Abstract
Background and objectives
The role of exercise in the management of inflammatory bowel disease (IBD) is inconclusive as most research focused on short or low-intensity exercise bouts and subjective outcomes. We assessed the effects of repeated prolonged moderate-intensity exercise on objective inflammatory markers in IBD patients.
Methods
In this study, IBD patients (IBD walkers, n = 18), and a control group (non-IBD walkers, n = 19), completed a 30, 40 or 50 km walking exercise on four consecutive days. Blood samples were taken at baseline and every day post-exercise to test for the effect of disease on exercise-induced changes in cytokine concentrations. A second control group of IBD patients who did not take part in the exercise, IBD non-walkers (n = 19), was used to test for the effect of exercise on faecal calprotectin. Both IBD groups also completed a clinical disease activity questionnaire.
Results
Changes in cytokine concentrations were similar for IBD walkers and non-IBD walkers (IL-6 p = .95; IL-8 p = .07; IL-10 p = .40; IL-1β p = .28; TNF-α p = .45), with a temporary significant increase in IL-6 (p < .001) and IL-10 (p = .006) from baseline to post-exercise day 1. Faecal calprotectin was not affected by exercise (p = .48). Clinical disease activity did not change in the IBD walkers with ulcerative colitis (p = .92), but did increase in the IBD walkers with Crohn’s disease (p = .024).
Conclusion
Repeated prolonged moderate-intensity walking exercise led to similar cytokine responses in participants with or without IBD, and it did not affect faecal calprotectin concentrations, suggesting that IBD patients can safely perform this type of exercise.
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are characterized by a clinical course with periods of active disease alternating with periods of remission [Citation1]. Environmental factors like exercise may influence the course of IBD [Citation2]. A recent study showed that IBD patients were significantly less physically active after their IBD diagnosis than before [Citation3]. Patients experience barriers to exercise due to IBD-related limitations such as fatigue, diarrhea, joint pain, weakness and fear for symptom exacerbation [Citation4]. However, small prospective studies showed that low-intensity exercise appears to be safe and well tolerated with minimal risk of symptom exacerbation in patients in remission or with mildly active disease [Citation2,Citation5,Citation6]. Moreover, a large observational study showed that patients in remission with higher exercise levels were significantly less likely to develop active disease after 6 months follow-up [Citation7].
Exercise may have beneficial effects on IBDby changing the intestinal microbiota and by release of cytokines, but the exact mechanism is unknown [Citation8,Citation9]. Exercise can enrich the microbiota diversity, stimulate proliferation of bacteria that modulate mucosal immunity and improve barrier functions of the gut, which all seem to be beneficial in an inflamed intestine [Citation10]. By contraction of skeletal muscles, myokines such as IL-6 are released which are known to exert anti-inflammatory effects and inhibit the release of proinflammatory cytokines such as IL-1β and TNF-α [Citation9]. It is known that cytokine concentrations can increase directly after exercise [Citation11–13]. In healthy individuals, this increase seems to depend on the intensity and duration of exercise [Citation10,Citation14]. In a previous study during the Nijmegen Four Days Marches, it was suggested that there is an adaptive response of the body to prolonged and repeated exercise, since they found a gradual decrease in cytokine concentration in healthy individuals after a peak on the first exercise day [Citation15]. It is unknown whether this pattern is the same in IBD patients and to what extent an increase in cytokine concentrations is actually a sign of IBD activity or whether it is all related to exercise. Therefore, it is of interest to also study faecal calprotectin as a more specific measure of IBD activity to be able to differentiate between exercise and disease effects. Faecal calprotectin is a marker of intestinal inflammation and correlates significantly with endoscopic disease activity [Citation16,Citation17]. So far, only a few studies on exercise in IBD patients assessed faecal calprotectin and they found no changes [Citation18–21]. Based on these studies on cytokines and faecal calprotectin, we expected that repeated prolonged exercise would not lead to a clinically relevant increase in inflammatory markers.
