgical intervention, i.e., colectomy or extensive small bowel resection, was an exclusion criterion.
External controls
As a reference population, a control group (n = 44) of apparently healthy individuals were recruited. Their health status was monitored by a questionnaire where each individual certified that they were not suffering from thyroid disease, heart and vascular disease, cancers, joint disease, diabetes, liver disease, lung disease, allergic disease, dermatitis or eczema, food allergy, IBD or other gastrointestinal disease, frequent urinary infection or other recent infection and not receiving any anti-inflammatory treatment. The median age (IQR) of the control group was 43.5 (33–53) years [Citation16].
Measurements of ECP and EDN in faecal samples
Stool samples were collected and stored at −70° C. Before analysis, the samples were thawed and faecal extracts were prepared as described previously [Citation16]. Faecal ECP was analysed using UniCAP (Phadia AB, Uppsala, Sweden) according to the manufacturer's protocol and EDN was measured by an in-house ELISA assay. The levels of markers in faeces were adjusted for water content [Citation16] and ECP and EDN levels were expressed as micrograms per gram of semidry faeces. Normal levels were set to: ECP ≤5.81 µg/g and EDN ≤2.15 µg/g. The intra-and inter-assay variations were less than 10% for all assays.
Histopathology and immunohistochemistry
Hematoxylin and eosin-stained sections from rectum were scored by an experienced pathologist and classified as histologic active or inactive, according to the Geboes histologic score [Citation17].
Immunohistochemical analyses for quantification of activated eosinophils were performed on biopsies from rectum in the group of twins earlier defined. From the biopsies that were formalin-fixed and embedded in wax, sections were cut and mounted on clean glass slides. Slides were then deparaffinized in xylene, rehydrated through decreasing concentrations of alcohol and finally rinsed in TBS Auto Wash Buffer (pH 7,6) (Biocare Medical, Concord, CA, USA). A monoclonal antibody to EPO (generous gift from Professor Per Venge, University of Uppsala, Sweden) was used to identify eosinophil granulocytes. Sections were incubated with the antibody overnight at room temperature in a humid chamber. The antigen-antibody complex was visualized by enzymatic reaction using a commercial HRP-polymer kit (MACH-1 Universal HRP-polymer kit, Biocare Medical, Concord, CA, USA) according to the protocol achieved from the manufacturer. The sections were mounted with Pertex (HistoLab Products AB, Västra Frölunda, Sweden). Ten high power fields (hpf) on each section were examined by two individuals in a blinded manner using a Leica DRMB microscope (x320) and EPO stained cells were counted. If the number of cells differed >3 standard deviations, they were counted a third time by another independent individual.
Statistical analyses
Continuous data are presented as median (inter-quartile range). Mean concentrations of faecal markers and median eosinophil counts for the different twin groups were calculated and estimates of the differences between the groups were analysed using regression analysis of a mixed model design with allowance for dependence within the twin pair. Twin pairs are considered randomly selected clusters, but twins within the cluster are dependent units. Therefore, the analysis must acknowledge this fact and the statistical model should be adjusted accordingly [Citation18]. To comply with the statistical assumptions, group comparisons had to consider the skew distributions and were, therefore, calculated based on the elogarithm of the concentrations and the median eosinophil counts, and then the results were transformed back to the original scale. The effect parameter is therefore the ratio of geometric means rather than differences [Citation19] and here denoted as Ratio. A statistically significant result at the 5% level is accordingly found if the 95% confidence interval for this ratio does not include the value 1.0. To specifically address the question of agreement of the concentrations of the faecal markers within the twin pairs, we calculated the intraclass correlation coefficient (ICC), based on the elogarithm of the concentrations, according to Dunn [Citation20]. The ICC coefficient not only depends on the degree of association between two measures but also reflects the equality between the measures. The ICC ranges from −1.0 to 1.0. A positive ICC is observed when there are small differences within the pairs but the means between the pairs differ substantially; a negative ICC is observed when the within-pair differences are greater than the between pair differences. To get insight into the influence of exposure to genetic and shared environmental risk factors, we compared the ICC for monozygotic and dizygotic twin groups with the ICC for external healthy controls. To generate pairs of the external controls (n = 44), we matched these individuals by age ± 6 years and sex. However due to differences in age we were only able to match 21 pairs. In particular, it is assumed from genetic theory that monozygotic twins share all genetic factors that influence a phenotype while dizygotic twins share one-half of their genes. Thus, the usual assumption is to expect the ICC for monozygotic MZ twins to be double that of dizygotic DZ twins [Citation21]. The statistical software used was SPSS version 26, SAS version 9.4 and STATA version 15.
