Abstract
Background
Effects of nutritional intake on inflammatory bowel disease (IBD) flare resolution are unknown. We hypothesised that nutritional factors during hospitalisation for acute severe IBD are associated with risk of subsequent relapse. We also studied risk factors for inadequate energy intake.
Methods
Patients admitted to the Karolinska Hospital Gastroenterology ward with IBD flare during 2015–2016 were retrospectively identified. In total, 91 patients were included. Data on nutrition, disease factors, inflammatory markers, and daily energy requirement were extracted. Requirement of new systemic steroid prescription, intensification of biological therapy, readmission, surgery, and calprotectin level were individually used as proxies for disease relapse. Follow-up was one year after discharge. Adjustments for age and sex were made where appropriate.
Results
Overall, 19%, 31%, and 45% of patients had days with energy intake <30, <50, and <70% of calculated requirement. Older age was associated with a higher number of days with energy intake <30, <50, and <70% of calculated requirement (regression coefficient 0.03, 0.04, 0.06 respectively, p = .012, .017, .008). The number of days with energy intake <30 and <70% of the calculated requirement and the length of the hospitalisation were associated with shorter time to new steroid prescription (hazard ratio 1.3, 1.1, 1.04 respectively, p = .016, .034, .011). CRP and calprotectin were not associated with relapse.
Conclusion
Older age is a predictor of inadequate energy intake during hospitalisation for acute severe IBD. Inadequate energy intake adjusted for age and sex during IBD flare was better predictor of time to the next steroid-requiring relapse than inflammatory markers.
Introduction
Inflammatory bowel diseases (IBD) include Crohn’s disease (CD) and ulcerative colitis (UC), which are chronic, incurable conditions that are often diagnosed in young adults, although 25–35% of IBD patients are over 60 years old [Citation1]. It is known that IBD in elderly population is often complicated by several factors, including for example polypharmacy, co-morbidities, treatment compliance, difficulties in differential diagnosis and risks of surgical complications [Citation2]. There has been longstanding interest from physicians, but also not least from patients in the association between nutrition and IBD and its importance has already been acknowledged widely [Citation3,Citation4]. Nutrition has been hypothesised to be an aetiological factor as well as a treatment option in IBD [Citation3,Citation4]. Also, IBD patients frequently suffer from malnutrition [Citation5]. However, most of the studies done so far on malnutrition in IBD investigate prevalence of malnutrition at a specific timepoint, e.g., at admission to hospital. However, to our knowledge, no studies have yet addressed the prevalence and effects of poor nutritional intake during an acute flare in hospital.
Consensus on the definition of malnutrition is lacking [Citation6]. The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends defining malnutrition as ‘a state resulting from lack of intake or uptake of nutrition that leads to altered body composition (decreased fat free mass) and body cell mass leading to diminished physical and mental function and impaired clinical outcome from disease’ [Citation6,Citation7]. Further, ESPEN recommends using malnutrition and undernutrition as synonyms [Citation6]. In this article, we use the term malnutrition with the definition by ESPEN.
Patients with IBD, particularly small intestinal CD are at risk of malnutrition due to malabsorption secondary to gut damage. However, the risk of malnutrition of patients is particularly high during active IBD [Citation8,Citation9] secondary to reduced nutritional intake due to food restrictive behaviour resulting from decreased appetite and desire to avoid symptoms or worsening of the flare [Citation8,Citation10]. Moreover, factors specific to the in-patient environment may further contribute to reduced nutritional intake including non-availability of the patients’ habitual diet, periods of fasting before endoscopy or other investigations and unpalatability of nutritional supplements. In addition, malnutrition is common in people over 60 years of age in general [Citation11].
Ananthakrishnan et al. [Citation12] found that severe hospitalisation, defined as need of non-elective bowel surgery during the inpatient period or over 7 days’ hospitalisation, was more common in patients with a diagnosis of malnutrition. Furthermore, according to data from the United States, the odds ratio (OR) for malnutrition among IBD hospitalisations compared with non-IBD hospitalisations was 5.57 after adjustment for age, comorbidity, sex, health insurance, income, and hospital characteristics [Citation13]. The specific effects of clinical malnutrition on gut inflammation are, however, unknown.
