Abstract
Objectives
Loss of response (LOR) to infliximab (IFX) remains a challenge in the management of inflammatory bowel diseases (IBD). Proactive dosing strategies to achieve and maintain predefined IFX trough levels (TL) may prevent LOR. We aimed to investigate the efficacy of dashboard driven IFX dosing compared to standard dosing in a prospective trial in IBD patients.
Methods
In this multicentre 1:1 ‘PRECISION’ trial, we randomized IBD patients in clinical remission (Harvey Bradshaw Index ≤4 for Crohn’s disease (CD) or a partial Mayo score ≤2 for ulcerative colitis (UC)) receiving IFX maintenance treatment. The precision group (PG) received IFX dosing guided by a Bayesian pharmacokinetic model, aiming to achieve and maintain a TL of 3 µg/ml by treatment (de)escalation as indicated by the dashboard. Patients in the control group (CG) continued treatment without dose adaptations. The primary endpoint was the proportion of patients in sustained clinical remission after 1 year.
Results
Eighty patients were enrolled (66 CD, 14 UC), and the median [interquartile range] age was 37 years [27–51]). After one year, 28/32 (88%) of patients in the PG were in sustained clinical remission versus 25/39 (64%) in the CG (p = .017). PG patients had lower median faecal calprotectin levels after 1 year (p = .031), whereas no significant differences in median CRP levels were found.
Conclusion
We demonstrated that the use of a Bayesian dashboard for IFX dosing in maintenance treatment for IBD reduced the incidence of LOR compared to standard dosing. Precision dosing also resulted in lower FCP levels.
ClinicalTrials.gov number
NCT02453776.
Introduction
The anti-tumour necrosis factor (TNF) antibody infliximab (IFX) is effective for the treatment of inflammatory bowel disease (IBD) [Citation1,Citation2]. For both Crohn’s disease (CD) and ulcerative colitis (UC), IFX is administered as intravenous infusions at weight-based dosing (5 mg/kg) starting with an induction schedule at week 0, 2, 6 and followed by 8-weekly maintenance treatment [Citation1,Citation2]. To achieve and maintain remission in IBD patients, adequate drug exposure is important [Citation1–3]. However, there is substantial variability in response and serum IFX concentrations among IBD patients at standard IFX doses, suggesting there may be benefit from personalized dosing based on clearance. It has been suggested that maintaining serum anti-TNF concentrations above predefined levels could lead to a reduction in primary non-response and secondary loss of response (LOR) rates [Citation4]. Moreover, suboptimal drug-exposure can lead to the development of anti-drug antibodies (ADA) [Citation5].
Several studies have investigated strategies to optimize IFX treatment, both during induction and maintenance treatment. Recent observational induction studies showed that IFX serum concentrations ≥28 µg/ml during the first 2 weeks of treatment and ≥15.0 µg/ml between week 2 and 6 are associated with higher mucosal healing rates in UC [Citation6–9]. During maintenance treatment, an association was reported between IFX trough levels (TL) (i.e., serum drug concentration measured just before the next infusion) of 3 µg/ml and improved clinical outcomes [Citation10–14].
It remains unclear whether exposure to higher IFX serum concentrations carries a risk of increased toxicity, but so far there is no evidence to support this. Observational data suggested that higher IFX TLs might be associated with an impaired quality of life, but this is yet to be confirmed [Citation15]. Unnecessary overexposure to TNF blockers also leads to higher treatment cost. Although personalized dosing based on serum TLs seems the most optimal strategy to prevent over and underdosing, the clinical benefit of this dosing strategy has not been demonstrated to date. In the TAXIT trial (Trough Concentration Adapted Infliximab Treatment), IBD patients in clinical remission on IFX maintenance therapy first entered an optimization phase, in which dose and/or interval adjustments were performed aiming to achieve IFX TLs within a certain therapeutic range (between 3 and 7 μg/ml) [Citation16]. After dose optimization, patients were randomized to continued TL-based dosing or clinically based dosing. No significant difference was observed in clinical remission rates (primary endpoint) between the two groups. Nonetheless, optimization resulted in a longer relapse-free survival and was more cost-effective. However, a Bayesian dashboard was not used during this optimization phase. In the more recent TAILORIX trial, anti-TNF-naïve CD patients with ulcerations at endoscopy received standard IFX induction treatment and were then randomized to further dosing based on symptoms alone or based on a combination of symptoms, biomarkers and serum concentrations of IFX [Citation17]. Proactive dose intensification was not superior to dose adjustments based on symptoms alone. This could, at least partly, be explained by the fact that patients in the ‘symptom-based dosing group’ were often dose intensified without objective evidence of active inflammation. Moreover, changes in the dosing frequency were not allowed. Both TAXIT and TAILORIX, did not use treatment algorithms incorporating factors that are known to affect IFX clearance, such as serum C-reactive protein (CRP), serum albumin, body weight, gender and ADA titers [Citation18,Citation19].