The study was approved by the Regional Ethics Review Board, Uppsala, Sweden.
Results
Twin cohort
In total, 58 twin pairs participated, of these 37 with Crohn’s disease (concordant monozygotic n = 6, discordant monozygotic n = 14, concordant dizygotic n = 1 and discordant dizygotic n = 16) and 21 pairs with ulcerative colitis (concordant monozygotic n = 1, discordant monozygotic n = 11 and discordant dizygotic n = 9). The median (interquartile range, IQR) disease duration of the twins with Crohn’s disease was 23.5 (18–32) years and the corresponding duration for twins with ulcerative colitis was 26.5 (22–38.5) years ().
Table 1. Clinical characteristics of twins with inflammatory bowel disease.
Faecal EDN and ECP – Crohn’s disease
To compare faecal concentrations of EDN and ECP between groups, a regression analysis of a mixed model design was used and the ratio with 95% confidence interval (95% CI) was calculated. Twins diagnosed with Crohn’s disease had higher levels of EDN (Ratio = 4.53, 2.39–8.66) and ECP (Ratio 1.71, 1.01–2.90) than external controls (, , Supplementary Table 1). Consistently, the diseased twins expressed higher levels of EDN (Ratio = 2.98, 1.65–5.37) and ECP (Ratio = 1.83, 1.24–2.70), when controlling for genetic predisposition and shared environmental exposure, i.e., when comparing with the healthy twin-siblings. Healthy twin-siblings had similar EDN (Ratio = 1.52, 0.79–2.94) and ECP-levels (Ratio = 0.93, 0.56–1.54) as external controls. To examine the influence of genetic vs shared environmental risk factors, we stratified the analyses for zygosity. The EDN and ECP levels in faecal samples from healthy twin siblings among monozygotic- and dizygotic pairs discordant for Crohn’s disease (Supplementary Table 1), did not differ from the corresponding levels in faecal samples from external controls ().
Figure 1. The median concentration (IQR) of faecal EDN and ECP in twins with Crohn’s disease and ulcerative colitis, their healthy siblings and external controls. EDN and ECP concentrations between groups were compared using a regression analysis of a mixed-model design, significances are shown with staples (*0.01< p ≤ .05, **.001< p ≤ .01, ***p ≤ .001).
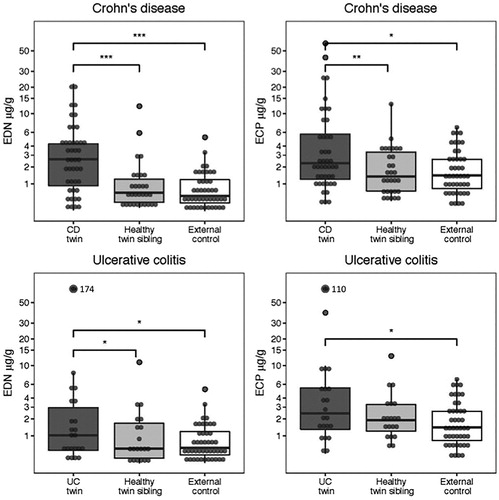
Table 2. The ratio of EDN and ECP respectively between different selected groups of twins and healthy controls. To compare EDN and ECP, a regression analysis of a mixed-model design was used and a ratio related to geometric means (Ratio) with 95% confidence interval (95% CI) was calculated.
