Abstract
Objective
Although scissor-type knives such as the Stag-Beetle (SB) Knife Jr are expected to result in a safe and easy colorectal endoscopic submucosal dissection (CR-ESD), information regarding the learning curve is lacking. Therefore, this study evaluated the learning curve with using SB Knife Jr.
Materials and methods
We retrospectively reviewed 507 CR-ESD procedures performed in 464 patients using SB Knife Jr. The primary endpoint was a learning curve to achieve a satisfactory complete resection rate. The secondary endpoints were learning curves to achieve a satisfactory en bloc resection rate, curative resection rate, and resection speed.
Results
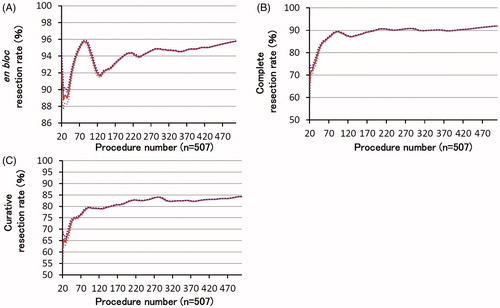
The complete, en bloc, and curative resection rates were 91.9%, 95.9%, and 84.0%, respectively. Moving average analysis showed that 39 cases were required for a complete resection rate of >80%, 41 for an en bloc resection rate of >90%, and 50 for a curative resection rate of >75%. We divided the procedure into three phases using the cumulative sum method: I, II, and III (cases 1–36, 37–119, and 120–507, respectively). Although we found no significant between-phase differences, the complete resection rate showed an increasing trend in Phase III (83.3 vs. 89.2 vs. 93.3%; p = .099). The en bloc resection rate (91.7 vs. 91.6 vs. 97.2%; p = .047) and resection speed (20.5 vs. 7.2 vs. 6.8 min/cm2; p < .001) were greater in Phase III. Despite the larger specimen size (27.3 vs. 38.2 vs. 40.4 mm; p < .001) and more severe fibrosis (p < .001) in Phase III, the procedure time was shorter (73.8 vs. 57.8 vs. 54.2 min; p = .041). The curative resection rate was not significantly different between phases.
Conclusions
SB Knife Jr enables safe and easy CR-ESD during the introductory period compared to the conventional tip-type knife and has an acceptable learning curve. Therefore, using this knife will encourage the widespread adoption of CR-ESD in Asian general hospitals and non-Asian countries.
Introduction
Colorectal endoscopic submucosal dissection (CR-ESD) allows en bloc resection of large and ulcerated colorectal superficial neoplasms, resulting in low rates of local recurrence, high-quality pathological specimens for accurate histological diagnosis, and curative resection of early carcinoma. However, CR-ESD has not been widely adopted in Asian general hospitals and non-Asian countries because of the technical challenges posed by the complex anatomic features of the large intestine [Citation1].
Novel scissor-type knives such as the Stag-Beetle Knife Jr (SB Knife Jr, Sumitomo Bakelite Co., Ltd, Tokyo, Japan) () enable the grasping of the target tissue, thus facilitating controlled dissection by the endoscopist and potentially preventing unexpected muscular layer injury. Therefore, SB Knife Jr does not require advanced endoscopic techniques and results in a safe and easy ESD.
A Japanese multicenter study reported good outcomes by general endoscopists using SB Knife Jr [Citation2]. SB Knife Jr has been demonstrated to be highly effective as an auxiliary device [Citation3], and we achieved favorable short- and long-term clinical outcomes using the device [Citation4]. SB Knife Jr has been reported to improve the self-completion rates for trainees and the R0 resection rates for CR-ESD [Citation5,Citation6]. Therefore, there is a possibility that the device will encourage the adoption of CR-ESD in Asian general hospitals and non-Asian countries in the future. Effective devices must not only enable a safe procedure but should also have an acceptable learning curve. However, information regarding the learning curve for SB Knife Jr is lacking. Adoption is further hindered by concerns regarding a slow procedure time when using SB Knife Jr due to the characteristics of the device. Therefore, the learning curve to achieve a satisfactory resection speed also needs to be validated.