Previous studies on exercise in IBD mainly focused on short bouts of exercise or low-intensity exercise, while health-enhancing effects of exercise are generally more noticeable after repeated bouts of exercise [Citation2,Citation5]. In addition, studies often explore patient well-being and disease activity without objective markers of inflammation. Therefore, the aim of this study was to assess the effects of repeated bouts of prolonged moderate-intensity walking exercise on inflammatory markers (i.e., cytokines and faecal calprotectin) in patients with IBD. Besides this, we investigated the effect of repeated prolonged moderate-intensity exercise on clinical disease activity.
Materials and methods
Study population
We included 19 IBD patients who participated in the 2019 edition of the Nijmegen Four Days Marches, the IBD walkers. Additionally, we included two control groups: a control group of 19 Nijmegen Four Days Marches participants without a history of IBD, the non-IBD walkers, to test for the effect of disease, and a control group of 19 IBD patients who did not participate in the exercise event, the IBD non-walkers, to test for the effect of exercise. IBD walkers and non-IBD walkers were recruited via the Nijmegen Exercise Study database of Radboud University Medical Centre, Nijmegen, The Netherlands, via social media and via word-of-mouth promotion. IBD non-walkers were recruited via the outpatient clinic of Hospital Gelderse Vallei, Ede, The Netherlands. Inclusion criteria for all IBD patients were age ≥18 years and diagnosis of CD or UC made by a gastroenterologist. IBD patients were excluded when they used specific biologicals (infliximab, adalimumab, golimumab, ustekinumab) as these might reduce cytokine concentrations. Non-IBD walkers were included when they were ≥18 years of age and they were excluded when they had a history of IBD or other gastro-intestinal diseases. The non-IBD walkers and IBD non-walkers were comparable with the IBD walkers for age (±5 years) and gender. This study was approved by the Medical Ethical Committee region Arnhem-Nijmegen (CMO registration number: 2019-5375) and by the Medical Ethical Committee of Wageningen University. All participants provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki and was registered at trialregister.nl as NL7872.
Study design
Measurements were performed 1 or 2 days prior to the first exercise day (baseline) and within 30-min post-exercise at the four consecutive exercise days. At baseline, body weight and height were measured, participant characteristics were registered, and questionnaires regarding physical activity and, in case of IBD participants, clinical disease activity were completed. Blood samples from IBD walkers and non-IBD walkers were collected at baseline and every exercise day directly after finishing to determine cytokine concentrations. Besides that, faecal samples were collected by IBD walkers and IBD non-walkers at baseline, at days 2 or 3 and at the end of the exercise event to determine calprotectin. Non-IBD walkers did not collect faecal samples. IBD walkers and non-IBD walkers walked 30, 40 or 50 km at a self-selected pace at four consecutive exercise days. Start and finish time were registered and used to calculate walking speed, without correction for breaks. Their heart rate (HR) was measured every 5 km during the first exercise day using a 2-channel ECG chest band system. Heart rate was used to estimate exercise intensity as a percentage of the maximum HR (exercise intensity = measured HR/expected maximal HR × 100%, where expected max HR = 208 – (0.7 × age)) [Citation22]. Heart rate measurements and blood sampling were not performed in IBD non-walkers. At the end of the exercise event, IBD walkers and IBD non-walkers completed a clinical disease activity questionnaire for the second time.
Participant characteristics and physical activity questionnaires
All participants completed a general questionnaire on demographics, level of education, smoking and medication use, and the validated Short Questionnaire to Assess Health enhancing physical activity (SQUASH) [Citation18]. IBD walkers and non-IBD walkers also completed a questionnaire on their preparations for the exercise event. IBD walkers and IBD non-walkers completed an extended general questionnaire with additional questions on type and extent of IBD, age of disease onset, number of flare-ups, previous IBD-related surgeries and their opinion on the influence of physical activity on their IBD.