In summary, IBD patients are at risk of malnutrition especially during active disease and potential immunomodulating and therapeutic effects of diet have been proposed [Citation3]. However, clinical effects of nutritional intake on the course of inflammation and healing during an acute, severe flare, are not fully elucidated, because earlier studies have addressed malnutrition at a specific time point. Specifically poor nutritional intake during acute disease has rarely been studied. Thus, in this study we evaluate the prevalence of poor nutritional intake during hospital stay for severe acute IBD flare and its effects on resolution of the inflammatory activity. Furthermore, from the clinical perspective it is important to detect risk factors for suboptimal energy intake to enable early detection and effective prevention of in-hospital malnutrition. Thus, risk factors of suboptimal energy intake during the inpatient period for acute, severe IBD were also studied.
Material and methods
Study design and population
All patients discharged from the gastroenterology ward at the Karolinska University Hospital with International Classification of Diseases, 10th Revision (ICD-10) diagnostic codes K50-52 for noninfective enteritis and colitis (that includes diagnoses UC, CD and IBD-unclassified) between 1.1.2015 and 28.2.2017 were identified. In total 385 discharge summaries (DS) were identified. Patients were required to have an acute severe flare of IBD (defined as hospital admission for intestinal inflammation in a patient with either an established diagnosis of IBD or confirmed IBD debut. In order to avoid including patients with IBD with self-reported symptoms during a hospital admission for another problem rather than acute severe flare of IBD it was required that the presence of the IBD ICD-code on the DS was combined with either a prescription of steroids (betamethasone, prednisolone, or budesonide administered either intravenously, orally or rectally) with continuous treatment lasting >24 h, or a new biological treatment, or acute IBD bowel surgery during the in-patient stay or confirmation that IBD flare was the main reason for patient’s hospitalisation as evaluated by a specialist gastroenterologist. The requirement for steroid treatment to be continued for >24 h was used to exclude patients where a diagnosis of acute IBD flare made by emergency room doctors was subsequently overturned by a specialist gastroenterologist who evaluates all patients within 24 h of admission.
If the admission to hospital was the first diagnosis of IBD (IBD debut) then the patient was only included if IBD was subsequently confirmed by histopathology, magnetic resonance tomography (MRT) or subsequent outpatient visit at the specialist IBD unit in Karolinska Solna where the diagnosis of IBD was recorded a second time.
The follow-up (FU) period was one year after discharge. Patients with total resection of the inflamed bowel tissue during the initial inpatient period were excluded. All the patients who fulfilled these inclusion criteria and were admitted during 2015–2016 and gave their consent were included in the study. Finally, every patient was included only once: readmissions were considered as an outcome of the first admission.
Data collection
Data were extracted from the hospital records and the national Swedish quality register for IBD (SWIBREG, www.swibreg.se). Laboratory data of inflammatory markers (first documented calprotectin during the admission, C-reactive protein (CRP) at admission and the total change of CRP during the inpatient period) and clinical data on age, sex, diagnosis, inflammation extent, disease behaviour (for CD), disease duration, previous surgery, number of bowel movements, length of stay, drug treatment at admission, drugs prescribed during the inpatient period, number of bowel movements at admission, body mass index (BMI) at admission, total change of the BMI during the inpatient period, use of PN during the inpatient period, and number and percentage of days with energy intake under 30, 50 and 70% of the calculated requirement were extracted. The following data were extracted for outcomes, summarized in : steroid prescriptions during the FU year, number of readmissions during the FU year, the highest calprotectin value during the first half of the FU year, start or intensification of a biological treatment during the FU year and surgery with bowel resection during the primary inpatient period or during the first 3 months following discharge. Outcomes were analysed individually, and no composite endpoint was defined. Only steroids administered orally or intravenously during the FU year were considered. Readmissions were required to be to a gastroenterology ward but were not analysed further, thus they could also include admissions for other reasons than IBD flare. The highest documented calprotectin during the first half of the FU year was used as a continuous outcome, meaning that its association with the hypothesised predictors was analysed with linear regression. Start or intensification of a biological treatment was defined as induction of a new biological therapy in a biologic-naïve patient, induction of a second, third or fourth line biological therapy in a patient that had previously been treated with another biological therapy (excluding changes to biosimilars), or shortening of the inter-dose interval and/or increase in the dosing of an existing biological therapy. Bowel resection surgery was defined as small bowel or colonic resection due to IBD.
