Abstract
Objectives
In acute portal vein thrombosis (PVT), a six-month anticoagulation treatment achieves complete recanalization in only 35%–45% of patients, but the predictors of poor treatment responses are unclear. We examined treatment outcomes in PVT and aimed to identify predictors of incomplete recanalization and portal hypertensive complications.
Materials and methods
This retrospective study comprised patients diagnosed with PVT between 2006 and 2015. Key exclusion criteria were liver cirrhosis, malignancy, and age <18.
Results
The final cohort comprised 145 patients, of whom 132 (92%) were primarily treated with anticoagulation. The 5-year cumulative incidence of complete recanalization was 42% and of portal hypertensive complications, 31%. Independent predictors of insufficient recanalization were sub-acute or chronic thrombosis (hazard ratio (HR) 3.1, 95% CI 1.6–5.8), while acute pancreatitis was a protective factor (HR 0.3, 95% CI 0.2 − 0.7). Independent predictors of incident portal hypertensive complications were as cites at baseline (HR 3.3, 95% CI 1.7–6.7), sub-acute or chronic thrombosis (HR 2.9, 95% CI 1.6–5.3), extension of thrombosis to the splenic or mesenteric vein (HR 2.6, 95% CI 1.2–5.7), myeloproliferative disease (HR 3.0, 95% CI 1.4–6.5), and anemia (HR 2.1, 95% 1.1–3.9), while acute pancreatitis was a protective factor (HR 0.1, 95% CI 0.03–0.5).
Conclusions
Etiology and age of thrombosis are associated with treatment responses in PVT. The presence of ascites at baseline, etiology, and extent of thrombosis, a non-acute thrombosis and anemia, are associated with the risk of portal hypertensive complications. Etiology and extent of thrombosis should be taken into account when determining the treatment (method) for PVT.
Introduction
Portal vein thrombosis (PVT) can be categorized according to age of thrombosis, completeness of obstruction, and extent of thrombosis. The aim of treatment was to determine the etiological factors, prevent the expansion of thrombosis, and restore portal vein circulation. Thrombosis restricted to the portal vein is usually treated with anticoagulation, and in acute thrombosis, early anticoagulation therapy attains complete recanalization in up to 35%–45% of patients [Citation1–3]. The role of anticoagulants in the treatment of chronic PVT is disputed [Citation4]. The effectiveness of anticoagulant treatment has been shown to be dependent, among other things, on the extent of the thrombosis, the presence of ascites, and the delay from onset of symptoms to beginning of treatment [Citation5–8]. If anticoagulant treatment fails, more invasive procedures can be considered. However, there is paucity of evidence to firmly guide when and which patients benefit from more aggressive treatment, e.g., thrombolysis. Extensive thrombosis, delayed start of anticoagulation treatment, and more than one thrombophilic risk factor have been suggested as indications for early more invasive treatment; however, the results have been inconsistent [Citation2,Citation9–15].
The aim of this study was to examine and compare treatment outcomes in patients treated for PVT in a large health care district, in order to find predictors of insufficient recanalization and portal hypertensive complications.
Materials and methods
Patients and methods
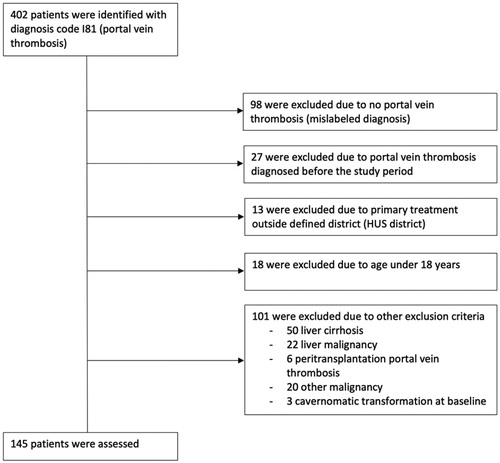
This retrospective cohort study included patients diagnosed with PVT in Helsinki and Uusimaa health care district’s hospitals (HUS). HUS-area serves a population of 1.6 million people in a defined geographical area of 9216 km2 in southern Finland and includes 18 secondary and one tertiary referral hospitals with approximately 2500 hospital beds. These public hospitals treat all referral patients within the area, whereas private referral hospitals treating these patients are non-existent. All HUS-area hospitals use the same electronic patient record system. Patients diagnosed with PVT in any of the HUS area hospitals between 2006 and 2015 were identified from electronic patient records by an electronic search for the International Classification of Diseases 10 (ICD-10) code I81 (PVT). Exclusion criteria were liver cirrhosis, malignancy, peritransplantation PVT, age below 18 years, primary treatment outside HUS district, and cavernous transformation at baseline. Patients with thrombus in the splenic or the superior mesenteric vein not extending to the portal vein were also excluded.