The aim of this study was to evaluate the learning curve with over 500 cases of CR-ESD performed using SB Knife Jr.
Materials and methods
Patients
We retrospectively reviewed the outcomes for 507 cases of CR-ESD performed on 464 patients using SB Knife Jr between May 2012 and June 2019 at Asahi General Hospital, Japan. This retrospective study was approved by the institutional review board of Asahi General Hospital (16 July 2019), and written informed consent for the procedure was obtained from all patients. Indications for CR-ESD were based on the guidelines proposed by the Japan Gastroenterological Endoscopy Society (JGES) [Citation7].
ESD procedure
All procedures were performed by a single colonoscopist with an experience of 419 colonic endoscopic mucosal resections and 68 gastric ESDs. An experienced nurse engaged in the endoscopy unit was the surgical assistant. Patients were admitted 1 day prior to CR-ESD and remained in the hospital for approximately 5 days. We performed bowel preparation using 2 L of isotonic polyethylene glycol electrolyte solution (Niflec; EA Pharma Co., Ltd, Tokyo, Japan) in the morning of the ESD procedure. A single endoscope attached to a transparent SB soft hood (Sumitomo Bakelite Co., Ltd) was used with carbon dioxide insufflation. A transparent hood was used to facilitate traction application on the mucosa to create space for submucosal dissection. We used a GIF-Q260J (Olympus Medical Systems, Tokyo, Japan) for rectal lesions, and a PCF-Q260JI (Olympus) or PCF-H290TI (Olympus) for lesions from the sigmoid colon to the cecum.
Only for uncleared margins such as sessile serrated lesions, we used an argon plasma coagulation probe for placement of marking dots along the target lesion circumference to indicate margins. First, we injected 0.4% sodium hyaluronate (MucoUp; Boston Scientific Japan, Tokyo, Japan) with a small amount of indigo carmine and epinephrine into the submucosal layer using a 26-gauge injection needle. Next, a circumferential incision was made from the anal side with SB Knife Jr. The knife had an open width of 4.5 mm and a length of 3.5 mm. For large lesions, a partial circumferential incision was often made to define the oral end of the lesion. The circumferential incision was extended, and submucosal dissection was performed; these steps were repeated in an alternating sequence until the entire lesion was resected. SB Knife Jr was also used for endoscopic hemostasis in bleeding situations. The dissected segment was grasped, and current was passed using a high-frequency generator (VIO300D; Erbe Elektromedizin, Tübingen, Germany). VIO300D was used with the following settings: endo-cut Q mode (effect 1, duration 1, interval 1) for mucosal incision and submucosal dissection, and a soft coagulation mode (effect 5, 40 W) for hemostasis. Sedatives and antibiotics were not routinely used.
Histopathological assessment
Histopathological diagnosis was performed in accordance with the guidelines for CR-ESD proposed by JGES [Citation7]. Complete resection was defined as en bloc resection where both the horizontal and vertical margins were negative. For en bloc resection specimens, we considered adenomas with unclear lateral margins and an expected lateral burning effect as margin negative. Curative resection was defined as complete resection and fulfillment of all four of the following characteristics: (I) differentiated or papillary carcinoma; (II) no lymphovascular invasion; (III) submucosal invasion depth <1000 µm (SM1); and (IV) grade 1 budding. We referenced the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer [Citation8]. The shape of the specimen was defined as elliptical, and the area of the lesion was calculated using the following formula: long axial radius × short axial radius × 3.14.
Adverse events
Delayed bleeding was defined as hematochezia requiring endoscopic hemostasis or blood transfusion or leading to a reduction of more than 2 g/dL in hemoglobin. Perforation was defined as a muscle layer defect during the procedure and free air findings on computed tomography (CT) after the procedure. CT was performed as needed, depending on abdominal tenderness and fever.