Faecal EDN and ECP – ulcerative colitis
Twins with ulcerative colitis had significant higher faecal levels of EDN (Ratio = 2.62, 1.20–5.69) and ECP (Ratio = 1.97, 1.04–3.74) than external controls. Consistently, they seemed to express higher levels of EDN (Ratio = 2.43, 1.13–5.24) and ECP (Ratio = 1.53, 0.92–2.53) than their healthy siblings (, , Supplementary Table 1). No difference was observed between healthy twin-siblings and healthy controls (EDN, Ratio = 1.08, 0.51–2.25 and ECP, Ratio = 1.29, 0.74–2.26). In addition, we stratified the analyses of twin pairs for zygosity. The EDN and ECP levels in faecal samples from healthy twin siblings among monozygotic- and dizygotic pairs discordant for Crohn’s disease (Supplementary Table 1), did not differ from the corresponding levels in faecal samples from external controls ().
Agreement on EDN and ECP levels within twin pairs
To evaluate the influence of genetic and shared environmental risk factors on the expression of eosinophilic faecal markers, concentrations of EDN and ECP within twin pairs were compared, using the ICC and stratifying for zygosity, concordance, or discordance for IBD status (). Within monozygotic twin pairs concordant for Crohn’s disease, a high agreement of EDN (ICC = 0.67) and ECP (ICC = 0.66) was observed. As there was only one dizygotic pair concordant for Crohn’s disease, ICC could not be calculated for this group. In addition, a high value (ICC = 0.63) was observed among monozygotic pairs discordant for Crohn’s disease with respect to ECP levels.
Table 3. Intraclass correlation coefficient for concentrations (elogarithm) of EDN and ECP in different groups of twins.
Eosinophil cell count in rectum
Immunohistochemistry with antibodies against eosinophil peroxidase (EPO) was performed on rectal biopsies from twins with Crohn’s disease (n = 31), twins with ulcerative colitis (n = 19) and healthy twin siblings among twin pairs discordant for Crohn’s disease (n = 20) and for ulcerative colitis (n = 17, ).
Figure 2. Immunohistochemical identification of eosinophils with EPO in rectal biopsies from: (a) twin with active Crohn’s disease, (b) healthy sibling among pair discordant for Crohn’s disease, (c) twin with active ulcerative colitis, (d) healthy sibling among pair discordant for ulcerative colitis
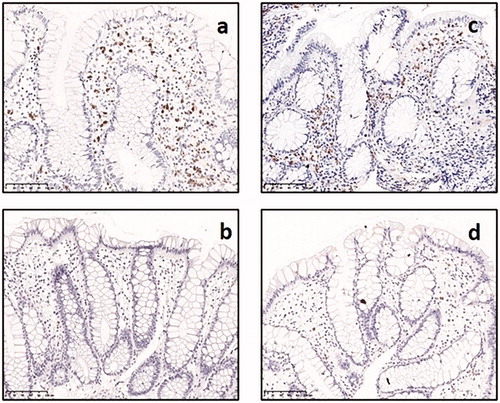
Twins with ulcerative colitis had higher median numbers of EPO+ eosinophils in the rectal mucosa (5.15 per hpf) than their healthy siblings (1.95 per hpf, Ratio = 2.21, 1.50–3.26, ). No difference was observed between the twins with Crohn’s disease (3.25 per hpf) and their healthy siblings (2.30 per hpf), with respect to number of EPO+ eosinophils (Ratio = 1.26, 0.90–1.76). Among the twins with Crohn’s disease, only four twins exhibited a histologically active inflammation in rectum, and the median eosinophil count in this specific group was 10.9 per hpf. The median number of eosinophils per hpf was 1.75 in healthy controls, but there were too few healthy controls (n = 5) to allow any statistical comparison with this group.