Institutional review board of HUS approved the study plan and gave permission to conduct the study. Patient records were analyzed and data regarding patient characteristics, hospital stay, treatment, and outcome were manually extracted. Last date of follow-up was defined as the date of last note in the electronic patient records. The patient records were reviewed up to 26 September 2019.
Definitions
The primary endpoints were insufficient recanalization and the incidence of portal hypertensive complications. Insufficient recanalization was defined as partial or total obstruction, or a cavernous transformation of the portal vein at last imaging. Portal hypertensive complications were defined as ascites, esophageal or fundus varices, or symptomatic portal biliopathy defined as jaundice needing an intervention together with compatible cholangiography findings.
Age of thrombosis was determined according to the anatomic-functional classification used by Sarin et al. [Citation26]. Acute thrombosis was defined either as a symptomatic thrombosis detected for the first time in a previously patent vein, the presence of hyperdense thrombus on imaging or absent or limited collateral circulation and dilated portal vein at the site of occlusion. Sub-acute thrombosis was defined similarly except that instead of symptomatic, it was asymptomatic. Chronic thrombosis was defined as no hyperdense thrombus, a previously diagnosed PTV on follow-up, portal cavernoma, or clinical features of portal hypertension.
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics version 23 (IBM Corp©, Armonk, NY, USA). Cox regression analysis was used for univariate and multivariate analyses. Variables with p values <.2 in univariate analysis were selected for multivariate analysis, except for variables that could be expected to cause multicollinearity and those with 10% or more variables missing. Kaplan–Meier estimates were used for survival or cumulative incidence of outcome events during follow-up. A two-tailed p-value below .05 was considered statistically significant.
Results
Patient characteristics and onset of disease
The initial extraction yielded 402 patients with a diagnosis of PVT. After exclusions, the final study cohort comprised 145 patients (). Information on patient characteristics and the onset of disease are shown in . The median age was 53 years, and the majority were male (n = 98, 68%). Of all patients, 83 (57%) had no comorbidities and 22 (15%) had anticoagulation or antithrombotic treatment at onset.
Table 1. Basic demographics and onset.
Etiology
Etiology was multifactorial in 43% (n = 63) of patients (). Most common etiological factors were thrombophilia (n = 86, 59%) and inflammatory disease (n = 66, 46%).The most common inflammatory disease was acute pancreatitis (n = 20, 14%). In 13 (9%) patients, no etiological factor was found.
Table 2. Prevalence of etiological factors.
Treatment and outcome
Baseline imaging results are shown in . Ascites was present in baseline imaging in 29% (n = 42). There was only 1 patient (0.7%) with varices at baseline. Diagnostic imaging was ultrasound in 30 (20%) patients, CT with contrast in 110 (76%) patients, and MRI in 6 (4%) patients. Last imaging was done less than 6 months from diagnosis in 35 (24%) patients, 6–9 months from diagnosis in 14 (10%) patients, 9–12 months in 5 (3%) patients, 12–18 months in 11 (8%) patients, and more than 18 months from diagnosis in 62 (43%) patients. No follow-up imaging was done in 18 (12%) patients after diagnosis.
Table 3. Characteristics of thrombosis at the time of diagnosis and treatment.