Meanwhile, a pharmacokinetic (PK) model for individualized IFX dosing has been created in which patient factors that contribute to the variability of IFX clearance are incorporated [Citation18,Citation19]. A Bayesian approach for population PK modelling can be used to predict which dose a patient should receive at which interval, to achieve and maintain a predefined serum TL. This so-called ‘model-based dosing’ has been used for many years by clinical pharmacists and pharmacologists, for example to dose antibiotics such as aminoglycosides [Citation20–23]. Dashboards are software systems that allow clinicians to apply model-based dosing by identifying an individual’s target TL. IFX doses are adjusted according to previously measured drug levels and relevant patient factors such as body weight and serum albumin levels to maintain effective drug concentrations in the serum. The aim of the PRECISION trial was to investigate the efficacy of dashboard driven IFX dosing compared to conventional dosing in IBD patients receiving IFX maintenance treatment over one year.
Methods
Study design and participants
This randomized controlled, multicentre trial was performed at one academic and two regional teaching hospitals in the Netherlands. Eligible patients were ≥ 18 years of age with a confirmed diagnosis of UC or CD who were in clinical remission, defined by a Harvey Bradshaw Index (HBI) of ≤ 4 for CD patients or a Partial Mayo (PM) score ≤ 2 points for UC. Patients receiving IFX treatment for at least 14 weeks could be included, regardless of the dosing schedule. Use of concomitant immunomodulators at stable dose was allowed. Patients with a history of fibro stenotic complications requiring endoscopic balloon dilatation and/or surgery in the past year were excluded. Patients treated with more than 5 mg/kg IFX per infusion and/or infusion intervals ≤ 7 weeks were considered to receive an intensified treatment regimen. Patients with an infusion interval ≥ 9 weeks and/or IFX doses <5 mg/kg were classified as receiving a de-intensified treatment regimen. The trial was performed according to the Declaration of Helsinki and the study protocol was approved by the Institutional Review Boards at all study sites. All patients provided written informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
Randomization and study procedures
Patients were randomly assigned (1:1) to receive dashboard driven IFX dosing (precision dosing group; PG) or to continue IFX maintenance treatment without adjustments of the dose and/or treatment interval (conventional dosing group; CG). Randomization was performed using a computer-generated randomization schedule by a team member not in charge of the clinical care of patients. Randomization was stratified by diagnosis, immunomodulator use and gender. In both groups, serum samples were collected before each IFX infusion and in the middle of the infusion interval (mid-infusion) for the measurement of serum CRP, serum albumin, serum IFX concentrations and ADA. In the PG, both measurements at trough and mid-infusion were used for the prediction. Serum IFX concentrations and ADA were measured at Sanquin Diagnostic Services (Amsterdam, the Netherlands), using a validated in-house bridging enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay antigen-binding test, respectively [Citation24,Citation25].