Table 4. The ratio of eosinophils counts defined as elogarithm (median) in rectal biopsies, identified by immunohistochemical staining, between different selected groups of twins. To compare eosinophil counts, a regression analysis of a mixed-model design was used and a ratio related to geometric means (Ratio) with 95% confidence interval (95% CI) was calculated.
Discussion
By exploring twin pairs with IBD, we could analyse the presence and activation status of eosinophils among healthy individuals who are genetically and environmentally predisposed to the disease, namely healthy twin-siblings among pairs discordant for ulcerative colitis or Crohn’s disease. Our data demonstrate that exposure to shared environmental and genetic risk factors do not lead to the activation of eosinophils, we could not observe any significant increase in activated eosinophils. These findings indicate that eosinophils do not play a primary role in early disease initiation of IBD.
Similar to many other chronic immune mediated diseases, the onset of clinical manifestations of IBD seems to be preceded by a pre-clinical phase of the disease. In this early phase of disease development, interactions between genetic and environmental risk factors translate into activation and propagation of immune-pathways that ultimately may lead to a subclinical inflammation as observed in some healthy first-degree relatives, including healthy twin siblings of patients with IBD [Citation11]. However, only in a few of these individuals the inflammation progresses into a clinical manifest colitis with activation of various immune cells, including neutrophils, eosinophils, lymphocytes, macrophages and mast cells. There is currently a knowledge gap with respect to which cell-types that are involved in the early preclinical phases of disease development.
Activation of neutrophils is seen as a hallmark of IBD, but the gastrointestinal tract represents a complex and sophisticated immune system, and the breakdown of the mucosal immune homeostasis in patients with IBD does not translate into activation of neutrophils only. Bischoff reported an increased count of eosinophils in patients with IBD already in 1996 [Citation22]. However, recent advances in our understanding of the mucosal immune system have revealed that eosinophils are recruited to the submucosa and activated in the lamina propria upon inflammation, a process regulated by IL-5 and chemotaxins [Citation23]. The activation leads to a release of eosinophil granule proteins into the lumen, as reflected by elevated levels of eosinophil granule proteins in both intestinal perfusion fluid [Citation24] and faeces [Citation16].
Based on analyses of myeloperoxidase in mucosal biopsies and calprotectin in faecal samples from the same twin cohort, we have previously demonstrated that neutrophils are activated in approximately two-thirds of healthy twin siblings in this cohort [Citation11]. There is data that indicate crosstalk between neutrophils and eosinophils. For example, neutrophils promote the activation of eosinophils via IL-33 [Citation25], a cytokine derived from epithelial cells that alerts the immune system of tissue or cell damage. IL-33 – and in particular its processed and mature forms – is highly bioactive driving the Th2 type immune response. The processing of IL-33 is carried out by cathepsin G and elastase released from neutrophils rapidly recruited to the injured tissue. It has recently been demonstrated that IL-33 generates eosinophil activation with adhesion, degranulation of EDN (previously known as eosinophil protein X (EPX)) and expression of surface protein with similar potency as IL-3, IL-5 or eotaxins [Citation25,Citation26]. In spite of our previously reported increased neutrophil activity and subclinical inflammation, defined by NFκB activity, in the healthy twin siblings [Citation11], we could not demonstrate any activation of the eosinophils in these twins. Interestingly, we recently reported increased levels of eotaxin-1 (CCL11), a strong chemoattractant of eosinophils in pre-diagnostic plasma samples from individuals who later in life developed ulcerative colitis and in samples from treatment-naïve incident ulcerative colitis patients [Citation27]. Taken together, these findings may suggest that eosinophil-driven mucosal inflammation is not triggered by shared genetic and environmental risk factors, but represents an element in a later phase of pre-diagnostic ulcerative colitis, i.e., before onset of clinically manifest disease.
In healthy individuals at homeostasis, eosinophils reside predominantly in the lamina propria of the gastrointestinal tract, exhibiting a gradient with increasing numbers in the distal direction. The maximal count is found in cecum and ascending colon, then the numbers decrease again with a low count in rectum [Citation28,Citation29]. Our immunohistochemical staining of eosinophils in the rectal mucosa confirmed that the eosinophils are present at low numbers in the unaffected twin-siblings, and significantly increased in the twins with ulcerative colitis.