First treatment modality was unfractionated heparin in 21 (15%) of patients, thrombolysis in 2 (1%), and surgery in 4 (3%) patients (). The rest (n = 111, 77%) had anticoagulation (other than unfractionated heparin) as first treatment or had no treatment (n = 7, 5%). Second intervention was needed in 14 (10%) patients, and a third or fourth was needed in 3 (2%) patients. Bowel resection, due to the development of intestinal infarction, was needed in 4 (3%) patients, on average 24 h (median) after PVT diagnosis. On three (2%) patients, it was the primary intervention and only one (1%) patient needed late bowel resection. In addition to these, four (3%) thrombectomies via laparotomy and two (1%) explorative laparotomies were done because either the clinical condition (n = 3) or CT findings (n = 2) raised suspicion of intestinal infarction.
Table 4. Outcomes and complications.
Kaplan–Meier estimates for complete recanalization were 21% at 6 months, 29% at 1 year, 35% at 2 years, and 42% at 5 years follow-up. For partial or complete recanalization, corresponding Kaplan–Meier estimates were 32% at 6-month, 45% at 1-year, 56% at 2-year, and 64% at 5-year follow-up. Kaplan–Meier estimates for cavernous changes were 1% at 6-month, 6% at 1-year, and 18% at 2-year follow-up.
Among patients with complete thrombosis at baseline, Kaplan–Meier estimates for complete and partial thrombosis at 2-year follow-up were 32% and 14%, respectively. For patients with baseline partial thrombosis, 25% progressed to complete thrombosis by 2 years.
Anticoagulation was continued for a median time of 8 months (IQR 5–12) and was continued indefinitely for 61 (42%) patients. Of those with a thrombophilic state, 22 (73%) had anticoagulation continued indefinitely, while the rest (27%) had it only continued temporarily.
Portal hypertensive complications
A total of 40 (28%) patients with either cavernous changes (n = 27), total obstruction (n = 6), or partial obstruction (n = 7) at the end of follow-up developed new-onset portal hypertensive complications: ascites in 11 (8%) patients (5 requiring treatment) and esophageal varices in 30 (21%), with 8 (6%) having variceal bleeding (esophageal). Symptomatic portal biliopathy developed in 2 (1%) patients. Of those 42 patients (29%) that had ascites at baseline, 1 developed ascites in follow-up and altogether 19 developed portal hypertensive complications. Kaplan–Meier estimates for the development of new-onset portal hypertensive complications at 5 and 10 years respectively were 31% and 42%. Gastroscopy was done to a total of 67 (46%) patients during follow-up, and all varices were confirmed with gastroscopy.
Complications and recurrences
Bleeding likely associated with anticoagulation was the only type of treatment complication observed (in the group). Gastrointestinal bleeding was the most common type of complication (n = 13, 9%), of which nine originated from the esophagus and four from the ventricle (fundus n = 3, corpus n = 1), all diagnosed by gastroscopy. Other bleeds (n = 12, 8%) included intramuscular hematomas (n = 5) and nose bleeds (n = 7). During follow-up, 5 (3%) patients had more than one bleeding episode ().
PVT recurrence developed in 6 (4%) patients after a median 2.4 years from baseline (IQR 0.9–10.2), and 8 (6%) patients had other thromboembolic conditions during follow-up ().
Multivariate analysis
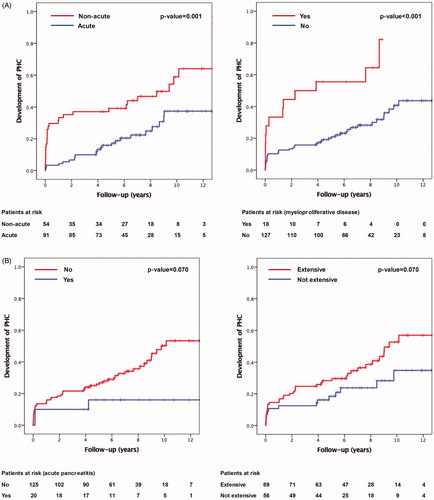
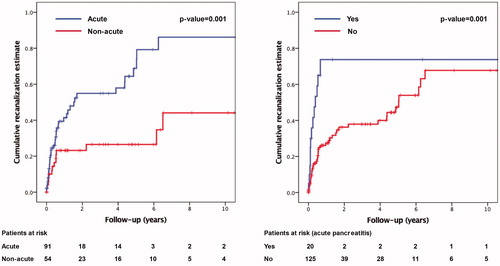
Sub-acute or chronic thrombosis (HR 3.1, 95% CI 1.6–5.8) was independently associated with insufficient recanalization, while acute pancreatitis at baseline (HR 0.3, 95% CI 0.2 − 0.7) was a protective factor. Kaplan–Meier estimates for complete recanalization at 3 years were 47% if the thrombosis was acute and 16% in sub-acute or chronic. If the patient had acute pancreatitis, the Kaplan-Meier estimate for recanalization at 3 years was 72% versus 29% if the patients did not have acute pancreatitis (, Supplementary Table 1).