Table 1. Outcomes used as a proxy of a flare or disease activity.
Patients’ daily energy need was calculated and documented during the inpatient period by a specialist dietitian or by another health care professional using the formula 30 kilocalories per kilogram, adjusted in cases of extreme values of BMI, age or physical activity. Importantly, the calculated energy need did not depend on the severity of the inflammatory activity. Dietary intake by the patients was assessed daily by ward nurses in conjunction with the patient and recorded in a structured data sheet under the supervision of a specialist dietitian. The difference between energy requirement and energy intake was calculated and then all data were entered into the electronic patient record. Patients with missing data were excluded from respective individual comparisons.
Statistical analyses
Demographic data were assessed with descriptive statistics. Descriptive statistics of centrality and spread of the data were calculated for ratio scale data (age, time of disease length etc.). Normality of ratio variables was tested by Shapiro–Wilk normality test. Absolute numbers within categories and proportions of the total available values in the study populations were presented for categorical data. Further analyses were performed using linear, logistical and Cox regression analyses with adjustment for age and sex except for the outcomes number of days with energy intake under 30, 50 and 70% of the calculated requirement. Linear regression analysis was used for the following outcomes: number of steroid prescriptions during the FU year, number of readmissions during the FU year and the highest calprotectin during the first half of the FU year (). Logistical regression analysis was used for the outcomes start or intensification of a biological therapy during the FU year and bowel resection surgery during the index admission or the first 3 months following discharge (). Cox regression analysis was used for the outcome time to the first steroid prescription during the FU year (). Pairs of predictors and outcomes with less than 50 observations were omitted except for the subgroup analyses. Further, stepwise backward variable selection analyses were done without statistical adjustments. Descriptive statistics were calculated, and illustrations were done with the statistical programme R and all the other analyses were done with STATA.
Ethical considerations
Ethical approval for the study was obtained by the local ethical committee in Stockholm (Dnr: 2018/2412-31), and informed consent was obtained from all participants.
Results
Patient characteristics
Supplementary Figure S1 details how inclusion and exclusion criteria were applied to build the studied patient group. The most common reasons for exclusion of patients were not fulfilling the stringent criteria for acute severe flare, absence of patient consent and that the same patient was presented multiple times (in which case only the first valid admission during the study period was included), Supplementary Figure S1. Baseline disease and demographic characteristics and prevalence of selected nutritional predictors in the whole included patient group are summarised and presented in . Among the study group one patient was excluded as an outlier (Supplementary Data, Tables 1S and 2S).
Table 2. Demographics, disease characteristics and nutritional predictors.
Prediction of inadequate nutritional intake during the inpatient stay
A total of 91 patients were included, and 19, 30 and 41 patients had at least one day with energy intake under 30, 50 and 70% of the calculated requirement respectively. Higher age was associated with an increased number of days with inadequate nutritional intake during the inpatient period, . Other tested predictors not significantly associated with these outcomes were sex, type of IBD, pure colitis vs small bowel involvement of inflammation (with or without colitis), newly diagnosed (yes/no), disease duration, earlier surgery, calprotectin, CRP at admission and the total change of the CRP during the inpatient period, the number of bowel movements and ongoing immunosuppressive or biological treatment, as well as the number of colonoscopies, sigmoidoscopies and upper endoscopies during the inpatient period (as separate predictors).
Table 3. Significant risk factors of inadequate nutritional intakeduring the inpatient period, (n = 91).
In the subgroup of patients with CD, higher CRP at day 1 predicted the number of days with energy intake under 30% of the calculated requirement and higher age at admission predicted the number of days with energy intake under 70% of the calculated requirement, see Table 3S in Supplementary Data.