Surveillance after the procedure
Surveillance after the procedure was based on the guidelines proposed by JGES and JSCCR [Citation7,Citation8]. Patients who underwent curative resection were followed up for 6–12 months using colonoscopy. Thereafter, patients were followed up every 3 years using colonoscopy, and CT scan if necessary, to detect local residual recurrence and metachronous lesions. The noncurative resection patients who rejected additional surgery were followed up every 6 months using colonoscopy, CT scan, and tumor marker tests.
Endpoint and outcome evaluation
The primary endpoint was a learning curve to achieve a satisfactory complete resection rate. The secondary endpoints were learning curves to achieve a satisfactory en bloc resection rate, curative resection rate, and resection speed. Further, we analyzed the short- and long-term outcomes such as adverse events, survival curves, and the local residual-recurrence rate.
Statistical analysis
The learning curve for the resection rate was evaluated using moving average analysis. The mean resection rate was calculated with sets of 20 cases and plotted against the number of procedures performed. A high-maturity level was defined as a complete resection rate of >80%, en bloc resection rate of >90%, and curative resection rate of >75% on the basis of the European Society of Gastrointestinal Endoscopy’s (ESGE) ESD training statement [Citation9]. The learning curve to achieve a satisfactory resection speed (min/cm2) was evaluated using the cumulative sum (CUSUM) method. CUSUM is the running total of differences between an individual case and the mean of all cases. This method enables the monitoring of a surgical procedure and evaluation of the learning curve during the training period. The resection speed was used for analysis as the index of technical progression. The resection speed was chronologically plotted from the earliest to the latest period of the procedure. The target value was set to 7.8 min/cm2 based on the mean value of the resection speed. A decision interval of 5 and a reference value of 0.5 were set in this study. In the CUSUM learning curve, the upper limit consists of deviations that are greater than the reference value. Values that exceed the decision interval are considered as outliers. The length of the procedure was divided into three phases (Phase I: learning phase; Phase II: experienced phase; Phase III: mature phase) according to the CUSUM charts. The three phases were defined as follows: Phase I: learning phase, from the earliest case to the maximum CUSUM value; Phase II: experienced phase, from the maximum CUSUM value to the decision interval; Phase III: mature phase, from the decision interval to the most recent case. Analyses among the phases were conducted using the chi-square test and analyses of variance. Survival analyses were performed using the Kaplan–Meier method. Cases with less than 180 days since the procedure were excluded from the survival analysis. These analyses were conducted at the Clinical Research Support Center of Asahi General Hospital. A two-sided p value of < .05 was considered to be statistically significant. All statistical analyses were performed using JMP® software version 14.0.2 (SAS Institute Japan, Tokyo, Japan).
Results
Patients’ clinical characteristics and short-term outcomes of CR-ESD
Patients’ clinical characteristics and the short-term outcomes of CR-ESD are presented in . Overall 464 patients were treated with 507 cases of ESD. The complete, en bloc, and curative resection rates were 91.9%, 95.9%, and 84.0%, respectively. Delayed bleeding occurred in 19 cases (3.7%), and all cases were treated with endoscopic hemostasis using clips. Six (1.2%) patients had perforations. Muscle layer defects during the procedure and free air findings on CT scan after the procedure were observed in all cases. These cases were managed by endoscopic closure using clips, and surgery was unnecessary.
Table 1. Baseline characteristics and procedure outcomes of all patients.
Learning curve for a satisfactory resection rate
The learning curve to achieve a satisfactory resection rate, determined using moving average analysis, is shown in . The positive slope indicated that the proficiency improved rapidly. Slopes for the complete and curative resection rates were positive, starting from the introductory period, and rapidly reached a plateau. The complete resection rate was >80% after 39 cases, whereas curative resection rate was >75% after 50 cases, and en bloc resection rate was >90% after 41 cases. Although the en bloc resection rate temporarily decreased, it remained at >90%.