Figure 2. Predictors of insufficient recanalization divided by (a) age of thrombosis and (b) etiology (acute pancreatitis).
Independent predictors of incident portal hypertensive complications were ascites at baseline (HR 3.3, 95% CI 1.7–6.7), sub-acute, or chronic thrombosis (HR 2.9, 95% CI1.6–5.3), extension of thrombosis to the splenic or mesenteric vein (HR 2.6, 95% CI 1.2–5.7), myeloproliferative disease (HR 3.0, 95% CI 1.4–6.5), and anemia (HR 2.1, 95% 1.1–3.9), while acute pancreatitis at baseline was a protective factor (HR 0.1, 95% CI 0.03–0.5).
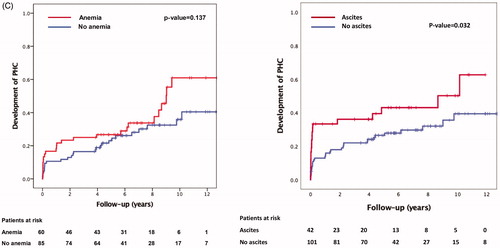
Kaplan–Meier estimates for the development of portal hypertensive complications at 5 years was 43% if ascites was present at baseline and 26% if it was not. In acute thrombosis, Kaplan–Meier estimate for the development of portal hypertensive complications was 22% and 47% in non-acute thrombosis. In a thrombosis extending to the superior mesenteric vein or the splenic vein, Kaplan–Meier estimates for the development of portal hypertensive complications at 5 years were 35% versus 25% in thrombosis only extending to the portal vein. If the patient had a myeloproliferative disease, Kaplan–Meier estimate for the development of portal hypertensive complications was 56% versus 27% if the patient did not have a myeloproliferative disease. If the patient had anemia, Kaplan–Meier estimate for the development of portal hypertensive complications at 5 years was 37% versus 27% if the patient did not have anemia at baseline. If the patient had acute pancreatitis, Kaplan–Meier estimate for the development of portal hypertensive complications at 5 years was 17% versus 33% if the patient did not have acute pancreatitis at baseline (, Supplementary Table 2).
Figure 3. (A) Predictors of portal hypertensive complications divided by (a) age of thrombosis and (b) etiology (myeloproliferative disease). (B) Predictors of insufficient recanalization divided by (a) etiology (acute pancreatitis) and (b) extent of thrombosis (two vs. one obstructed vein). (C) Predictors of insufficient recanalization divided by (a) anemia and (b) ascites.
Survival
Median clinical follow-up time was 6.9 years (IQR 5.3–9.4, range 3.8–13.7) and 12 (8%) patients died within 90 days. Deaths were due to PVT (n = 1), myocardial infarction (n = 3), sepsis (n = 2), pancreatitis (n = 1), and pneumonia (n = 1). The rest (n = 4) were unknown. Kaplan–Meier survival estimates were 90%, 88%, and 82% at 1 year, 3 years, and 5 years, respectively. Two PVT-related deaths occurred in follow-up, which were 8 days (bowel ischemia) and 4.5 years after diagnosis (bowel ischemia due to the development of a new PVT).
Discussion
Treatment of PVT remains a challenge, as we found that complete recanalization under mainly anticoagulation therapy is achieved in only 42% of patients and 31% develop new portal hypertensive complications in a 5-year follow-up period (ascites, esophageal varices, or symptomatic portal biliopathy). Non-acute thrombosis was an independent risk factor for insufficient recanalization, and acute pancreatitis was a protective factor. Ascites at baseline, non-acute thrombosis, thrombosis extending to the superior mesenteric vein or the splenic vein, myeloproliferative disease, and anemia were risk factors for portal hypertensive complications, while acute pancreatitis was a protective factor. Thus, patients with these risk factors might be candidates for more aggressive therapy, i.e. thrombolysis via either superior mesenteric artery or transjugular intrahepatic portosystemic shunt (TIPS) [Citation16–19].