In unadjusted multivariant regression analyses with stepwise selection, age at admission (p = .006) and length of the inpatient period (p = .15) were the most important predictors of inadequate nutritional intake in the whole patient group, (R2 = 21%). This indicates that 21% of the variation in the number of days with energy intake <50% of the calculated requirement can be explained by variations of these predictors in the final model. Other predictors not associated with malnutrition were immunosuppressive or biological medication at admission, CRP at admission, first documented BMI, use of PN during the inpatient period, diagnosis (CD/UC/IBD-unclassified), number of colonoscopies during the admission, earlier disease duration at admission and sex.
Factors predicting relapse of inflammation
We defined a set of clinical outcomes as proxy markers of disease relapse, . Inadequate nutritional intake and length of the inpatient period were significantly associated with time to the first steroid prescription in Cox regression analyses adjusted for age and sex (, ). Immunosuppressive or biological medication at admission as well as total weight gain during the inpatient period were significantly associated with the number of readmissions in linear regression analyses adjusted for age and sex (). All other predictors including first documented calprotectin, CRP at admission, the total change of CRP during the inpatient period, BMI at admission, use of PN during the inpatient period, mean percentage of energy intake of the calculated requirement over the whole in-patient period, number of days with energy intake under 50% of the calculated requirement and proportion of days with energy intake under 30, 50 and 70% of the calculated requirement, were not significantly associated with any of the outcomes, . Thus, patients undergoing surgery during the index admission or within 3 months from the discharge were not more likely to have poor nutritional intake than the rest of the study population.
Figure 1. Kaplan Meier curves on steroid free survival in patients with respect to number of days inadequate energy intake. (a) The upper panel shows survival curves for patients with no days with energy intake under 30% of calculated requirement and in patients with at least one day with energy intake under 30% of calculated requirement. Difference between survival is statistically significant with p < 0.03. Curves are adjusted for age and sex. The table under the Kaplan Meier curves shows numbers at risk in each category at respective time points. (b) The lower panel shows survival curves for patients with 0, 1, 2 to 3 and 4 or more days with energy intake under 70% of calculated requirement. Differences between curves for patients with 0 days and patients with 1, 2 to 3 or 4 or more days with energy intake under 70% of calculated requirement are not statistically significant (p = 0.6, 0.4, and 0.1 respectively). Curves are adjusted for age and sex. The table under the Kaplan Meier curves shows numbers at risk in each category at respective time points.
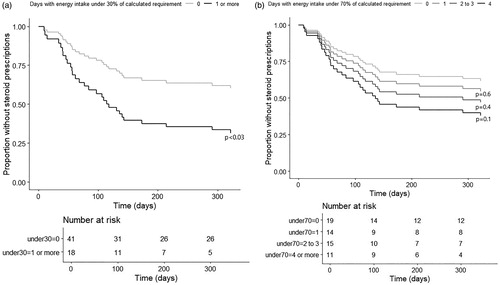
Table 4. Factors significantly associated with disease course.
In unadjusted stepwise backward variable selection regression analyses, the number of days with energy intake under 30% of the calculated requirement was the most significant predictor for time to the first steroid prescription, (p = .012, HR = 1.3). No other predictors were significant in these analyses. However, for the number of steroid prescriptions during the FU year no predictors were found significant. Other predictors that were tested in these two multivariant models were length of stay, number of days with energy intake under 50 and 70% of the calculated requirement, immunosuppressive or biological medication at admission, age at admission, CRP at admission, first documented BMI, use of PN during the inpatient period, diagnosis (CD/UC/IBD-unclassified) and sex.
Discussion
This study has demonstrated that inadequate nutritional intake in patients during inpatient admission for acute IBD flare is a significant problem, particularly among older patients and may have implications for achievement or durability of remission after acute IBD flare.
