Abstract
Background
Roux-en-Y gastric bypass (RYGB) can cause multiple food intolerances and gastrointestinal complaints are frequently reported after dairy consumption. We aimed to determine the prevalence of lactose malabsorption and intolerance, and complaints associated with dairy consumption in daily life, before and after RYGB.
Method
The lactose breath test (LBT) and lactose tolerance test (LTT) was performed in 84 patients awaiting RYGB surgery and 84 patients after surgery. Gastrointestinal symptoms at baseline and after testing were recorded. Lactose malabsorption was defined as a positive LBT and/or LTT. Lactose intolerance as a positive test combined with an increase of gastrointestinal complains. Dairy consumption in daily life and successive gastrointestinal complaints were registered via a questionnaire. Results of preoperative and postoperative patients were compared.
Results
Lactose malabsorption was present in 15 (17.9%) of the preoperative patients and in 25 (29.8%) of the postoperative patients (OR 2.46; 95%CI: 1.08–5.59; p = .03). Of the preoperative patients 6 (7.1%) patients met the criteria for lactose intolerance, compared to 8 (9.5%) patients in the postoperative group (OR 1.48; 95%CI 0.48–4.57; p = .50). Twelve (14.3%) preoperative patients indicated to have gastrointestinal complaints after dairy consumption in daily life versus 45 (53.6%) postoperative patients (p < .01).
Conclusion
This study shows no increase in patients with proven lactose intolerance after RYGB compared to preoperative patients. Gastrointestinal complaints after dairy consumption in daily life were far more frequently reported by RYGB patients. It is unlikely that all reported gastrointestinal complaints are actually caused by lactose. Other ingredients in dairy, like fat, are possibly contributory.
Introduction
Roux-en-Y gastric bypass (RYGB) for morbid obesity leads to a reduced gastrointestinal uptake of some nutrients due to bypassing the majority of the stomach, duodenum and proximal jejunum. A decreased calcium intake and uptake is often seen, with responsive elevated plasma parathyroid hormone levels and diminished vitamin D levels [Citation1,Citation2]. Longer term after RYGB an increase in osteoporosis can be found [Citation3]. After surgery, patients are advised to increase their protein and calcium intake to compensate for the reduced uptake [Citation4]. Dairy products contain relatively large amounts of calcium and protein and are therefore an important part of the postoperative diet.
RYGB can cause multiple food intolerances. Many patients experience gastrointestinal complaints after consuming carbohydrate and fat dense food [Citation5,Citation6]. Complaints are also frequently reported after dairy consumption, like nausea, bloating and diarrhoea [Citation7], which can lead to avoiding dairy products. Lactose malabsorption may be the cause of this intolerance. It has been described after Billroth gastroduodenostomy and gastrojejunostomy [Citation8,Citation9].
Lactose is a disaccharide that can be found in milk from mammals, and in products derived from this milk. Human intestines have difficulties absorbing this disaccharide so the intestinal villi secrete lactase. This enzyme has highest activity in the jejunum and gradually decreases towards the proximal and distal gut, it cleaves lactose into galactose and glucose, which can be absorbed [Citation10]. If there is insufficient hydrolysis of lactose, the remaining lactose is fermented by gut bacteria, mainly into fatty acids and hydrogen gas. This can cause gastrointestinal complaints like bloating, flatulence, diarrhoea and pain, i.e. lactose intolerance [Citation7]. The altered anatomy after RYGB may contribute to intolerance, but could also influence lactose test characteristics by a more rapid glucose absorption directly after ingestion of lactose and/or alteration of gut microbiome [Citation11–13].
Whether the increase of gastrointestinal complaints after dairy consumption can be attributed to lactose malabsorption and intolerance in RYGB patients remains to be determined. This study was designed to determine the prevalence of lactose malabsorption and lactose intolerance before and after RYGB, to evaluate whether change in anatomy after RYGB has an influence on lactose test characteristics and to gain more insight in patients’ dairy consumption and possible related complaints in daily life.
Methods
An observational study was performed in two major bariatric surgery hospitals in The Netherlands, by the same investigators, same surgical team and using the same methods and equipment in both hospitals. Patients after RYGB (exposed) and patients awaiting RYGB surgery with a body mass index (BMI) of ≥40 kg/m2 or a BMI of ≥35 kg/m2 with obesity related comorbidities (non-exposed), were approached for participation in this study at the outpatient clinic or by telephone. Postoperative patients and patients before surgery were matched for age (plus or minus 5 years) and gender on group level.