EASL guidelines recommend anticoagulation therapy in all patients with acute PVT [Citation20], while ACCP guidelines recommend anticoagulation in symptomatic patients only [Citation21]. It is noteworthy that neither guideline recommend thrombolysis for treatment of PVT, even though, as we show here, one-third will develop portal hypertensive complications and over half of PVTs are not completely recanalized within 5 years. ESVS guidelines conclude that there is not yet enough evidence to support recommendations for endovascular therapies. Both ESVS and ESTES guidelines recommend endovascular intervention for patients with venous acute mesenteric ischemia if the patients’ condition deteriorates during medical therapy, although ESVS guidelines recommend this only if the patient is a poor surgical candidate [Citation22,Citation27].
Only a few studies have analyzed risk factors for insufficient recanalization. In a European prospective multicenter study, Plessier et al. [Citation6] analyzed 102 patients with acute, non-cirrhotic PVTs, and identified ascites and thrombosis extending to the splenic vein as independent risk factors for insufficient recanalization. In fact, recanalization of the portal vein did not occur in any of the patients with both of these risk factors [Citation6]. In our material, ascites and extensive thrombosis did not reach statistical significance as risk factors for insufficient recanalization. There were 23 (16%) patients with ascites and thrombosis extending to the splenic vein. Of these, 7 (30%) recanalized completely, 2 achieved partial recanalization (9%), and 14 (61%) achieved no recanalization.
However, both ascites at baseline and extensive thrombosis (extending to the superior mesenteric vein or the splenic vein) were risk factors for the development of portal hypertensive complications.
In another retrospective multicenter study in Spain, Turnes et al. [Citation5] included 38 patients with acute non-cirrhotic, non-malignant PVTs and found a delay in initiating anticoagulation associated with lack of recanalization. Patency of portal vein was achieved in 39%−44% of patients treated with anticoagulation in these studies [Citation5–6], and portal hypertensive complications occurred in 55% [Citation5]. Our results are in line with these reports, but we also identified acute pancreatitis as a protective factor. The protective effect of acute pancreatitis is most likely due to the transitory nature of inflammation as a hypercoagulative state. Out of the 20 patients with acute pancreatitis, 3 had a genetic mutation and none had a myeloproliferative disease. Taking out those with a double diagnosis did not change the correlation between acute pancreatitis and outcome.
Recently, there has been increasing interest toward interventional treatment of acute PVT. A systematic review comprising 399 patients with PVT reported outcomes of TIPS combined with either catheter-directed thrombolysis or thrombectomy or both. TIPS was technically feasible in 95% of patients with 10% complication rate and 79% 12-month recanalization rate [Citation17]. However, most of the patients in this meta-analysis were cirrhotic (92%).
Few studies have reported outcomes of interventional treatment of non-cirrhotic, non-malignant PVT. Klinger et al. [Citation14] treated 17 consecutive patients with a combination of transjugular thrombectomy, local thrombolysis, and used combined TIPS selectively. Recanalization was achieved in 94%, 2-year patency rate was 88%, and none developed portal hypertensive complications. A total of 94% had concomitant superior mesenteric vein and 88% splenic vein thrombosis in addition to complete PVT. A report by Wolter et al. [Citation19] included seven patients with non-cirrhotic non-malignant PVT in whom TIPS combined with local thrombolysis and thromboaspiration was used with patency rate of 71%. All patients had splenic vein and/or superior mesenteric vein thrombosis in addition to complete PVT. Thus, both of these series used invasive intervention mainly on patients who had risk factors for both insufficient recanalization (complete PVT) and portal hypertensive complications (concomitant superior mesenteric vein and/or splenic vein thrombosis).