Days with inadequate nutritional intake occurred frequently in these patients despite the availability and frequent use of PN and frequent involvement of a specialist dietitian in the patients’ care, demonstrating that achieving adequate nutrition in patients admitted to hospital with acute IBD is challenging. The finding that inadequate nutrition in patients with IBD is a common problem is in line with previous findings by others [Citation14], but now we show that this is not just a background factor for IBD flare but an ongoing problem during the flare. We have shown that higher age is a predictor of inadequate nutritional intake amongst IBD patients with severe active flare independent of disease duration. Previously, patients over the age of 65 have more frequently been registered with malnutrition ICD-9-CM code 263.x in discharge codes [Citation15]. However, these ICD-codes at discharge do not differentiate between pre-existing malnutrition that was present when the patient was admitted and malnutrition occurring during the hospital stay. In contrast, our study has specifically shown that the number of days with suboptimal energy intake whilst the patient is in hospital is associated with increasing age. Interestingly, in one study in general gastroenterology patients, where malnutrition was defined as BMI under 20 kg/m2 or at least 10% weight loss during the last six months, younger age was found as a risk factor of malnutrition [Citation16] suggesting that the association between inadequate nutritional intake and age during acute flare may be specific to IBD. On the other hand, malnutrition is common generally in elderly population [Citation11]. Also, conflicting results are likely to occur due to different definitions for malnutrition. Though the aetiology is multifactorial, many of the factors contributing to malnutrition in the elderly population act by reducing food intake [Citation11]. In conclusion, clinicians should be vigilant in monitoring nutritional intake in older patients during their inpatient stay with acute IBD. Also, it should be recognised that malnutrition is an additional factor, among several others, complicating IBD in elderly population also during a severe flare [Citation2].
In CD patients, male gender and CRP at admission predicted inadequate nutritional intake. This is in keeping with a previous study showing that in hospitalised adolescents with CD, male gender was a risk factor for malnutrition [Citation17]. The association between CRP at admission and the number of days with suboptimal intake indicates that for patients with CD at least, the severity of inflammation may increase the risk for inadequate nutritional intake. Food avoidance has been shown to be more frequent during active disease [Citation9], but to our knowledge it has not been previously demonstrated whether severity of inflammation may be directly related to inadequate nutritional intake.
Patients with more days with inadequate nutritional intake had shorter time to their first steroid prescription after discharge from hospital, indicating that inadequate nutritional intake may impair recovery from acute IBD flare necessitating intensified steroid treatment. Paradoxically, we found that weight gain during the inpatient period was associated with the number of readmissions during the FU year. However, we speculate that during the short period of hospitalisation, weight gain may reflect fluid accumulation, secondary to steroid treatment and inflammation, rather than gain of fat free mass reflecting general nutritional status. Also, weight gain during the inpatient period might reflect inadequate hydration status at admission which is then corrected. A study that differentiates between body weight attributable to water, fat and muscle mass would be able to better discern this.
To our knowledge, associations between inadequate nutritional intake during an active flare and durability of remission have not been previously studied. Most of the studies done so far characterising nutrition in IBD patients have focused on retrospective nutritional data in outpatients or nutritional status at admission to or at discharge from hospital. Such data can only reveal the patients’ habitual nutritional status but does not reveal the impact of dietary intake during the IBD flare. For example, Nguyen et al. [Citation13] defined malnutrition as occurrence of Clinical Modification of the International Classification of Diseases, 9th Revision (ICD-9-CM) codes for protein-calorie malnutrition in the medical case summaries at discharge and showed that malnutrition is associated with increased in-hospital mortality, length of stay, and total charges among IBD patients [Citation13]. However, in this study it is not possible to evaluate the contribution of the nutritional intake during the acute flare compared with the contribution of the patient’s background nutritional status prior to the acute flare. Thus, the present study highlights a unique opportunity for physicians to intervene in improving patients’ nutrition during acute flare with potential impacts on clinical outcome.
Using one diagnosis as a marker does not provide detailed information about the degree or severity of malnutrition, nor whether the patient suffers from deficiencies in specific components of the diet such as calorie- and/or protein deficiency. Furthermore, low vitamin D levels are associated with clinical relapse [Citation18], and zinc deficiency is found to be associated with increased risk of IBD-related hospitalizations, surgeries, and complications in CD and risk of IBD-related hospitalisations for UC [Citation19], indicating adverse effects of micronutrient malnutrition on the disease course. It may be speculated that adequate nutritional intake might positively affect bowel healing by, for example, enabling intestinal bacteria to produce more butyrate that protects colonocytes by acting as an energy source for them [Citation20]. Also, glutamine is a food component that can act as a direct energy source for enterocytes, even though its clinical effects for gut barrier function are yet inconclusive [Citation4,Citation21]. There is also further preclinical data on specific nutritional components having effects on gut barrier or inflammatory cascades though evidence on clinical effect is lacking [Citation4,Citation21,Citation22]. However, effects of EEN demonstrate that nutritional factors can have also clinical significance [Citation3]. Future studies of the impact of diet during acute IBD flare should collect more detailed dietary data prospectively and in addition use several methods to define nutritional status in order to better understand how nutrition during flare affects both the process of mucosal healing and clinical outcomes.