Exclusion criteria were the consumption of >60 grams of alcohol (6 units) per day, recent use of antibiotics (<28 days before test), diabetes mellitus and active gastrointestinal (GI) diseases: coeliac disease, inflammatory bowel disease, gastrointestinal infection or other gastrointestinal disease that may influence lactase activity.
All postoperative patients had laparoscopic RYGB surgery, at least 11 months before study participation. A 4 × 8 cm gastric pouch, 50 cm biliary limb and 150 cm antecolic, antegastric alimentary limb was created.
Ethical approval
This study was carried out in accordance with the ethical standards of the Helsinki Declaration. All patients provided written informed consent. Data was encrypted before storage and analysis. The medical ethics committee of the MC Slotervaart/Reade in Amsterdam approved this study.
Data collection
Patient files were used to collect patient characteristics weight (loss), BMI, date of surgery, gender and age. After inclusion, patients were asked about their and their parents country of origin, as this can be of influence on the prevalence of lactose intolerance. Patients were categorized for Northwest European (NW-E) country of origin and other country of origin. At baseline, patients completed a questionnaire to determine their dairy consumption in real life (type of products and frequency), type of complaints they experience after dairy consumption in daily life and whether they consider themselves lactose intolerant. Prior to and after the lactose tests, patients scored their gastrointestinal symptoms on a second questionnaire. The following eight symptoms were scored on a 5 point Likert scale, of which total scores were calculated (minimum-maximum score 0–32 points): diarrhoea, nausea, abdominal pains, bloating, vomiting, belching, borborygmi and flatulence. The questionnaires are shown in the supplementary data.
Lactose malabsorption testing
Lactose malabsorption was measured in two ways, with the lactose hydrogen breath test (LBT) and lactose tolerance test (LTT), at the same time.
For the LBT, patients were given a lactose solution of 25 gram in 125 mL water. Before and 30, 60, 90 and 120 min after the consumption of this lactose solution, exhaled air was collected (AlveoSampler, Quintron, Milwaukee, USA) and hydrogen and methane concentrations were determined directly after each breath sample collection (MicroLyzer, Quintron). The MicroLyzer was used according to the manufacturers users instructions and frequently calibrated. An increase of 20 parts per million for hydrogen and 10 parts per million for methane relative to the baseline measurement was considered a positive outcome for lactose malabsorption [Citation14].
Simultaneously, for the LTT, before and 30, 60, 90 and 120 min after consumption of the lactose solution, capillary glucose levels were determined (HemoCue Glucose 201 DM, HemoCue AB, Angelholm, Sweden). A rise of less than 1.1 mmol/L (20 mg/dL) relative to the baseline measurement was considered a positive outcome for lactose malabsorption [Citation14].
Patients were asked not to eat and drink at least 12 h, not to smoke at least 2 h and not to exercise prior to testing.
Statistical analyses
Descriptive statistics were used for patients characteristics in both groups. Data was checked for normal distribution using graphical estimation and Shapiro–Wilk test. Student’s t-test was used to compare continuous, normally distributed variables, either independent or paired, based on the (in)dependency of data. Non-parametric tests were used for non-normally distributed data. The Wilcoxon signed rank test or McNemar test was used for paired data and Mann–Whitney U or Fisher’s Exact Test for independent variables, based on whether data is continuous or categorical.
Characteristics lactose malabsorption tests:
Median values for hydrogen and methane, and mean glucose levels were determined with descriptive statistics per time point for all patients and separately for positive and negative test outcome. Preoperative and postoperative hydrogen, methane and glucose levels at the different time-points were compared (Bonferroni correction for multiple testing was applied, alpha 0.003), as were the pre and postoperative differences in glucose rise and fall between time-points.
Lactose malabsorption:
Lactose malabsorption is defined as a positive result on the LBT and/or LTT. Proportions of preoperative and postoperative patients with lactose malabsorption were determined with descriptive statistics. Using binary logistic regression the association with operation status, corrected for country of origin (NW-E or other), was determined. To gain more insight in the influence of RYGB on test characteristics, results of the LBT and LTT are also reported separately. Correlations between the tests, preoperative and postoperative, were determined with Spearmans rank correlation.