Blood samples
Blood samples were taken at baseline and within 30 min post-exercise at the four consecutive exercise days. A venous blood sample was collected in a 10 mL EDTA vacutainer (Becton-Dickinson, NJ). The vacutainer was put on melting ice water and centrifuged at 1200 rcf for 15 min at 4 °C. Plasma was then transferred to polypropylene tubes and stored at −80 °C until analysis. IL-6, IL-8, IL-10, IL-1β and TNF-α concentrations were determined using the MesoScale Discovery (MSD) MULTI-SPOT Assay System [Proinflammatory Panel 1 (human) Kits, K15049D] according to the manufacturers’ instructions. The lower detection limits varied per plate and were 0.136–0.432 (IL-6), 0.052–0.138 (IL-8), 0.039–0.213 (IL-10), 0.013–0.080 (IL-1β) and 0.066–0.229 (TNF-α) pg/ml. All values below these lower detection limits were considered as missing’s. The percentages of missing values were 3.8% for IL-8 and TNF-α, 8.6% for IL-6 and IL-1β and 20.5% for IL-10. These values were imputed during statistical analysis. All standards for the calibration curve, control samples, and 10% of the plasma samples from participants were measured in duplicate. Accuracy and precision were evaluated by controls across multiple runs and multiple lots as described in the manufacturer’s protocol.
Faecal samples
Faecal samples were collected at baseline, halfway and at the end of the exercise event. Participants were provided with materials and instructions to collect the samples at home. Samples were stored in a refrigerator before transfer to the study laboratory for analysis. Faecal calprotectin was determined using a sandwich enzyme-linked immunosorbent assay (ELISA). Faecal calprotectin concentrations for this assay ranged from 0 to 2500 µg/g.
Clinical disease activity questionnaire
IBD walkers and IBD non-walkers completed a clinical disease activity questionnaire at baseline and at the end of the last exercise day. Depending on their type of IBD, this was the Patient Harvey Bradshaw Index (P-HBI) for CD and the Patient Simple Clinical Colitis Activity Index (P-SCCAI) for UC [Citation23,Citation24].
Statistical analysis
Normally distributed data are presented as mean ± SD, skewed data as median with interquartile range (IQR) and categorical data as frequency with proportion. Differences between groups for participant and exercise characteristics were analyzed with a chi-square test for categorical data, and an independent samples t-test for continuous data. One-way analysis of variance (ANOVA) was used to examine differences in exercise characteristics over time within groups. Post-hoc analyses were performed using the Bonferroni multiple comparisons test. The main outcomes, cytokine responses and faecal calprotectin, were analysed using linear mixed models to account for missing values, after log10 transformation to obtain normality [Citation25]. Participant was used as a random factor and sampling days, groups and their interaction were used as fixed factors. A random intercept was used with an unstructured covariance structure for cytokines and an identity covariance structure for faecal calprotectin for covariance of time points. Baseline values were used as a reference. Changes in clinical disease activity scores were analyzed with a Wilcoxon signed-ranks test within groups and with an independent samples t-test and a Mann–Whitney U test between groups. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics version 24.
Results
Participant and exercise characteristics
In total, 56 participants were included in the data analysis: 18 IBD walkers, 19 non-IBD walkers and 19 IBD non-walkers. One male IBD walker dropped out after the first exercise day, due to a fall, and was excluded from further analysis. Another IBD walker withdrew during the second exercise day due to abdominal pain and frequent loose stools. Since this might have been related to her IBD, she was included in further analysis. The mean age of the study population was 54 ± 12 years and the average BMI of all participants was 26.0 ± 3.8 kg/m2. In each group 11 participants were female. No significant differences were found between IBD walkers and non-IBD walkers or IBD non-walkers regarding participant characteristics, except for training distance during the last two weeks before the walking event (IBD walkers vs non-IBD walkers, p = .03, ). The average heart rate during the first exercise day was 114 ± 12 bpm, resulting in an average exercise intensity of 67 ± 8%, which fulfills the definition of moderate-intensity exercise [Citation26]. This is supported by the subjective rate of perceived exertion which was 5.0 [IQR 3.0–7.0], indicating that participants scored the prolonged walking exercise as a moderate activity [Citation27]. No differences in heart rate and exercise intensity were found between IBD walkers and non-IBD walkers (p = .96 and p = .99, respectively). Walking distance did not differ between groups (p = .35) and walking speed did also not differ between groups or between days (p = .50 and p = .15, respectively, ). IBD non-walkers were more often treated with immunosuppressants compared to IBD walkers (6 vs 47%, p = .004). Furthermore, IBD non-walkers reported not to experience an effect of physical activity on their IBD, while IBD walkers were positive about the effect of physical activity ().