As age was found to be a predictor of poor nutritional intake during the inpatient period, one could speculate that age could also be a confounding factor in the association between poor nutritional intake and higher number of steroid prescriptions during the FU year. For example, elderly patients could be subjected for multiple steroid prescriptions because of reluctance to initiate immunomodulatory or biological therapy. However, we adjusted all the analyses predicting relapse of inflammation for age and sex, eliminating possible confounding effects of these factors. Thus, age is a predictor of poor nutritional intake during an IBD flare, but poor nutritional intake during a flare predicts future relapse of the inflammation independent of age.
The severity of inflammation may affect both appetite and risk of relapse and thus confound the association between nutritional intake and flare recurrence. Mechanisms by which inflammation can reduce appetite include increased proinflammatory cytokines and central serotonin release, as shown in a rat colitis model [Citation10]. However, confounding effect of inflammation in our study is not supported by our results since direct markers of disease severity such as CRP and number of bowel movements were associated neither with inadequate nutritional intake in the whole cohort nor with flare recurrence. However, the length of the inpatient period was associated with the time to the first steroid prescription, and immunosuppressive or biological medication at admission was associated with future readmissions, both of which may be interpreted as a proxy for disease severity. Nevertheless, the number of days with inadequate nutritional intake was not significantly associated with length of the inpatient period which is evidence against confounding between these two factors. In conclusion, although confounding effect of disease severity cannot be totally excluded, our findings suggest that the association between inadequate nutritional intake and quicker relapse is independent from the disease severity.
One limitation of this study is the use of the arbitrary cut-offs of energy intake under 70, 50 and 30% of the calculated requirement. However, there is no available data to suggest which level of deficit in nutritional intake might lead to adverse outcomes. Thus, these arbitrary limits were applied.
The outcomes detailed in are proxy markers for relapse of the inflammation. However, the use of these proxy markers was necessitated by the retrospective design of the study. Only oral or intravenous steroids were included for this outcome (not local preparations) to capture more severe inflammatory activity.
Due to retrospective study design, we were not able to characterise patients’ nutritional intake more detail. This is an important limitation since, for example, adequate intake of micro- and macronutrients might be equally or even more important for achievement of durable remission. In addition, we were not able to distinguish the calories gained from enteral vs parenteral intake which precluded analysis of the relative importance of these two routes of nutrition. Further, we were not able to define whether patients fulfil the criteria for malnutrition. Further limitations of the study include small sample size and that the study was done with data from only one centre. Also, due to retrospective study design it was challenging to obtain patients’ consent resulting in exclusions for that reason.
One individual was excluded from the analysis after visual inspection of the data as she was deemed an extreme case that may falsely affect the analyses, see Supplementary Data.
Conclusions
Inadequate nutritional intake during a severe flare of IBD is a common problem and may be a predictor of quicker relapse, independent of markers of inflammatory activity. This motivates close monitoring and correction of patients’ nutritional intake during IBD flare. Moreover, we have found that older age is a predictor of inadequate nutritional intake, indicating that this group of IBD patients should be especially carefully monitored during acute IBD flare. The results of this study motivate future larger, prospective studies, which would also permit investigation of the mechanisms that might underlie this finding.
Supplemental Material
Download MS Word (35.6 KB)Acknowledgements
Andrea Discacciati and Henrike Häbel from Biostatistics Core Facility at Karolinska Institutet have contributed to statistical analyses by providing assistance with methodology selection and use of statistical software.