Lactose intolerance:
Lactose intolerance is defined as a positive LBT and/or LTT combined with any increase of total gastrointestinal symptom score in reference to the baseline score. Descriptive statistics were used to determine baseline and post-test total scores on the gastrointestinal symptoms questionnaire for preoperative and postoperative patients, and number of patients with increasing scores in both groups. Using binary logistic regression the association between increasing scores and operation status was determined. Subsequently, with descriptive statistics, proportions of patients who met the criteria for lactose intolerance were determined. Again, using binary logistic regression the association with operation status, corrected for country of origin, was determined.
Daily life dairy consumption:
Descriptive statistics are also used to report results on the questionnaires on daily life dairy consumption and proportions of self-reported lactose intolerance in the preoperative and postoperative group. Differences in proportions are compared.
After the inclusion of 100 patients, 50 per group, an interim analysis was carried out because of the roughly estimated postoperative proportion of lactose intolerant patients, on the outcome of difference in objectified lactose intolerance. According to the method of O’Brien-Fleming an α of 0.0054 was used for the interim analysis. No difference was found, therefore the study was continued and completed. An α of 0.0492 is used for the final analysis in this paper.
Data were analysed using SPSS Statistics (version 25, IBM, Armonk, New York, USA).
Results
Study population: Between August 2018 and November 2019 a total of 340 patients were approached for participation in this study in certain time periods at the outpatient clinic or by telephone and 168 patients were included in the study. Patient characteristics are presented in . Non-participating patients were younger than the included patients (mean 42.9 vs 46.7 years old, p < .01) but did not differ in sex ratio.
Table 1. Patient characteristics.
Characteristics lactose malabsorption tests
The lactose hydrogen breath test (LBT) and lactose tolerance test (LTT) was carried out in 84 postoperative and 84 preoperative patients. Median hydrogen, methane and mean glucose levels per time point are presented in respectively, for all pre and postoperative patients and subdivided for test outcome. Baseline hydrogen levels of preoperative patients differed from postoperative patients’ baseline hydrogen (p < .001). Methane and glucose levels at all time-points differed between patients before and after surgery (all p < .001), except for glucose at 30 min (p = .004).
Figure 1. Median hydrogen (A), methane (B) and mean glucose (C) levels per time point for all pre and postoperative patients and subdivided for test outcome. PPM: parts per million; mmol/L: millimoles per litre; BL: baseline; min: minutes; LBT: lactose breath test; LTT: lactose tolerance test; neg: negative test outcome; pos: positive test outcome.
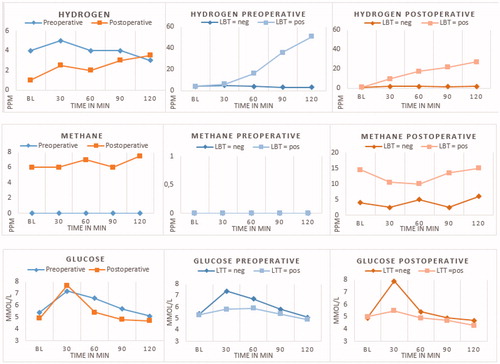
As expected, glucose levels after lactose intake rose more rapidly in postoperative patients than in preoperative patients, as difference in glucose value from baseline to 30 min after lactose intake rose with 1.8 mmol/L in preoperative and 2.8 mmol/L in postoperative patients (p < .01, mean difference 0.95; 95%CI 0.62–1.27). Subsequently, a decline of 0.6 mmol/L glucose was seen in preoperative patients from 30 − 60 min after lactose intake, this was 2.3 mmol/L in postoperative patients (p < .01, mean difference 1.71, 95% CI: 1.41–2.00).
Prevalence lactose malabsorption
The prevalence of lactose malabsorption was 17.9% in the preoperative group and 29.8% in the postoperative group. After adjustment for country of origin the odds of having lactose malabsorption was 2.46 higher in the post-operative group compared to the pre-operative group (95%CI: 1.08–5.59; p = .03, ). Among the patients with a Northwest European country of origin, presence of lactose malabsorption was 9.3% before surgery and 27.3% after surgery.
Table 2. Exact numbers and odds ratio’s for lactose malabsorption, increase in complaints during lactose test and lactose intolerance.
shows the distribution of positive and negative LBT and LTT among the preoperative and postoperative patients. Eleven (13.1%) preoperative patients had a positive LBT compared to 22 (26.2%) postoperative patients. Also 11 preoperative patients had a positive LTT compared to 8 (9.5%) postoperative patients. We observed a correlation between LBT and LTT among preoperative patients (r = 0.58, p < .01) and postoperative patients (r = 0.27, p = .01).