Table 1. Characteristics of the study population consisting of IBD walkers, compared to non-IBD walkers for effect of disease and to IBD non-walkers for effect of exercise.
Table 2. IBD specific characteristics, IBD walkers compared to IBD non-walkers.
Cytokines
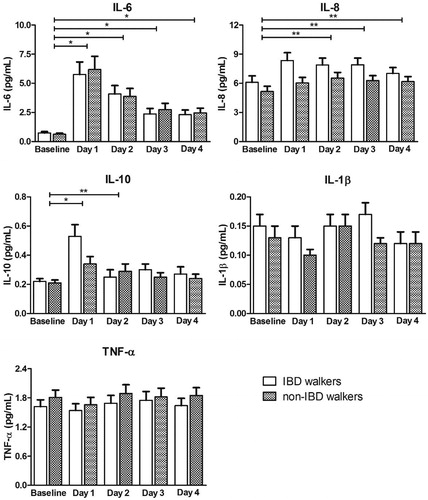
No differences in cytokine concentrations were found between IBD walkers and non-IBD walkers (F-test: IL-6 p = .95; IL-8 p = .07; IL-10 p = .40; IL-1β p = .28; TNF-α p = .45) in the linear mixed model analysis. From baseline until post-exercise day 4, all IL-8 concentrations were higher in IBD walkers compared to non-IBD walkers, though not significant (p = .07, /Supplemental Table 1). When combining the two groups, differences between days were found (F-test: IL-6 p < .001; IL-8 p = .012; IL-10 p < .001; IL-1β p = .008; TNF-α p = .018). For IL-6 and IL-10, a significant increase was seen between baseline and post-exercise day 1 (p < .001 and p = .006, respectively). Hereafter, both concentrations decreased, and from day 3 onwards IL-10 did not significantly differ anymore from baseline (p = .21), while IL-6 remained significantly higher throughout the days compared to baseline (p < .001, /Supplemental Table 1). IL-8 concentrations increased between baseline and post-exercise day 1 in both groups and further increased on exercise day 2 in non-IBD walkers. From post-exercise day 2 onwards, IL-8 concentrations remained quite stable and significantly higher compared to baseline (p = .033, p = .029 and p = .040, respectively, /Supplemental Table 1). Concentrations of IL-1β and TNF-α stayed rather stable during the event and concentrations did not differ from baseline (all p-values ≥ .10, /Supplemental Table 1).
Figure 1. Estimated marginal mean cytokine concentrations (pg/mL) after back transformation, at baseline and days 1 to 4, for IBD walkers and non-IBD walkers. Cytokine concentrations are presented in picogram per millilitre. *p < .01, **p < .05. Data are presented as mean ± standard error and were derived from a linear mixed model analysis. All statistical tests were performed on the log10 scale. Hereafter, data were back transformed for presentation in this figure. Differences between groups were never statistically significant and therefore groups were combined to test for differences between days, using baseline as reference. IBD: inflammatory bowel disease; IL: interleukin; TNF: tumor necrosis factor.
Faecal calprotectin
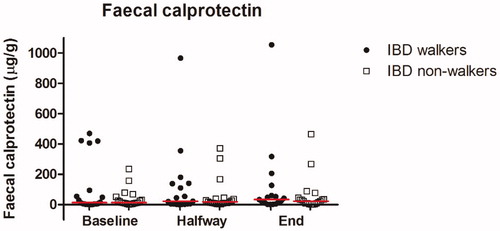
Faecal calprotectin concentrations showed a wide-range and skewed distribution, ranging from 0 to 1054 µg/g (). With the use of linear mixed models, no differences were found between IBD walkers and IBD non-walkers (F-test: p = .73) nor overtime (F-test: p = .48). A remarkable increase was observed in one IBD walker and in two IBD non-walkers. Faecal calprotectin concentrations of the IBD walker increased from 419 at baseline to 1054 μg/g at the end of the exercise event. Despite this increase, the participant was able to complete the exercise event. Concentrations of the two IBD non-walkers increased from baseline to the end of the exercise event from 234 to 465 μg/g and from 156 to 266 μg/g ().