Disclosure statement
Katja Anneli Kulmala has nothing to disclose. Jan Björk has nothing to disclose. Sara Andersson reports personal fees from Mylan/Meda AB, personal fees from Ferring AB, personal fees from MediahusetiGöteborg, outside the submitted work. Ann-Sofie Backman reports personal fees from JANSSEN-CILAG AB, personal fees from Takeda, personal fees and non-financial support from Ferring, personal fees from Tillotts, outside the submitted work. Michael Eberhardson reports personal fees from Janssen, personal fees from Takeda, grants and personal fees from MSD, personal fees from AbbVie, personal fees from Tillotts, personal fees and other from Ferring, personal fees from Pfizer, personal fees from Orion Pharma, other from BMID AB, outside the submitted work. Francesca Bresso has nothing to disclose. Charlotte RH Hedin reports speaker fees from JANSSEN-CILAG AB, speaker fees from Takeda, outside the submitted work.
References
- Sturm A, Maaser C, Mendall M, et al. European Crohn’s and Colitis Organisation topical review on IBD in the elderly. J Crohns Colitis. 2017;11:263–273.
- Arnott I, Rogler G, Halfvarson J. The management of inflammatory bowel disease in elderly: current evidence and future perspectives. Inflamm Intest Dis. 2018;2:189–199.
- Sigall-Boneh R, Levine A, Lomer M, et al. Research gaps in diet and nutrition in inflammatory bowel disease. A topical review by D-ECCO Working Group [Dietitians of ECCO]. J Crohns Colitis. 2017;11:1407–1419.
- Bischoff SC, Escher J, Hébuterne X, et al. ESPEN Practical Guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr. 2020;39:632–653.
- Mijac DD, Jankovic GL, Jorga J, et al. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315–319.
- Cederholm T, Barazzoni R, Austin P, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49–64.
- Sobotka L, Allison SP, Forbes A, et al. Basics in clinical nutrition. 4th ed. Prague: GALÉ; 2011.
- Gassull MA, Cabre E. Nutrition in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2001;4:561–569.
- Casanova MJ, Chaparro M, Molina B, et al. Prevalence of malnutrition and nutritional characteristics of patients with inflammatory bowel disease. J Crohns Colitis. 2017;11:1430–1439.
- Ballinger A, El-Haj T, Perrett D, et al. The role of medial hypothalamic serotonin in the suppression of feeding in a rat model of colitis. Gastroenterology. 2000;118:544–553.
- Eder P, Niezgodka A, Krela-Kazmierczak I, et al. Dietary support in elderly patients with inflammatory bowel disease. Nutrients. 2019;11:1421.
- Ananthakrishnan AN, McGinley EL, Binion DG, et al. A novel risk score to stratify severity of Crohn’s disease hospitalizations. Am J Gastroenterol. 2010;105:1799–1807.
- Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis. 2008;14:1105–1111.
- Wedrychowicz A, Zajac A, Tomasik P. Advances in nutritional therapy in inflammatory bowel diseases: review. World J Gastroenterol. 2016;22:1045–1066.
- Ananthakrishnan AN, McGinley EL, Binion DG. Inflammatory bowel disease in the elderly is associated with worse outcomes: a national study of hospitalizations. Inflamm Bowel Dis. 2009;15:182–189.
- Gheorghe C, Pascu O, Iacob R, et al. Nutritional risk screening and prevalence of malnutrition on admission to gastroenterology departments: a multicentric study. Chirurgia (Bucur). 2013;108:535–541.
- Dotson JL, Bricker JB, Kappelman MD, et al. Assessment of sex differences for treatment, procedures, complications, and associated conditions among adolescents hospitalized with Crohn’s disease. Inflamm Bowel Dis. 2015;21:2619–2624.
- Gubatan J, Moss AC. Vitamin D in inflammatory bowel disease: more than just a supplement. Curr Opin Gastroenterol. 2018;34:217–225.
- Siva S, Rubin DT, Gulotta G, et al. Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. InflammBowel Dis. 2017;23:152–157.
- Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526.
- Hering NA, Schulzke JD. Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis. 2009;27:450–454.
- Ramakers JD, Mensink RP, Schaart G, et al. Arachidonic acid but not eicosapentaenoic acid (EPA) and oleic acid activates NF-kappaB and elevates ICAM-1 expression in Caco-2 cells. Lipids. 2007;42:687–698.