Patients in the CG continued IFX maintenance treatment without dose adaptations. Patients in the PG received IFX dosing guided by a Bayesian PK model, aiming to achieve and maintain an IFX TL of 3 µg/ml by treatment optimization as indicated by the dashboard system. Infusion intervals could vary between every 4 to 12 weeks and doses could vary between 1 to 10 mg/kg, respectively. At each IFX infusion, clinical disease activity was scored using the HBI for CD and the PM score for UC patients. Faecal calprotectin (FCP) levels were evaluated at baseline, after 6 months and at the end of the study at 12 months. At baseline and at the end of the study, quality of life was scored using the EQ5D and SF-36 questionnaires.
Dashboard system
A dashboard system (iDose, Projections Research Inc.) developed for dosing of therapeutic antibodies in IBD patients was used to determine optimal dosing regimens. For this study, an IFX target TL of 3 μg/ml was used as specified in the protocol. The dashboard utilizes a combination of Bayesian updating, Bayesian forecasting and Bayesian model averaging [Citation26]. Based on the population PK model incorporated in the dashboard, interpretation of a measured concentration has contributions from the underlying pharmacology (described by a model), variability (arising from different patient factors), and prior knowledge. The more individual patient data entered into the system, the more accurate the prediction becomes for the individual patient. Hence, without measured IFX levels and ADA, the first prediction is based only on the available factors known to affect IFX PK as determined by the underlying population PK model (the Bayesian Prior). The PK model forms a framework for providing a typical concentration–time curve. For an accurate prediction, IFX serum concentrations, ADA levels, serum CRP levels, serum albumin levels (all at trough and in the middle of two infusions), together with patient-specific variables such as body weight and gender were used. The dashboard system used in this study employs a two-compartment population PK model of IFX and is based on 7169 observations in 788 patients (655 IBD, 117 rheumatoid arthritis, and 16 patients with Kawasaki’s disease) that participated in various controlled clinical trials evaluating the efficacy and safety of IFX [Citation27].
Sample size calculation
A Fisher’s exact test with a 0.050 two-sided significance level had 80% power to detect a difference between the PG with an expected proportion of sustained clinical remission (p1) of 0.94 and CG with an expected proportion of sustained clinical remission (p2) of 0.66 (odds ratio of 0.098), leading to a sample size of 35 patients per group. Accounting for a dropout rate of approximately 15%, it was decided to include a total of 80 patients, with a 1:1 allocation ratio. The expected sustained clinical remission rate of 0.94 in the intervention group was based on data from the TAXIT trial [Citation16]. After an initial optimization phase in which IFX dose and or interval were modified to achieve IFX TLs of 3–7 μg/ml, 7% of the included IBD-patients who received TL-based IFX dosing required rescue therapy (defined as the need for an intervention based on clinical symptoms). The expected sustained clinical remission rate of 0.66 in the control group is based on the reported need for treatment intensification in 23–46% of patients receiving anti-TNF therapy within 12 months [Citation28].
Outcome parameters and follow-up
The primary endpoint was the proportion of patients who received per protocol treatment and remained in clinical remission (PM score ≤ 2 for UC or HBI ≤ 4 for CD at all study visits) for 1 year (per protocol analysis). Criteria for failure and treatment discontinuation included clinical flare (worsening of clinical symptoms defined as HBI >4 or PMS >2 on two consecutive visits with a maximum interval of 4 weeks and/or treatment-associated adverse events. All randomized patients were evaluated in the intention-to-treat analysis. Secondary endpoints included the proportion of patients in clinical remission at 6 months, biochemical remission (FCP <250 µg/g and CRP <5.0 mg/L) at 6 and 12 months, and the incidence of (serious) adverse events. Quality of life was evaluated at 6 and 12 months. Lastly, the time to clinical relapse was evaluated using survival analysis.
Statistical analysis
The primary endpoint was analysed in the patients without protocol deviations, representing the per protocol population. In addition, all randomized patients were included in the intention-to-treat analysis. Statistical programs GraphPad Prism version 7.03 and IBM SPSS Statistics 25 were used for statistical analysis. Additional evaluations were conducted using R Statistical Software (version 3.5.3, https://www.R-project.org/); utilizing libraries such as MASS (version 7.3-47), survival (version 2.41-3), Hmisc (version 4.0-3), tidyverse (version 1.2.1), broom (version 0.4.3), doBy (version 4.5-15), dplyr (version 0.7.4), linpk (version 1.0), tableone (version 0.8.1) along with ggplot and gridExtra packages for visualizing the data. Data are stated as median (interquartile range) unless mentioned otherwise. The Fisher’s Exact or Chi-Square test was used for univariate analysis of discrete variables.