Figure 2. Column scatter of faecal calprotectin concentrations (μg/g) at baseline, halfway (day 2 or 3) and at the end of the exercise event, for IBD walkers and IBD non-walkers. Faecal calprotectin concentrations are presented in microgram per millilitre. All statistical tests were performed on the log10 scale using baseline as a reference. Lines represent medians. IBD: inflammatory bowel disease.
Clinical disease activity
P-SCCAI scores, ranging from 0 to 13, were not different between UC walkers and UC non-walkers at baseline and at the end of the exercise event (p = .42 and p = .20, respectively), nor did they change within groups (p = .92 and p = .75) or between groups (p = .81, ). P-HBI scores ranging from 0 to 15, were also not different between CD walkers and CD non-walkers at baseline and at the end of the exercise event (p = .82 and p = .10, respectively), nor did they change over time in CD non-walkers (p = .50). Yet, P-HBI scores increased significantly over time in CD walkers (p = .024) and therefore became significantly different between CD walkers and CD non-walkers (p = .046), indicating that clinical disease activity worsened during the exercise event in participants with CD ().
Table 3. Clinical disease activity scores at baseline and at the end of the exercise event, for IBD walkers and IBD non-walkers, UC and CD separately.
Discussion
We found that repeated bouts of prolonged moderate-intensity exercise results in comparable changes in cytokine concentrations in IBD walkers and non-IBD walkers, suggesting that the cytokine response is only affected by exercise rather than disease activity. Furthermore, stable and comparable faecal calprotectin in IBD walkers and IBD non-walkers also suggests that repeated prolonged moderate-intensity exercise does not lead to disease exacerbation. These objective findings are supported by self-reported P-SCCAI scores, though not by P-HBI scores. Our results suggest that repeated prolonged moderate-intensity exercise does not appear to have harmful effects on disease activity in UC and probably not in CD either. It seems that IBD patients can safely perform this type of exercise without significant exacerbation of inflammation.
To the best of our knowledge, this is the first study to investigate the effect of repeated prolonged moderate-intensity exercise on IBD patients. At the moment it is unknown which exercise type, intensity and duration is safe and beneficial in IBD patients. Our study revealed that cytokine responses to repeated prolonged moderate-intensity exercise in IBD patients were comparable to the responses in non-IBD controls and these responses are in line with the literature. In two comparable studies, cytokine concentrations increased and decreased in a similar manner as cytokine concentrations in our study [Citation15,Citation28]. In these studies and ours, a peak in IL-6 and IL-10 concentrations were seen post-exercise day 1 compared to the other exercise days despite rather similar durations of exercise on the four consecutive exercise days. This might be due to the training adaptation of the body [Citation29]. The strong increase of IL-6 and IL-10 between baseline and post-exercise day 1 indicates a substantial increase in production and secretion of myokines which is primarily regulated by skeletal muscles. Therefore, the increase in IL-6 and IL-10 might be explained by the walking exercise rather than other factors such as gender, fitness level or (type of) IBD [Citation11,Citation29]. Contrary to IL-6, IL-8 and IL-10, there were barely any changes in IL-1β and TNF-α between baseline and the four consecutive exercise days, which is also in line with the literature. The majority of studies showed that the concentrations of IL-1β and TNF-α remain unchanged following exercise [Citation13,Citation30]. Two studies specifically investigated cytokine responses in IBD patients. A pilot study in 15 paediatric CD patients and 15 healthy controls showed that IL-6 increased significantly in both groups during 60 minutes of moderate-intensity cycling, but not after short bouts of high-intensity cycling. Within 60 minutes after exercise, IL-6 returned to baseline. No change was found in TNF-α comparable to our observations [Citation30]. The fact that IL-6 only increased after moderate-intensity cycling could be explained by the production and secretion of myokines by skeletal muscles, which is predominantly influenced by the duration rather than the intensity or mode of exercise [Citation29]. A randomized cross-over trial in 17 IBD patients found no changes in IL-6, IL-8, IL-10 and TNF-α after eight weeks of moderate-intensity aerobic and resistance training three times per week [Citation31]. As cytokines seem to return to baseline within minutes to hours after exercise, changes might have been missed due to the timing of blood sampling since they sampled before and after the 8-week intervention period. Our study with a prolonged duration of exercise and blood sampling within 30 min post-exercise therefore adds new insights to the current literature on cytokines in IBD patients.