CUSUM learning curve for satisfactory resection speed
The CUSUM learning curve for the resection speed (min/cm2) is shown in . The maximum CUSUM value was obtained in case number 37. The intersection point for the CUSUM learning curve and the decision interval corresponded to case numbers 119 and 120. Phase I (learning phase) corresponded to case numbers 1–36, Phase II (experienced phase) corresponded to case numbers 37–119, and Phase III (mature phase) corresponded to case numbers 120–507. The slope for CUSUM in Phase I was positive, indicating that the proficiency of the clinician was immature. CUSUM in Phase II had a negative slope, suggesting that the proficiency had improved rapidly. The slope was close to a plateau in Phase III, indicating maturation.
Figure 3. CUSUM resection speed (min/cm2) against procedure number. The red line shows the decision interval, whereas the purple line indicates the outlier. CUSUM: cumulative sum.
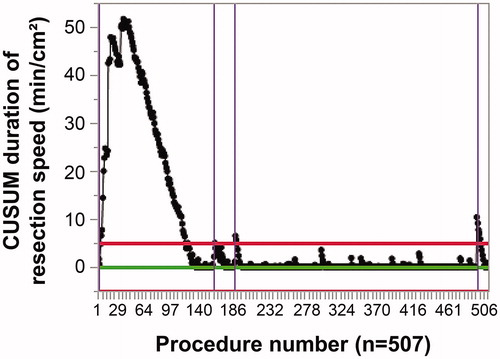
Three cases in Phase III were considered as outliers. The first was for case number 157, in which a depressed lesion protruded in the rectum. Although the estimated submucosal invasion depth was ≥1000 µm (SM2), the patient refused surgery. The procedure time was 57 min because of severe fibrosis and bleeding. The tumor size was 11 mm × 8 mm, the invasion depth was SM2, and the results for lymphovascular invasion and the margins were negative.
The second was for case number 182. The lesion also protruded in the rectum. The patient refused surgery despite the estimation of SM2 invasion. The procedure time was 67 min because of severe fibrosis and bleeding. The tumor size was 20 mm × 14 mm, the invasion depth was SM2, lymphovascular invasion was positive, and the margins were negative.
The third was for case number 492. The lesion was a 15 mm × 8 mm flat-type adenoma in the cecum. Because of paradoxical scope movement and an intradiverticular lesion, the procedure time was 124 min.
Learning curve for satisfactory short-term outcomes, including resection speed and adverse events among the three phases
The tumor and procedure characteristics among the three phases are shown in . Although we found no significant between-phase differences, the complete resection rate showed an increasing trend from Phases I to III (83.3 vs. 89.2 vs. 93.3%; p = .099). The en bloc resection rate (91.7 vs. 91.6 vs. 97.2%; p = .047) and resection speed (20.5 vs. 7.2 vs. 6.8 min/cm2; p< .001) were significantly greater from Phases I to III. Despite the larger specimen size (27.3 vs. 38.2 vs. 40.4 mm; p< .001) and more severe fibrosis (p< .001) in Phase III, the procedure time was shorter (73.8 vs. 57.8 vs. 54.2 min; p = .041). In addition, the curative resection rate (75.0 vs. 80.7 vs. 86.1%; p =.151) increased during the mature phase but was not significantly different between phases.
Table 2. Tumor and procedure characteristics among the three phases.
The adverse events encountered are summarized in . The number of cases with delayed bleeding and perforation were not significantly different between phases.