Interventional treatment was used scarcely in our material. There were five endovascular procedures (three thrombolysis and two thrombectomies) and four surgical operations (thrombectomy via laparotomy). Although there has been concern of safety of interventional treatment, we did not have any complications from neither endovascular nor surgical procedures. Thrombolysis was performed through a catheter in the superior mesenteric artery accessed through the femoral artery. Two of these patients achieved full recanalization, while one developed cavernous changes. One patient developed portal hypertensive complications. Endovascular thrombectomy was performed through a transhepatic puncture, and both of these patients achieved full recanalization and did not develop portal hypertensive complications. All of those with surgical thrombectomies achieved complete recanalization and did not develop portal hypertensive complications.
Although interventional treatment options for PVT have showed promising results in recent studies [Citation9,Citation14,Citation15,Citation19,Citation23], there is no consensus on which patients benefit most from these [Citation2]. Since anticoagulation is a safe and effective option in the majority of cases, utilization of more invasive methods should be evaluated carefully [Citation3,Citation8,Citation24].
Chronic thrombosis has been suspected to recanalize poorly with anticoagulation treatment. This was true in our study as well, as we found both sub-acute and chronic thrombosis to be predictors of insufficient recanalization.
Thrombosis extending to the splenic or superior mesenteric vein was found to predict incident portal hypertensive complications but did not reach statistical significance as a predictor of insufficient recanalization. In localized PVT, formation of porto-portal collaterals may restore portal flow in a few weeks. Conversely, restoration of venous flow in thrombosis extending to the splenic or mesenteric veins likely requires the formation of larger porto-systemic collaterals, and despite these collaterals, significant portal hypertension may still persist and thereby predispose to portal hypertensive complications.
The study by Sogaard et al. [Citation3] concluded that follow-up of patients with PVT should be well organized, due to the high risk of development of portal hypertension. We agree with this statement and see room for improvement in our own health care district. Our study included 35 patients with no follow-up imaging (or gastroscopy) performed after six months. Though it is not possible to recommend optimal follow-up time based on our study alone, we believe gastroscopy should be performed on all patients at the latest 3 months from diagnosis.
This study has limitations. As a retrospective study, there is an inherent risk of information bias and misclassifications. Incidence and prognosis might be underestimated due to misdiagnosis and miscoding of patients with PVT. For example, there is clinical knowledge to support that some patients with, for example a simultaneous pancreatitis diagnosis, might not be recorded with a separate I81 diagnosis code. Due to this some of the patient fitting, the criteria might not be included in the search.
The radiological follow-up period varied a lot within our sample and up to 24% of patients had no follow-up imaging done after 6 months from diagnosis. PVT is known to achieve recanalization after 6 months, and therefore, lack of follow-up imaging might cause results to seem more pessimistic than they really are. We tried to minimize the potential effect of this by using Kaplan–Meier survival analysis in our outcome analyses.
There are several guidelines for the treatment of PVT [Citation17,Citation21,Citation22,Citation25], yet it is still somewhat unclear which patients and types of PVTs are in risk of insufficient recanalization. In our study, we found that patients with non-acute PVTs were less likely to recanalize sufficiently, whereas acute pancreatitis was a protective factor from this. We also found that patients with ascites at baseline, a myeloproliferative disease, a non-acute, extensive PVT, and anemia at baseline were more likely to develop portal hypertensive complications, whereas acute pancreatitis was a protective factor from this. Based on our findings, the age, etiology, and extent of thrombosis should be considered when deciding on the treatment and follow-up of PVT.
Supplemental Material
Download PDF (89.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Manzano-Robleda MDC, Barranco-Fragoso B, Uribe M, et al. Portal vein thrombosis: what is new? Ann Hepatol. 2015;14(1):20–27.
- Lang SA, Loss M, Wohlgemuth WA, et al. Clinical management of acute portal/mesenteric vein thrombosis. Viszeralmedizin. 2014;30(6):394–400.
- Sogaard KK, Astrup LB, Vilstrup H, et al. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol. 2007;7(1):34.
- Handa P, Crowther M, Douketis JD. Portal vein thrombosis. Clin Appl Thromb Hemost. 2014;20(5):498–506.