Results
Patients
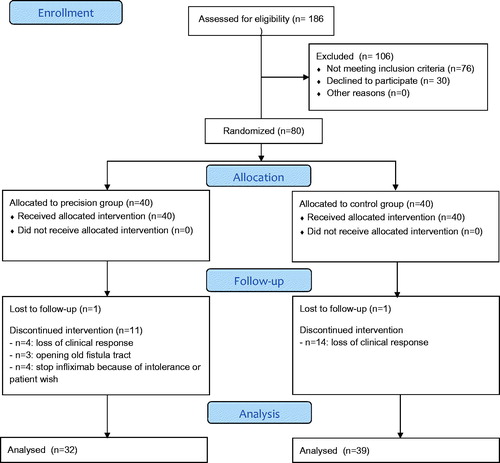
Between May 2015 and November 2017, 186 IBD patients receiving IFX maintenance treatment were screened of which 80 patients were randomized (40 patients in each treatment group) (Table 1)). Median [IQR] serum CRP levels at baseline were 2.0 mg/L [0.9–5.3] in the PG and 2.1 mg/L [1.0–6.5] in the CG. Median baseline [IQR] FCP levels were 110 µg/g [27–434] in the PG and 150 µg/g [41–626] in the CG (p = .637). The median (interquartile range, IQR) IFX treatment duration prior to inclusion was 4 years (2.0–6.8) and 40% of patients received concomitant immunomodulator therapy at baseline. Baseline patient characteristics per treatment group are shown in . The reasons for treatment failure are listed in the CONSORT flow diagram ().
Table 1. Baseline characteristics per treatment group.
Clinical outcomes
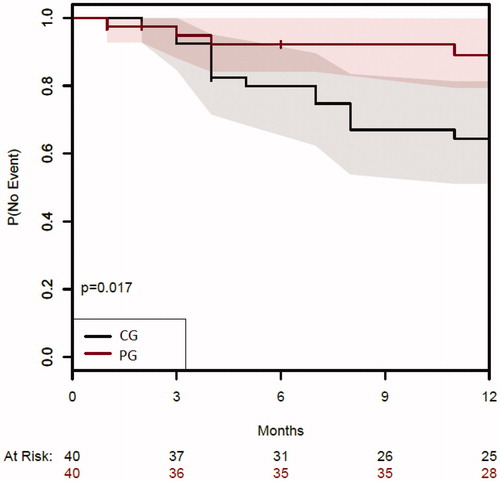
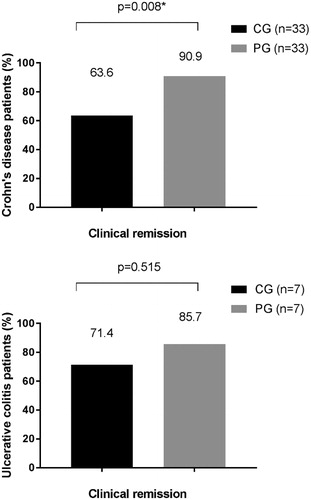
Based on per protocol analysis, dashboard driven IFX dosing resulted in a higher proportion of patients in sustained clinical remission compared to conventional dosing during one year of follow-up [(28/32 (88%) versus 25/39 (64%)] (p = .017) (). As reflected in the CONSORT diagram, nine patients were censored because of IFX intolerance and/or the patient’s preference to switch treatment (n = 4) or for the opening of an old perianal fistula after dose de-escalation (n = 3). In these three cases, MRI confirmed that the fistula tracts were pre-existing as shown on earlier imaging and were therefore not considered as new perianal fistulas (and hence not recorded in the HBI). Two patients were lost to follow-up (1 in each group). In the intention to treat analysis, 25 out of 40 patients (63%) in the CG versus 28 out of 40 patients (70%) in the PG met the primary endpoint (p = .637). Subgroup analysis per disease type (CD vs UC), demonstrated a statistically significant difference in sustained clinical remission rates between the two treatment groups in CD patients (p = .008) ().