The IBD twin cohort is a unique material but one limitation of this study is the relatively low number of twins enrolled. The analyses of faecal EDN and ECP in healthy twin siblings and healthy external controls generated fairly similar results both when mono- and dizygotic twins where analysed all together or separately among pairs discordant for Crohn’s disease. When comparing within-pair agreement between monozygotic and dizygotic pairs discordant for disease status, an agreement was only seen in terms of ECP levels among monozygotic pairs discordant for Crohn’s disease. One may speculate that the lack of differences could be related to the limited number of twins as a result from dividing for zygosity. The group of Crohn’s disease twins where highly heterogenous in location and behaviour of disease. In accordance with previous data on concentrations of antibodies to some microbial antigens, such as anti-Saccharomyces cerevisiae (ASCA), Pseudomonas fluorescens-related protein (anti-I2), Escherichia coli outer membrane porin C (anti-OmpC), a high ICC-value was observed for levels of EDN and ECP among monozygotic pairs concordant for Crohn’s disease [Citation30,Citation31]. Previous twin studies have demonstrated pronounced differences between twins with IBD, especially Crohn’s disease, and their healthy twin siblings with respect to the composition of gut microbiota [Citation32–34]. If these dissimilarities contributed to the observed differences in the activation of eosinophils can only be speculated on, since gut microbiota was not examined in this study. Another important limitation of the study, is that we were not able to examine the influence of non-shared environmental factors. Thus, it cannot be precluded that non-shared environmental factors, such as smoking and dietary habits during adulthood, play a role in the activation of eosinophils.
In summary, our new data indicate that eosinophils, as opposed to neutrophils, are not active in the early phase of subclinical mucosal inflammation. In light of these findings, activation of eosinophils must be considered as a consequence of inflammation rather than a primary event, triggered by exposure to genetic and shared environmental risk factors alone. The interplay between immune cells is complex and further studies are needed to characterize the chain of early events that ultimately leads to clinically manifest IBD.
| Abbreviations | ||
| EDN | = | Eosinophil derived neurotoxin |
| ECP | = | Eosinophil cationic protein |
| EPO | = | Eosinophil peroxidase |
| IBD | = | inflammatory bowel disease. |
Supplemental Material
Download MS Word (14.3 KB)Acknowledgement
We thank Ingrid Stolt for skilful assistance in preparations of samples and assessment of eosinophil markers. We also thank Åke Öst for histological assessment.
Disclosure statement
JH has served as speaker and/or advisory board member for AbbVie, Celgene, Celltrion, Ferring, Gilead, Hospira, Janssen, MEDA, Medivir, MSD, Novartis, Olink Proetomics, Pfizer, Prometheus Laboratories, Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, and UCB. He also received grant support from Janssen, MSD and Takeda. IS has received lecture fees from Meda and Nutricia. MC has served as a speaker and/or advisory board member for Janssen, Pfizer, Takeda and Vifor Pharma. For the remaining authors none were declared.
Additional information
Funding
References
- Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94(1):155–165.
- Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):362–381.
- Filippone RT, Sahakian L, Apostolopoulos V, et al. Eosinophils in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(7):1140–1151.
- Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014;5:570.
- Venge P. The eosinophil and airway remodelling in asthma. Clin Respir J. 2010;4(Suppl 1):15–19.
- Blanchard C, Rothenberg ME. Chapter 3: biology of the eosinophil. Adv Immunol. 2009;101:81–121.
- Lampinen M, Rönnblom A, Amin K, et al. Eosinophil granulocytes are activated during the remission phase of ulcerative colitis. Gut. 2005;54(12):1714–1720.
- Lampinen M, Backman M, Winqvist O, et al. Different regulation of eosinophil activity in Crohn's disease compared with ulcerative colitis. J Leukoc Biol. 2008;84(6):1392–1399.