- Turnes J, García-Pagán JC, González M, et al. Portal hypertension-related complications after acute portal vein thrombosis: impact of early anticoagulation. Clin Gastroenterol Hepatol. 2008;6(12):1412–1417.
- Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51(1):210–218.
- Condat B, Pessione F, Helene Denninger M, et al. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology. 2000;32(3):466–470.
- Rodriguez-Castro KI, Vitale A, Fadin M, et al. A prediction model for successful anticoagulation in cirrhotic portal vein thrombosis. Eur J Gastroenterol Hepatol. 2019;31(1):34–42.
- Rosenqvist K, Eriksson LG, Rorsman F, et al. Endovascular treatment of acute and chronic portal vein thrombosis in patients with cirrhotic and non-cirrhotic liver. Acta Radiol. 2016;57(5):572–579.
- De Santis A, Moscatelli R, Catalano C, et al. Systemic thrombolysis of portal vein thrombosis in cirrhotic patients: a pilot study. Dig Liver Dis. 2010;42(6):451–455.
- Hollingshead M, Burke CT, Mauro MA, et al. Transcatheter thrombolytic therapy for acute mesenteric and portal vein thrombosis. J Vasc Interv Radiol. 2005;16(5):651–661.
- Semiz-Oysu A, Keussen I, Cwikiel W. Interventional radiological management of prehepatic obstruction of the splanchnic venous system. Cardiovasc Intervent Radiol. 2007;30(4):688–695.
- Ganger DR, Klapman JB, McDonald V, et al. Transjugular intrahepatic portosystemic shunt (TIPS) for budd-chiari syndrome or portal vein thrombosis: review of indications and problems. Am J Gastroenterol. 1999;94(3):603–608.
- Klinger C, Riecken B, Schmidt A, et al. Transjugular local thrombolysis with/without TIPS in patients with acute non-cirrhotic, non-malignant portal vein thrombosis. Dig Liver Dis. 2017;49(12):1345–1352.
- Gerwing M, Wilms C, Heinzow H, et al. Escalating interventional recanalization therapy in non-cirrhotic, non-malignant acute portal vein thrombosis. Eur J Gastroenterol Hepatol. 2019;31(12):1584–1591.
- Liu K, Li W, Du X, et al. Comparison of systemic thrombolysis versus indirect thrombolysis via the superior mesenteric artery in patients with acute portal vein thrombosis. Ann Vasc Surg. 2017;39:264–269.
- Rodrigues SG, Sixt S, Abraldes JG, et al. Systematic review with meta‐analysis: portal vein recanalisation and transjugular intrahepatic portosystemic shunt for portal vein thrombosis. Aliment Pharmacol Ther. 2019;49(1):20–30.
- Wang C, Wei L, Niu H, et al. Agitation thrombolysis combined with catheter-directed thrombolysis for the treatment of non-cirrhotic acute portal vein thrombosis. WJG. 2018;24(39):4482–4488.
- Wolter K, Decker G, Kuetting D, et al. Interventional treatment of acute portal vein thrombosis. Rofo. 2018;190(8):740–746.
- European Association For The Study Of The Liver. EASL clinical practice guidelines: vascular diseases of the liver. J Hepatol. 2015;64(1):179–202.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352.
- Tilsed JVT, Casamassima A, Kurihara H, et al. ESTES guidelines: acute mesenteric ischaemia. Eur J Trauma Emerg Surg. 2016;42(2):253–270.
- Luo J, Yan Z, Wang J, et al. Endovascular treatment for nonacute symptomatic portal venous thrombosis through intrahepatic portosystemic shunt approach. J Vasc Interv Radiol. 2011;22(1):61–69.
- Hall TC, Garcea G, Metcalfe M, et al. Impact of anticoagulation on outcomes in acute non-cirrhotic and non-malignant portal vein thrombosis: a retrospective observational study. Hepatogastroenterology. 2013;60(122):311–317.
- Maurice JB, Brodkin E, Arnold F, et al. Validation of the baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol. 2016;65(5):899–905.
- Sarin S, Philips CA, Kamath P, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology. 2016;151(4):574–577.
- Björck M, Koelemay M, Acosta S, et al. Editor's choice - management of the diseases of mesenteric arteries and veins: clinical practice guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:460–451.