Long-term outcomes among the three phases
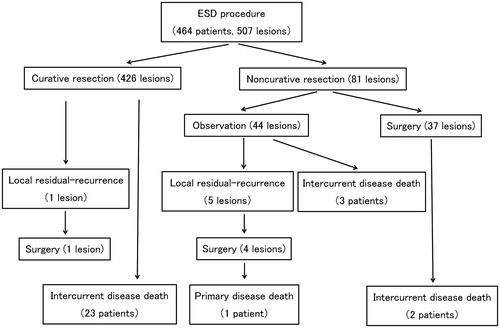
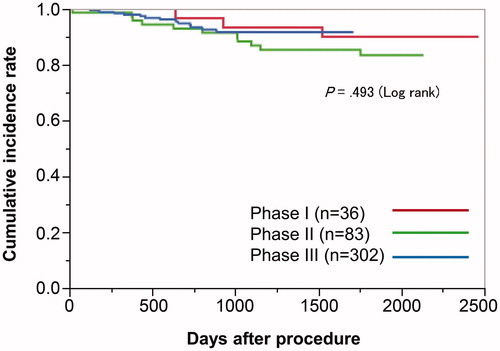
The survival curve, patient flowchart, and local residual-recurrence analysis are presented in and . The mean follow-up period was 831 ± 679 days. Curative resection was performed for 426 lesions, and noncurative resection was performed for 81 lesions. Among the noncurative resection group, 44 lesions were monitored, and additional surgery was performed for 37 lesions. Among the 507 cases, six lesions (1.2%) had local residual recurrence during a follow-up period of 118 days. Local residual recurrence occurred for one lesion in the curative resection group, and for five lesions in the noncurative resection group. The patient with local residual recurrence in the curative resection group had a laterally spreading, granular-type (nodular-mixed type) tumor in the sigmoid colon. Although the invasion depth was SM1 and lymphovascular invasion results and the margins were negative, local residual recurrence occurred on postoperative day 183. We performed additional surgery, and the pathological diagnosis was complete resection. Local residual recurrence was observed for none of the lesions in Phase I, three lesions (3.6%) in Phase II, and three lesions (0.8%) in Phase III. Overall 29 patients died during the follow-up period, and one death was due to colorectal cancer. There were no significant differences among the phases in the local residual recurrence and survival curves as shown in and .
Discussion
This study involved more than 500 cases of CR-ESD performed using the scissor-type SB Knife Jr. Using this knife, the complete, en bloc, and curative resection rates and adverse events have favorable outcomes and are acceptable, even during the introductory period. With regard to the learning curve to achieve a satisfactory resection rate, 39 cases were required for a complete resection rate of >80%, 41 cases were necessary to achieve an en bloc resection rate of >90%, and 50 cases were required for a curative resection rate of >75%. These results indicated that CR-ESD using SB Knife Jr resulted in good short-term outcomes in relatively few cases. In addition, despite the increase in the difficulty of cases encountered such as severe fibrosis, the proficiency improved rapidly after 37 cases and the speed reached a plateau at 120 cases. These results indicated that the length of the procedure can be reduced with experience.
The complete, en bloc, and curative resection rates had favorable outcomes and were acceptable even during the introductory period. In addition, the considerably low rate of adverse events in the introductory period suggests that the procedure is easy and safe. These results are similar to those reported previously with scissor-type knives and indicated that CR-ESD using SB Knife Jr resulted in good short-term outcomes [Citation2–6,Citation10–13]. Although positive outcomes with CR-ESD have been reported in high-volume centers in Asia (notably Japan) [Citation14–19], the adoption of CR-ESD has not been widespread in general hospitals because of the technical challenges posed by the complex anatomic features of the large intestine. A Japanese multicenter prospective cohort study demonstrated that the risk of adverse events was reduced in high-volume centers [Citation15]. In addition, a Japanese multicenter retrospective study reported positive outcomes in low-volume centers, although the en bloc resection rates were reported to be lower than the rates in high-volume centers [Citation20]. Although the reports on the use of CR-ESD and its favorable outcomes are gradually increasing in non-Asian countries, ESD still does not have a satisfactory performance level [Citation21–30]. Favorable results such as high complete, en bloc, and curative resection rates during the introductory period, indicate that using SB Knife Jr might benefit the adoption of CR-ESD in developing facilities such as Asian general hospitals and non-Asian countries.
The learning curve to achieve a satisfactory resection rate was favorable. The ESGE’s ESD training statement recommends a complete resection rate of >80%, en bloc resection rate of >90%, and curative resection rate of >75% as a high-maturity index [Citation9]. Moving average analysis shows that 39 complete resection, 41 en bloc resection, and 50 curative resection cases are required for the maturation of CR-ESD proficiency using SB Knife Jr. Previous reports regarding the adoption of CR-ESD using the conventional tip-type knife indicated that 25–40 cases were necessary to achieve a high maturity level in safety, self-completion, and resection speed, and 40–100 cases were necessary for resection quality [Citation31–38]. Therefore, our results suggest that SB Knife Jr has the same or better requirements to achieve proficiency in comparison with the conventional tip-type knife.