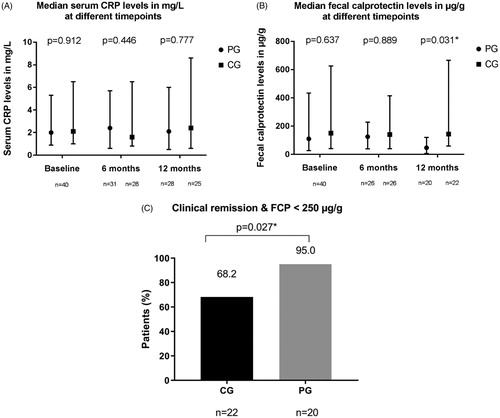
Biochemical outcomes
Serum CRP and FCP levels at baseline, 6 and 12 months are shown in . After 1 year, the median FCP levels were significantly lower in patients in the PG (47 µg/g [6–120], n = 20) compared to the CG (144 µg/g [59–666] (p = .031, n = 22) (). Furthermore, combining clinical remission rates and FCP levels <250 µg/g at one year resulted in a statistically significant difference between the two groups, in favour of the PG (p = .027) ().
Figure 4. Serum CRP (4 A) and faecal calprotectin (4B) levels per treatment group at baseline, 6 and 12 months. Proportion of patients in clinical remission with a faecal calprotectin level <250 µg/g (4 C). Cut offs for normal serum CRP and FCP: 5 mg/L and 250 µg/g respectively. PG: precision dosing group; CG: conventional dosing group; CRP: C-reactive protein; FCP: faecal calprotectin; n = number of patients; *p < .05, statistically significant.
Pharmacokinetic analysis and immunogenicity
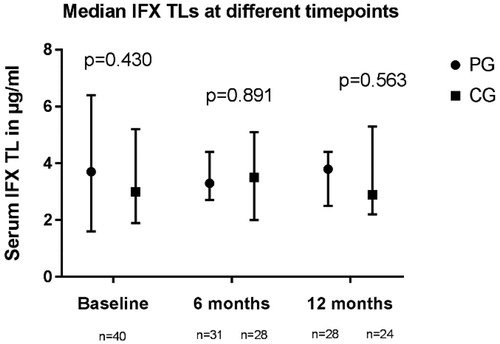
Median [IQR] IFX TLs at baseline were 3.7 µg/ml [1.6–6.4] and 3.0 [1.9–5.2] µg/ml in the PG (n = 40) and CG (n = 40), respectively (). After one year, median TLs were 3.8 µg/ml [2.5–4.4] in the PG n = 28) and 2.9 µg/ml [2.2–5.3] in the CG (n = 24) (p = .563). Two patients, both allocated to the PG, were ADA positive at baseline with associated TLs below the lower limit of quantification (0.03 µg/ml). Both patients were intensified to 10 mg/kg IFX every 4 weeks according to the model’s guidance and experienced a mild infusion reaction during the first infusion at higher dose. One patient continued IFX treatment with antihistaminic pre-medication and reached therapeutic TLs with undetectable ADA. The second patient stopped IFX treatment because of sustained clinical remission and patient preference.
Dosing interventions in PG
In the PG, treatment de-escalation by either lowering the IFX dose and/or increasing the dosing interval was applied in 20 out of 40 patients, which was successful in 70% of patients meaning that they could continue IFX treatment using these dosing regimens. In all patients receiving dashboard guided dosing, the first two dose recommendations and adjustments were the most significant. From that point onwards, recommendations were stable, although predictions using the dashboard system were made before every infusion. In 14 out of 40 patients (35%), the dashboard advised treatment intensification by decreasing dosing intervals and/or increasing the dose. In only 6 out of 40 patients (15%), no change in dosing regimen was advised. In 3 out of 20 patients, this change in regimen led to re-opening of perianal fistulas and in 3 patients to secondary LOR.