- Torres J, Burisch J, Riddle M, et al. Preclinical disease and preventive strategies in IBD: perspectives, challenges and opportunities. Gut. 2016;65(7):1061–1069.
- Keita ÅV, Lindqvist CM, Öst Å, et al. Gut barrier dysfunction: a primary defect in twins with Crohn’s disease predominantly caused by genetic predisposition. J Crohns Colitis. 2018;12(10):1200–1209.
- Zhuhlina Y, Hahn-Strömberg V, Shamikh A, et al. Subclinical inflammation with increased neutrophil activity in healthy twin siblings reflect environmental influence in the pathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(8):1725–1731.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667.
- Magnusson PK, Almqvist C, Rahman I, et al. The Swedish Twin Registry: establishment of a biobank and other recent developments. Twin Res Hum Genet. 2013;16(1):317–329.
- Silverberg MS1, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl a):5A–36A.
- Lichtenstein P, De Faire U, Floderus B, et al. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252(3):184–205.
- Peterson CG, Eklund E, Taha Y, et al. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterology. 2002;97(7):1755–1762.
- Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404–409.
- Carlin JB, Gurrin LC, Sterne JA, et al. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34(5):1089–1099.
- Bland JM, Altman DG. Statistics Notes: the use of transformation when comparing two means. BMJ. 1996;312(7039):1153.
- Piegorsch WW. Design and analysis of reliability studies: the statistical evaluation of measurement errors. Graham Dunn, Oxford University Press, New York, 1989. Statist Med. 1991;10(1):156–158.
- Bouchard TJ, McGue M. Genetic and environmental influences on human psychological differences. J Neurobiol. 2003;54(1):4–45.
- Bischoff SC, Wedemeyer J, Herrmann A, et al. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996;28(1):1–13.
- Rothenberg ME, Mishra A, Brandt EB, et al. Gastrointestinal eosinophils. Immunol Rev. 2001;179(1):139–155.
- Carlson M, Raab Y, Peterson C, et al. Increased intraluminal release of eosinophil granule proteins EPO, ECP, EPX, and cytokines in ulcerative colitis and proctitis in segmental perfusion. Am J Gastroenterol. 1999;94(7):1876–1883.
- Lefrançais E, Roga S, Gautier V, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci Usa. 2012;109(5):1673–1678.
- Angulo EL, McKernan EM, Fichtinger PS, et al. Comparison of IL-33 and IL-5 family mediated activation of human eosinophils. PLoS One. 2019;14(9):e0217807.
- Bergemalm D, Andersson E, Karlin P, et al. Markers of systemic inflammation in preclinical ulcerative colitis. Abstract United European Gastroenterol J. 2019;7(8 suppl):111.
- DeBrosse CW, Case JW, Putnam PE, et al. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006;9(3):210–218.
- Matsushita T, Maruyama R, Ishikawa N, et al. The number and distribution of eosinophils in the adult human gastrointestinal tract: a study and comparison of racial and environmental factors. Am J Surg Pathol. 2015;39(4):521–527.
- Amcoff K, Joossens M, Pierik MJ, et al. Concordance in Anti-OmpC and Anti-I2 indicate the influence of genetic predisposition: results of a European Study of Twins with Crohn's Disease. J Crohns Colitis. 2016;10(6):695–702.
- Halfvarson J, Standaert-Vitse A, Järnerot G, et al. Anti-Saccharomyces cerevisiae antibodies in twins with inflammatory bowel disease. Gut. 2005;54(9):1237–1243.
- Dicksved J, Halfvarson J, Rosenquist M, et al. Molecular analysis of the gut microbiota of identical twins with Crohn’s disease. Isme J. 2008;2(7):716–727.
- Willing B, Halfvarson J, Dicksved J, et al. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn’s disease. Inflamm Bowel Dis. 2009;15(5):653–660.
- Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139(6):1844–1854.