Table 3. Lactose breath test and lactose tolerance test results.
Prevalence increased complaints and lactose intolerance
Gastrointestinal complaints increased after lactose intake in 22.6% of preoperative patients and 27.4% of postoperative patients (adjusted OR 1.22; 95%CI: 0.61–2.44; p = .58; ). Of those patients with increasing complaints, most had a mild increase of <5 points. Two (2.4%) preoperative patients had a substantial increase of gastrointestinal complaints (>5 points) after consumption of the lactose solution. Among patients after surgery this was 4/84 (4.8%, p = .68).
No difference in lactose intolerant patients before (7.1%) and after surgery (9.5%) was found (OR 1.48; 95%CI 0.48–4.57; p = .50; ), corrected for country of origin. Subanalysis for NW-E country of origin patients showed a higher presence of lactose intolerance after surgery, but it did not reach statistical significance (before surgery 2.7%, after surgery 10.4%; p = .10). Of the preoperative patients with lactose intolerance, four patients had 1 point increase, one patient had 2 points increase and one patient’s score increased 8 points. Six postoperative patients with lactose intolerance had an increase of ≤4 points on GI complaint score, one patients’ score increased 7 points and one patient had an increase of 11 points.
Daily life dairy consumption
Patients were asked before lactose testing if they thought lactose containing products causes them gastrointestinal complaints. Twelve (14.3%) preoperative patients indicated this, of which 3 tested positive on the lactose tests, versus 45 (53.6%) postoperative patients (p < .01), of which 15 tested positive. Bowel cramps was the most frequently reported complaint, shows all most frequently reported GI complaints. Even though, 71 (84.5%) preoperative and 77 (91.7%) postoperative patients use dairy products on a daily basis. The most frequently used dairy products among preoperative and postoperative patients were milk (78.6% and 76.2% respectively), yoghurt (79.8% and 73.5%) and cheese (both 92.9%). Complaints after milk consumption were reported by 34.5% of all postoperative patients, complaints after ice-cream by 33.3% and after whipped cream by 29.8%, which were the most often reported products postoperatively. This was milk (9.5%) in the preoperative group.
Table 4. Most frequently reported complaints after dairy consumption.
Discussion
This study shows no increase in patients with proven lactose intolerance after Roux-en-Y gastric bypass compared to a morbidly obese patients before bariatric surgery. Furthermore, gastrointestinal complaints after lactose intake occurred in the same proportion of postoperative as preoperative patients. However, a difference was seen for lactose malabsorption between patients after RYGB and patients with morbid obesity, when adjusted for country of origin.
Interestingly, most postoperative patients continue to use dairy on a daily basis, even though they more frequently report bowel cramps and other gastrointestinal complaints after intake of dairy products.
This is the first study to investigate lactose intolerance after RYGB. Lactose intolerance originates from lactose malabsorption because of primary or secondary lactase deficiency. Where primary lactase deficiency is due to decreasing lactase production in the intestines, starting months after birth and regulated by the lactase-gene on chromosome 2 [Citation15], secondary lactase deficiency is caused by several gastrointestinal diseases. These lead to mucosal damage, resulting in a reduction of lactase production [Citation16,Citation17]. Several studies were performed after Billroth gastroduodenostomy and gastrojejunostomy which showed an increase in lactose intolerance postoperatively [Citation8,Citation9]. It was suggested that the gastrointestinal changes caused diminished lactase production. RYGB could have similar effects. The results of this study contradict this.
The lactose hydrogen breath test (LBT) and lactose tolerance test (LTT) are the two most frequently used tests in clinical practise and were therefore used in this study. Over 25% of postoperative patients tested positive on the LBT, twice as many as preoperative patients and with an even larger difference in the Non-Northwest European subgroup. In the normal population, the LBT has a sensitivity and specificity of 88 and 85 percent respectively [Citation14], but whether this is altered after RYGB is unknown. Furthermore, a positive lactose LBT may not only be the result of lactase deficiency, leading to lactose fermentation into hydrogen and methane by colonic bacteria. Gut microbiome changes are seen after RYGB and (subclinical) small intestinal bacterial overgrowth (SIBO) after RYGB is reported [Citation18]. SIBO can influence lactose breath testing [Citation19]. We did not exclude SIBO before lactose testing in this study, which may have led to more positive results on the lactose breath test. If so, this probably further decreases the number of patients with true lactose intolerance/malabsorption because of lactase deficiency. The relatively high methane levels we found in postoperative patients might be due to microbiome changes after RYGB.