Safety and quality of life
In the PG, 2 patients were diagnosed with pneumonia, one of whom was hospitalized for intravenous antibiotics. Three patients in the PG, known with perianal disease, presented with re-opening of prior perianal fistula tracts after dose de-escalation. In all patients, the fistula tracts closed after returning to their previous treatment schedule apart from the trial. Adverse events are listed in . SF36 and EQ5D scores at baseline and 12 months for quality of life are shown in Supplementary Figures 6 and 7, respectively.
Table 2. (Serious) adverse events to anti-TNF treatment.
Discussion
The PRECISION study is the first randomized prospective trial evaluating clinical benefit from personalized IFX dosing in IBD patients. Dashboard guided IFX dosing resulted in a significantly higher proportion of patients maintaining clinical remission during one year of follow-up compared to patients who continued IFX treatment without proactive dose adjustments. After one year of follow-up, patients in the PG had significantly lower median FCP levels compared to patients in the CG.
IFX dose reduction was possible in 20/40 patients because of ‘supra-therapeutic’ TLs (>3 µg/ml) and 70% of patients could continue treatment with at these reduced dosing regimens. These results are in line with the previously published TAXIT study, in which the majority of patients (93%) with supra-therapeutic IFX TLs could safely be dose de-escalated to a TL between 3 and 7 µg/ml. However, in our study IFX dose reduction led to re-opening of perianal fistulas in 3 CD patients, suggesting that a TL of 3 µg/ml is probably insufficient for fistula control. This confirms a recent observation by Yarur et al. [Citation29] in which it was demonstrated that patients with closed perianal fistula on average had significantly higher median serum IFX levels compared to patients with actively draining fistulas [15.8 vs. 4.4 µg/ml, respectively (p < .0001)]. Another study showed a more favourable fistula response in CD patients with higher median IFX TLs during induction treatment compared to patients with lower median TLs [Citation30]. This is in line with our own observations that both for IFX and adalimumab higher serum concentrations are needed for fistula healing than for clinical remission [Citation31]. Although dashboard guided dosing is probably also useful for CD patients with perianal fistulising disease, higher target concentrations are to be recommended for this subpopulation.
Indeed, since treatment goals often differ between IBD patients and might also change during the disease course, personalized treatment is highly relevant. The results of this study are in line with recently published retrospective analyses showing improved outcomes in IBD patients receiving proactive monitoring of anti-TNF serum concentrations compared to a reactive TDM-based approach [Citation32–35]. Factors that are helpful for treatment personalization are those influencing the clearance of therapeutic antibodies in IBD patients. For IFX serum albumin concentrations, serum CRP, gender and body weight are most relevant [Citation18,Citation19]. For physicians, it is most challenging to take these factors into account while dosing individual patients. As a consequence, most patients receive under- or overdosing as it was seen in the first stage of TAXIT. This also reflects the fact that the drug development process for therapeutic antibodies in many cases has been suboptimal, aiming for a ‘one size fits all’ dose. Body weight is the only factor that determines dosing of certain biologics, and in clinical practice calculated doses are often rounded depending on drug quantity supplied per vial, which is suboptimal.
Besides patient-related factors, dashboard systems also include other factors that influence IFX PK, such as previous IFX doses, prior IFX serum concentrations and ADA levels. Individual target serum concentrations can be identified and the dashboard provides dose recommendations.
Dubinsky and colleagues recently demonstrated that dosing recommended by a PK dashboard system differs from standard care dosing in paediatric IBD patients receiving IFX [Citation36]. In the PRECISION trial, we set the target IFX TL at 3 µg/ml based on earlier literature and recommendations. However, the target can be manually adjusted by the physician.