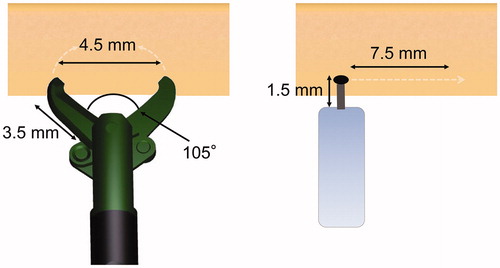
The good learning curve to attain a satisfactory resection speed indicated that SB Knife Jr overcomes the issue of slow resection speed, which is considered a drawback for such devices. The factors that affect slow resection speed when using scissor-type knives such as SB Knife Jr are as follows. First, precise grasping of the target tissue and dissection require multiple steps [Citation10]. Second, use of the appropriate rotation technique by the surgical assistants is essential [Citation10,Citation11]. While using the conventional tip-type knife, clinicians need to move the endoscope to the target tissue; therefore, surgical assistants do not require an in-depth knowledge of CR-ESD. In contrast, while performing CR-ESD with SB Knife Jr, surgical assistants need to rotate the forceps to hold the target tissue. Thus, the assistants require the same degree of knowledge and rotation techniques as the clinician. The schema for SB Knife Jr and the conventional tip-type knife is shown in . SB Knife Jr has a forceps length of 3.5 mm, an opening width of 4.5 mm, and an opening angle of 105°. Assuming a fan-shaped dissection surface, a single grasp and dissection action enables the clinician to cut approximately 11.2 mm2 of tissue. In contrast, assuming a rectangular dissection surface, the tip-type knife with a 1.5 mm forceps length requires a horizontal movement of approximately 7.5 mm to cut the same length of tissue. In the difficult-to-operate large intestine, these movements require advanced endoscopic techniques. SB Knife Jr can also function as a hemostatic forceps and enables hemostasis or precoagulation without device replacement. These characteristics enable clinicians to shorten the procedure time after a relatively short training period. This validation study showed that full proficiency with the device can be acquired within 120 cases, and the issue of slow resection speed, which is considered a drawback, can be eliminated. The resection speed and procedure time were not inferior to those in previous reports using the conventional tip-type knife and were superior at the mature stage [Citation15–19].
Figure 6. Schema for SB Knife Jr and the conventional tip-type knife. SB Knife Jr has a forceps length of 3.5 mm, an opening width of 4.5 mm, and an opening angle of 105°. Assuming a fan-shaped dissection surface, a single grasp and dissection action enables the clinician to cut approximately 11.2 mm2 of tissue. In contrast, assuming a rectangular dissection surface, the tip-type knife with a 1.5 mm forceps length requires a horizontal movement of approximately 7.5 mm to cut the same length of tissue. In the difficult-to-operate large intestine, these movements require advanced endoscopic techniques.
There were several limitations in this study. This was a single-center retrospective study, and all procedures were performed by one operator. The applicability of these favorable outcomes to other operators or institutions is limited. However, the learning curve for CR-ESD using SB Knife Jr was the focus of our study. Validation of the learning curve with a single operator eliminates the potential for technical performance variations among operators and enables evaluation of the pure learning curve. In addition, although CR-ESD using the scissor-type knife is gradually increasing, penetration rates remain low. Our previous study included 247 cases [Citation4], and this sample size was the largest among all studies previously conducted on CR-ESD using scissor-type knives [Citation13]. Furthermore, few previous reports for CR-ESD using SB knife Jr exist in Western countries [Citation27]. Therefore, even if this study with >500 CR-ESD cases was conducted by one operator, we believe it to be acceptable and extremely significant.