We found comparable faecal calprotectin concentrations in IBD walkers and IBD non-walkers and no change over time. This is in line with the results of a randomized clinical trial on moderate-intensity running and two pilot studies on yoga classes and high-intensity interval training or moderate-intensity continuous training. In these studies, all types of exercise were performed three or four times per week during 8–12 weeks and no significant changes in faecal calprotectin were found [Citation19–21]. Even though repeated prolonged moderate-intensity exercise did not significantly change faecal calprotectin concentrations in our study, noticeable changes were observed in some participants. The observed increase in calprotectin concentrations in three participants (one IBD walker and two IBD non-walkers) may be a sign of disease exacerbation, since calprotectin is a sensitive biomarker of intestinal inflammation [Citation16,Citation17]. However, as an increase was seen in both groups, it is not likely that the repeated bouts of prolonged moderate-intensity exercise caused this increase. It has been hypothesized that calprotectin concentrations depend on the amount of blood in stool and stool consistency, which varies widely between stools in IBD patients with active disease and causes natural day-to-day variation in faecal calprotectin [Citation32–35]. Thus, the fact that a considerable number of participants had active disease is a likely explanation for the high variability of faecal calprotectin concentrations in our study.
To date, the majority of studies examined the effect of exercise on IBD by subjective measures such as self-perceived disease activity and quality of life. It was found that low intensity exercise significantly improves self-perceived disease activity in IBD patients in remission or with mildly active disease [Citation36,Citation37]. Regarding moderate-intensity exercise, self-perceived disease activity did not change in the previously mentioned randomized controlled trial on moderate-intensity running or in the randomized cross-over trial on moderate-intensity aerobic and resistance training [Citation19,Citation31]. Another randomized controlled trial also found no change in self-perceived disease activity after 10 weeks of mind-body therapy including moderate-intensity exercise in 30 UC patients in remission of mildly active disease [Citation38]. In this study, we found that changes in P-HBI scores were higher in walking CD patients compared to the CD non-walkers, suggesting worsening of clinical disease activity during the exercise event. However, these changes were not reflected in faecal calprotectin. Therefore, the chance that clinical disease activity increased due to the exercise event is small, since faecal calprotectin better correlates with endoscopic disease activity than clinical disease activity questionnaires, especially in patients with colonic disease which applied to the majority of our patient group [Citation39–41].
The strengths of this explorative study are the use of objective inflammatory markers on the systemic as well as the intestinal level combined with self-reported validated questionnaires, in comparable groups in terms of age, gender and disease type. Moreover, the inclusion of CD as well as UC with a variety of disease states widens the applicability of the results. However, some limitations should be taken into account. First, due to the explorative nature of this study no proper sample size calculation was performed and the sample size was relatively small. As a result, it was not possible to compare subgroups (CD vs UC or remission vs active disease) or to correct for confounders such as BMI or IBD specific medication. However, our sample size was large enough to detect a change in calprotectin of 40 µg/g as calculated in a post-hoc power analysis. Second, we excluded IBD patients that used specific biologicals which resulted in a select group of participants regarding medication use that does not accurately reflect the current IBD population. Though only a minority of the IBD population uses biologicals. Third, during the exercise event, we only took post-exercise blood samples. Therefore, we do not know whether cytokine concentrations completely recovered overnight. Also, baseline blood samples were not collected on the same day and same time of day across participants, which could have led to some variability due to normal daily variation in cytokine concentrations. However, these biases were equal for IBD walkers and non-IBD walkers and are therefore not likely to have affected cytokine responses. Fourth, we did not find harmful effects during the marches, though it would have been interesting to have follow-up data of the weeks or months after the marches to see whether harmful effects would appear at a later time.