We found no difference in proportion of positive LTT’s between the postoperative and preoperative group. Apparently there is sufficient lactase activity after RYGB to hydrolyse lactose and to raise blood glucose with at least 1.1 mmol/L in most patients, but the sensitivity of the LTT probably decreases after RYGB. In the normal population, the LTT has a sensitivity and specificity of 94% and 90% respectively [Citation14]. However, glucose kinetics are known to be altered after RYGB. Patients after surgery have similar times to postprandial peak glucose concentrations but peak concentrations are often far higher and also subsequently decline faster than in patients before surgery [Citation20–22]. This is in line with the glucose curves in this study. Consequently, after RYGB, less lactose has to be hydrolysed into glucose to reach the 1.1 mmol/L glucose increase required for a negative LTT.
This is the first study to use the LBT and LTT in this population. No gold standard is available, unfortunately, to easily validate the LBT and LTT in the RYGB population. Determining whether cut-off values of the LBT and LTT, or time points to measure, need to be adjusted after RYGB and how this influences the sensitivity and specificity of the tests is desirable and will help drawing firmer conclusions about true lactose malabsorption and intolerance. In future research, patients with proven lactase deficiency and sufficiency should be tested before and after RYGB surgery as this can give more insight in LBT and LTT validity. The LTT results seem to be more in line with the limited gastrointestinal complaints patients report after lactose solution intake. We expect that, maybe after adjustment of the cut-off value, the LTT will be the preferable test after RYGB.
The population prevalence of lactose malabsorption varies per country and ethnicity as various ethnic groups maintain lactase production throughout life. It ranges from 0–5% in Northwest European countries to almost 100% in parts of Asia and Africa [Citation23]. In The Netherlands and additionally in the area of the study hospitals there is nowadays a mixed ethnic population. Several patients with other countries of origin than Northwest European (mainly Surinam, Moroccan and Dutch Antilles) were included because we aimed to test a random population sample. The known higher prevalence of lactose intolerance in many parts of the world compared to Northwest European, can explain the higher than expected proportion of positive lactose tests before surgery.
Several procedural decisions require comment. To enhance acceptability of the tests, shortened lactose tests to determine lactose malabsorption was used with a follow-up of 120 min after lactose intake. In all patients who reported to have gastrointestinal complaints after dairy consumption in daily life, the complaints started between 0–60 min after dairy intake. We do not think that this shortening had relevant influence on the results. Furthermore, the lactose tolerance test is less dependent on longer follow-up.
Lactose doses of 25 grams in 125 mL solution or 50 grams in 250 mL solution for lactose intolerance testing are used in studies and clinical practise. For this study, 25 grams in 125 mL was used as this is the advised dose [Citation19], a more physiological dose and easier to consume after RYGB. For the LTT, capillary blood was used as finger-pricks are better accepted by patients but may have less sensitivity compared to venous blood testing [Citation24].
While a large proportion of patients after RYGB stated to have gastrointestinal complaints after diary consumption in daily life, interestingly, only few patients had gastrointestinal complaints after intake of the lactose solution, even though they were aware of the high lactose dosage they consumed. Like in clinical practice, the LBT and LTT were not blinded or placebo controlled, which is a potential limitation.
Many different gastrointestinal complaints were registered in over a third of postoperative patients after dairy intake in daily life. However, almost all patients still use dairy on a daily bases in order to have enough protein and calcium intake, which is advised by dieticians. This may explain why patients use dairy more frequently after, than before surgery. Apparently, in our study population, complaints are not severe enough to leave dairy out of the diet.
This study shows that it is unlikely that all reported gastrointestinal complaints RYGB patients have after dairy consumption in daily life, are actually caused by lactose. Other ingredients in dairy, like fat, are possibly contributory. Moreover, if, together with the dairy products, other foods are consumed that may cause complaints, the symptoms can easily be attributed to the wrong product. Many products and ingredients are notorious after RYGB when it comes to postprandial gastrointestinal complaints. Lactose does, for most patients, not seem to be an important one.