In conclusion, SB Knife Jr enables safe and easy CR-ESD during the introductory period compared to the conventional tip-type knife and also has an acceptable learning curve. SB Knife Jr enables clinicians to safely achieve acceptable short-term outcomes with relatively few cases and to develop a satisfactory resection speed with experience. Using SB Knife Jr does not require advanced endoscopic techniques as the conventional tip-type knife, leading to safe and easy CR-ESD for novices. Therefore, we believe that using SB Knife Jr will encourage the widespread use of CR-ESD in developing facilities such as Asian general hospitals and non-Asian countries in the future. Multicenter prospective studies in non-Asian countries are needed to confirm its efficacy.
Author contributions
A.M. and T.K designed the study, and wrote the draft of the manuscript. Y.S. contributed to analysis and interpretation of data. M.K., A.N., E.I., H.S., Y.S., and K.S. assisted in the preparation of the manuscript. All authors approved the final version of the manuscript.
Acknowledgments
This study was supported by the Clinical Research Center at Asahi General Hospital, Asahi, Japan. We thank all support team members.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kuwai T, Tamaru Y, Kusunoki R, et al. Submucosal injection solutions for ESD: separating the winners from the losers. Dig Dis Sci. 2019;64(10):2699–2700.
- Homma K, Otaki Y, Sugawara M, et al. Efficacy of novel SB knife Jr examined in a multicenter study on colorectal endoscopic submucosal dissection. Dig Endosc. 2012;24(Suppl 1):117–120.
- Oka S, Tanaka S, Takata S, et al. Usefulness and safety of SB knife Jr in endoscopic submucosal dissection for colorectal tumors. Dig Endosc. 2012;24(Suppl 1):90–95.
- Kuwai T, Yamaguchi T, Imagawa H, et al. Endoscopic submucosal dissection of early colorectal neoplasms with a monopolar scissor-type knife: short- to long-term outcomes. Endoscopy. 2017;49(9):913–918.
- Shiga H, Endo K, Kuroha M, et al. Endoscopic submucosal dissection for colorectal neoplasia during the clinical learning curve. Surg Endosc. 2014;28(7):2120–2128.
- Yamashina T, Takeuchi Y, Nagai K, et al. Scissor-type knife significantly improves self-completion rate of colorectal endoscopic submucosal dissection: single-center prospective randomized trial. Dig Endosc. 2017;29(3):322–329.
- Tanaka S, Kashida H, Saito Y, et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32(2):219–239.
- Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42.
- Pimentel-Nunes P, Pioche M, Albéniz E, et al. Curriculum for endoscopic submucosal dissection training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2019;51(10):980–992.
- Akahoshi K, Okamoto R, Akahane H, et al. Endoscopic submucosal dissection of early colorectal tumors using a grasping-type scissors forceps: a preliminary clinical study. Endoscopy. 2010;42(5):419–422.
- Akahoshi K, Akahane H. A new breakthrough: ESD using a newly developed grasping type scissor forceps for early gastrointestinal tract neoplasms. World J Gastrointest Endosc. 2010;2(3):90–96.
- Nawata Y, Homma K, Suzuki Y. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for large superficial colorectal tumors. Dig Endosc. 2014;26(4):552–555.
- Yoshida N, Dohi O, Inoue K, et al. Efficacy of scissor-type knives for endoscopic mucosal dissection of superficial gastrointestinal neoplasms. Dig Endosc. 2020;32(1):4–15.
- Niimi K, Fujishiro M, Kodashima S, et al. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010;42(9):723–729.
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72(6):1217–1225.
- Saito Y, Kawano H, Takeuchi Y, et al. Current status of colorectal endoscopic submucosal dissection in Japan and other Asian countries: progressing towards technical standardization. Dig Endosc. 2012;24(Suppl 1):67–72.
- Lee EJ, Lee JB, Lee SH, et al. Endoscopic submucosal dissection for colorectal tumors-1,000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc. 2013;27(1):31–39.
- Shigita K, Oka S, Tanaka S, et al. Long-term outcomes after endoscopic submucosal dissection for superficial colorectal tumors. Gastrointest Endosc. 2017;85(3):546–553.