In conclusion, we found a comparable change in cytokine concentrations after repeated prolonged moderate-intensity exercise in IBD walkers compared to non-IBD walkers, and stable and comparable faecal calprotectin in IBD walkers compared to IBD non-walkers. These results suggest that IBD patients can safely perform this type of exercise without significant exacerbation of inflammation. More studies, preferably with larger sample sizes and a follow-up period, are needed to further investigate the effects of this type of exercise.
| Abbreviations | ||
| BMI | = | body mass index |
| CD | = | Crohn’s disease |
| ELISA | = | enzyme-linked immunosorbent assay |
| HR | = | heart rate |
| IBD | = | inflammatory bowel disease |
| IL | = | interleukin |
| IQR | = | interquartile range |
| P-HBI | = | Patient Harvey Bradshaw Index |
| P-SCCAI | = | Patient Simple Clinical Colitis Activity Index |
| SD | = | standard deviation |
| SQUASH | = | Short Questionnaire to Assess Health enhancing physical activity |
| TNF | = | tumor necrosis factor |
| UC | = | ulcerative colitis |
Supplemental Material
Download MS Word (28.1 KB)Acknowledgements
We would like to thank all participants, and all student and faculty volunteers for their practical assistance in data collection. Special thanks to master students Vicky de Ruijter and Anne de Korte for their help with participant recruitment. We also would like to acknowledge laboratory technician Nhien Ly for analysing the cytokine concentrations and statistician dr. João Paulo for her help with the statistical analysis.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–1657.
- Engels M, Cross RK, Long MD. Exercise in patients with inflammatory bowel diseases: current perspectives. Clin Exp Gastroenterol. 2018;11:1–11.
- Gatt K, Schembri J, Katsanos KH, et al. Inflammatory bowel disease [IBD] and physical activity: a study on the impact of diagnosis on the level of exercise amongst patients with IBD. J Crohns Colitis. 2019;13(6):686–692.
- DeFilippis EM, Tabani S, Warren RU, et al. Exercise and self-reported limitations in patients with inflammatory bowel disease. Dig Dis Sci. 2016;61(1):215–220.
- Narula N, Fedorak RN. Exercise and inflammatory bowel disease. Can J Gastroenterol. 2008;22(5):497–504.
- Eckert KG, Abbasi-Neureither I, Koppel M, et al. Structured physical activity interventions as a complementary therapy for patients with inflammatory bowel disease – a scoping review and practical implications. BMC Gastroenterol. 2019;19(1):115.
- Jones PD, Kappelman MD, Martin CF, et al. Exercise decreases risk of future active disease in patients with inflammatory bowel disease in remission. Inflamm Bowel Dis. 2015;21(5):1063–1071.
- Codella R, Luzi L, Terruzzi I. Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis. 2018;50(4):331–341.
- Bilski J, Mazur-Bialy A, Brzozowski B, et al. Can exercise affect the course of inflammatory bowel disease? Experimental and clinical evidence. Pharmacol Rep. 2016;68(4):827–836.
- Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017:3831972.
- Pedersen BK, Steensberg A, Fischer C, et al. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc Immunol Rev. 2001;7:18–31.
- Brown M, McClean CM, Davison GW, et al. The acute effects of walking exercise intensity on systemic cytokines and oxidative stress. Eur J Appl Physiol. 2018;118(10):2111–2120.
- Suzuki K, Nakaji S, Yamada M, et al. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8:6–48.
- Pedersen BK. Special feature for the olympics: effects of exercise on the immune system: exercise and cytokines. Immunol Cell Biol. 2000;78(5):532–535.
- Terink R, Bongers CCWG, Witkamp RF, et al. Changes in cytokine levels after prolonged and repeated moderate intensity exercise in middle-aged men and women. Transl Sports Med. 2018;1(3):110–119.
- D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(12):2218–2224.
- Kostas A, Siakavellas SI, Kosmidis C, et al. Fecal calprotectin measurement is a marker of short-term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. WJG. 2017;23(41):7387–7396.
- Wendel-Vos GC, Schuit AJ, Saris WH, et al. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J Clin Epidemiol. 2003;56(12):1163–1169.