Supplemental Material
Download Zip (36.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Karefylakis C, Naslund I, Edholm D, et al. Vitamin D status 10 years after primary gastric bypass: gravely high prevalence of hypovitaminosis D and raised PTH levels. Obes Surg. 2014;24(3):343–348.
- Switzer NJ, Marcil G, Prasad S, et al. Long-term hypovitaminosis D and secondary hyperparathyroidism outcomes of the Roux-en-Y gastric bypass: a systematic review. Obes Rev. 2017;18(5):560–566.
- Raoof M, Naslund I, Rask E, et al. Effect of gastric bypass on bone mineral density, parathyroid hormone and vitamin D: 5 years follow-up. Obes Surg. 2016;26(5):1141–1145.
- Bosnic G. Nutritional requirements after bariatric surgery. Crit Care Nurs Clin North Am. 2014;26(2):255–262.
- Graham L, Murty G, Bowrey DJ. Taste, smell and appetite change after Roux-en-Y gastric bypass surgery. Obes Surg. 2014;24(9):1463–1468.
- Boerlage TC, van de Laar AW, Westerlaken S, et al. Gastrointestinal symptoms and food intolerance 2 years after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Br J Surg. 2017;104(4):393–400.
- Suarez FL, Savaiano DA, Levitt MA. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333(1):1–4.
- Gudmand-Hoyer E, Asp NG, Skovbjerg H, et al. Lactose malabsorption after bypass operation for obesity. Scand J Gastroenterol. 1978;13(6):641–647.
- Gudmand-Hoyer E, Jarnum S. Milk intolerance following gastric surgery. Scand J Gastroenterol. 1969;4(2):127–132.
- Newcomer AD, McGill DB. Distribution of disaccharidase activity in the small bowel of normal and lactase-deficient subjects. Gastroenterology. 1966;51(4):481–488.
- Aron-Wisnewsky J, Prifti E, Belda E, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68(1):70–82.
- Paganelli FL, Luyer M, Hazelbag CM, et al. Roux-Y gastric bypass and sleeve gastrectomy directly change gut microbiota composition independent of surgery type. Sci Rep. 2019;9(1):10979.
- Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22(2):228–238.
- Marton A, Xue X, Szilagyi A. Meta-analysis: the diagnostic accuracy of lactose breath hydrogen or lactose tolerance tests for predicting the North European lactase polymorphism C/T-13910. Aliment Pharmacol Ther. 2012;35(4):429–440.
- Bayless TM, Paige DM, Bedine MS. Lactose intolerance. N Engl J Med. 1995;333(20):1358–1359.
- Corlew-Roath M, Di Palma JA. Clinical impact of identifying lactose maldigestion or fructose malabsorption in irritable bowel syndrome or other conditions. South Med J. 2009;102(10):1010–1012.
- Mishkin B, Yalovsky M, Mishkin S. Increased prevalence of lactose malabsorption in Crohn's disease patients at low risk for lactose malabsorption based on ethnic origin. Am J Gastroenterol. 1997;92(7):1148–1153.
- Machado JD, Campos CS, Lopes Dah Silva C, et al. Intestinal bacterial overgrowth after Roux-en-Y gastric bypass. Obes Surg. 2008;18(1):139–143.
- Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the North American Consensus. Am J Gastroenterol. 2017;112(5):775–784.
- Jacobsen SH, Bojsen-Moller KN, Dirksen C, et al. Effects of gastric bypass surgery on glucose absorption and metabolism during a mixed meal in glucose-tolerant individuals. Diabetologia. 2013;56(10):2250–2254.
- Sessa L, Guidone C, Gallucci P, et al. Effect of single anastomosis duodenal-ileal bypass with sleeve gastrectomy on glucose tolerance test: comparison with other bariatric procedures. Surg Obes Relat Dis. 2019;15(7):1091–1097.
- Van Olden CC, Van de Laar AW, Meijnikman AS, et al. A systems biology approach to understand gut microbiota and host metabolism in morbid obesity: design of the BARIA Longitudinal Cohort Study. J Intern Med. 2020. DOI:10.1111/joim.13157
- Storhaug CL, Fosse SK, Fadnes LT. Country, regional, and global estimates for lactose malabsorption in adults: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(10):738–746.
- Dominguez Jimenez JL, Fernandez Suarez A. Correlation between capillary and venous blood glucose in the lactose tolerance test. Digest Dis Sci. 2016;61(1):208–214.