- Yamada M, Saito Y, Takamaru H, et al. Long-term clinical outcomes of endoscopic submucosal dissection for colorectal neoplasms in 423 cases: a retrospective study. Endoscopy. 2017;49(3):233–242.
- Boda K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for colorectal tumors: a large multicenter retrospective study from the Hiroshima GI Endoscopy Research Group. GastrointestEndosc. 2018;87(3):714–722.
- Farhat S, Chaussade S, Ponchon T, SFED ESD study group, et al. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy. 2011;43(8):664–670.
- Akintoye E, Kumar N, Aihara H, et al. Colorectal endoscopic submucosal dissection: a systematic review and meta-analysis. Endosc Int Open. 2016;04(10):E1030–E1044.
- Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86(1):74–86.
- Spychalski M, Skulimowski A, Dziki A, et al. Colorectal endoscopic submucosal dissection (ESD) in the West - when can satisfactory results be obtained? A single-operator learning curve analysis. Scand J Gastroenterol. 2017;52(12):1442–1452.
- Ebigbo A, Probst A, Römmele C, et al. Step-up training for colorectal and gastric ESD and the challenge of ESD training in the proximal colon: results from a German Center. Endosc Int Open. 2018;6(5):E524–E530.
- Daoud DC, Suter N, Durand M, et al. Comparing outcomes for endoscopic submucosal dissection between Eastern and Western countries: a systematic review and meta-analysis. World J Gastroenterol. 2018;24(23):2518–2536.
- Yamamoto S, Radomski T, Shafazand M. Implementation of mentor-assisted colorectal endoscopic submucosal dissection in Sweden; learning curve and clinical outcomes. Scand J Gastroenterol. 2018;53(9):1146–1152.
- Rönnow CF, Uedo N, Toth E, et al. Endoscopic submucosal dissection of 301 large colorectal neoplasias: outcome and learning curve from a specialized center in Europe. Endosc Int Open. 2018;6(11):E1340–E1348.
- Zhang X, Ly EK, Nithyanand S, et al. Learning curve for endoscopic submucosal dissection with an untutored, prevalence-based approach in the United States. Clin Gastroenterol Hepatol. 2020;18(3):580–588.e1.
- Ramos-Zabala F, Parra-Blanco A, Beg S, et al. Feasibility and learning curve of unsupervised colorectal endoscopic submucosal hydrodissection at a Western Center. Eur J Gastroenterol Hepatol. 2020;32(7):804–812.
- Hotta K, Oyama T, Shinohara T, et al. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endosc. 2010;22(4):302–306.
- Sakamoto T, Saito Y, Fukunaga S, et al. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum. 2011;54(10):1307–1312.
- Ohata K, Ito T, Chiba H, et al. Effective training system in colorectal endoscopic submucosal dissection. Dig Endosc. 2012;24(Suppl 1):84–89.
- Białek A, Pertkiewicz J, Karpińska K, et al. Treatment of large colorectal neoplasms by endoscopic submucosal dissection: a European single-center study. Eur J Gastroenterol Hepatol. 2014;26(6):607–615.
- Yang DH, Jeong GH, Song Y, et al. The feasibility of performing colorectal endoscopic submucosal dissection without previous experience in performing gastric endoscopic submucosal dissection. Dig Dis Sci. 2015;60(11):3431–3441.
- Shiga H, Kuroha M, Endo K, et al. Colorectal endoscopic submucosal dissection (ESD) performed by experienced endoscopists with limited experience in gastric ESD. Int J Colorectal Dis. 2015;30(12):1645–1652.
- Jeon HH, Lee HS, Youn YH, et al. Learning curve analysis of colorectal endoscopic submucosal dissection (ESD) for laterally spreading tumors by endoscopists experienced in gastric ESD. Surg Endosc. 2016;30(6):2422–2430.
- Shiga H, Ohba R, Matsuhashi T, et al. Feasibility of colorectal endoscopic submucosal dissection (ESD) carried out by endoscopists with no or little experience in gastric ESD. Dig Endosc. 2017;29(Suppl 2):58–65.