- Klare P, Nigg J, Nold J, et al. The impact of a ten-week physical exercise program on health-related quality of life in patients with inflammatory bowel disease: a prospective randomized controlled trial. Digestion. 2015;91(3):239–247.
- Arruda JM, Bogetz AL, Vellanki S, et al. Yoga as adjunct therapy for adolescents with inflammatory bowel disease: a pilot clinical trial. Complement Ther Med. 2018;41:99–104.
- Tew GA, Leighton D, Carpenter R, et al. High-intensity interval training and moderate-intensity continuous training in adults with Crohn's disease: a pilot randomised controlled trial. BMC Gastroenterol. 2019;19(1):19.
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156.
- Bennebroek Evertsz F, Hoeks CC, Nieuwkerk PT, et al. Development of the patient Harvey Bradshaw index and a comparison with a clinician-based Harvey Bradshaw index assessment of Crohn's disease activity. J Clin Gastroenterol. 2013;47(10):850–856.
- Bennebroek Evertsz F, Nieuwkerk PT, Stokkers PC, et al. The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: a comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis. 2013;7(11):890–900.
- Molenberghs G, Bijnens L, Shaw D. Linear mixed models and missing data. In: Verbeke MG, editor. Linear mixed models in practice. New York: Springer; 1997. p. 191–274.
- Riebe D, Ehrman JK, Liguori G, et al. ACSM's guidelines for exercise testing and prescription. Philadelphia: Wolters Kluwer; 2018. p. 143–179.
- Scherr J, Wolfarth B, Christle JW, et al. Associations between Borg's rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013;113(1):147–155.
- Verheggen R, Eijsvogels TMH, Catoire M, et al. Cytokine responses to repeated, prolonged walking in lean versus overweight/obese individuals. J Sci Med Sport. 2019;22(2):196–200.
- Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33.
- Ploeger H, Obeid J, Nguyen T, et al. Exercise and inflammation in pediatric Crohn's disease. Int J Sports Med. 2012;33(08):671–679.
- Cronin O, Barton W, Moran C, et al. Moderate-intensity aerobic and resistance exercise is safe and favorably influences body composition in patients with quiescent Inflammatory Bowel Disease: a randomized controlled cross-over trial. BMC Gastroenterol. 2019;19(1):29.
- Cremer A, Ku J, Amininejad L, et al. Variability of faecal calprotectin in inflammatory bowel disease patients: an observational case-control study. J Crohns Colitis. 2019;13(11):1372–1379.
- Du L, Foshaug R, Huang VW, et al. Within-stool and within-day sample variability of fecal calprotectin in patients with inflammatory bowel disease: a prospective observational study. J Clin Gastroenterol. 2018;52(3):235–240.
- Calafat M, Cabre E, Manosa M, et al. High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis. 2015;21(5):1072–1076.
- Lasson A, Stotzer PO, Ohman L, et al. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis. 2015;9(1):26–32.
- Ng V, Millard W, Lebrun C, et al. Low-intensity exercise improves quality of life in patients with Crohn's disease. Clin J Sport Med. 2007;17(5):384–388.
- Loudon CP, Corroll V, Butcher J, et al. The effects of physical exercise on patients with Crohn's disease. Am J Gastroenterol. 1999;94(3):697–703.
- Elsenbruch S, Langhorst J, Popkirowa K, et al. Effects of mind-body therapy on quality of life and neuroendocrine and cellular immune functions in patients with ulcerative colitis. Psychother Psychosom. 2005;74(5):277–287.
- Gracie DJ, Williams CJ, Sood R, et al. Poor correlation between clinical disease activity and mucosal inflammation, and the role of psychological comorbidity, in inflammatory bowel disease. Am J Gastroenterol. 2016;111(4):541–551.
- Walsh A, Kormilitzin A, Hinds C, et al. defining faecal calprotectin thresholds as a surrogate for endoscopic and histological disease activity in ulcerative colitis-a prospective analysis. J Crohns Colitis. 2019;13(4):424–430.
- Ricanek P, Brackmann S, Perminow G, IBSEN II Study Group, et al. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46(9):1081–1091.