The use of computer programs to individualize dosing, leading to higher treatment efficacy, shorter hospitalization stays and lower costs, has been mostly restricted to clinical pharmacists and pharmacologists due to the relative complexity of its implementation [Citation37]. However, the use of a dashboard system also allows clinicians to individualize dosing in the consulting room. The dose recommended by the dashboard system might be lower than the on-label dosing regimen, leading to cost savings. In our study, 50% of the patients in the PG had a TL >3 µg/ml and the majority of these patients (70%) could be safely dose de-escalated.
The first two dose recommendations by the dashboard had most impact on the dosing schedule. Probably, this was because the patients in this study were adults with stable disease control on IFX maintenance treatment. Using dashboard guided dosing in patients during IFX induction treatment or in patients with LOR during maintenance treatment, will likely result in more frequent dose adaptations. During induction, recommended serum concentrations of IFX are also different from those during maintenance. In ulcerative colitis, for instance, IFX concentrations above 25–28 µg/ml have been associated with better outcomes.
The dashboard offers guidance on not only the IFX dose to be given, but also on the most optimal date for the infusion. Conveniently, it is possible to change this date based on the patient’s or physician’s discretion, e.g., during vacation times, because the dashboard offers dose recommendation for other potential infusions dates.
Our study population represents a real-life cohort consisting of patients on IFX maintenance treatment from academic and regional hospitals. The standard IFX dosing regimen according to the label for these patients is 5 mg/kg IFX every 8 weeks. At entry in the study, 28% of the patients in the PG and 33% of patients in the CG received IFX treatment at an intensified dosing regimen with either a higher dose or a shortened infusion interval. This reflects clinical practice in most centres, where empiric dose adjustments are performed mainly in patients with signs of clinical and/or biochemical suboptimal response or LOR.
Using a dashboard requires some training for the operator. Therefore, the dashboard system in this study was used by one trained researcher (AS), who provided dose recommendations to all participating centres to minimize inter-observer bias. However, the training that is needed does not take more than a couple of hours, so it can be anticipated that implementation in clinical practice could happen without foreseeable problems.
The strengths of our study were its prospective design, the availability and incorporation of mid infusion serum concentrations, the dose guidance/adjustment at every infusion and the central coordination of dosing across centres using a validated dashboard. Systematic evaluation of clinical disease activity using the HBI for CD and PM score for UC was performed at every study visit and FCP was used as a surrogate marker of mucosal inflammation.
Our study has also several limitations. It was decided not to perform endoscopies to minimize invasive procedures in these stable IFX responders, also because FCP is considered a reliable measure of endoscopic disease activity [Citation38]. An additional limitation is the fact that we used a drug-sensitive assay to detect ADA. However, low ADA levels in the presence of drug (not detectable by a drug-sensitive assay) are often transient and are considered to be clinically irrelevant [Citation39]. Also, in the PG, patients were dose intensified if TLs were below 3 µg/ml, irrespective of their biochemical markers or clinical disease activity. It is possible that these patients received unnecessary dose adaptations and that they would have remained in clinical remission if these changes were not made. Finally, we used an IFX target concentration of 3 µg/ml based on previous publications. Ungar et al. [Citation40] however proposed an optimal window of 6–10 µg/ml to achieve mucosal healing. Most likely, the optimal IFX target TL is not only patient but also target dependent and might hence vary during the disease course. Future research should focus on different cut-off levels for specific outcome measures and treatment goals, such as mucosal healing, perianal fistula closure and histological remission.
Since there is some evidence that an intensified IFX dosing regimen may increase primary and sustained response rates in patients with severe UC, the efficacy of dashboard driven dosing should be further investigated in IBD patients during induction treatment [Citation6,Citation7,Citation41]. Patients losing response to IFX due to increased clearance with low serum drug levels will probably benefit most from this approach.
In conclusion, this randomized controlled trial evaluated the efficacy of dashboard guided dosing of IFX in IBD patients during maintenance treatment. A significantly higher percentage of patients receiving dashboard guided IFX dosing maintained clinical remission during one year of follow-up compared to patients who did not receive proactive dose adjustments. Moreover, patients in the intervention group had significantly lower FCP levels. In the majority of patients with TLs >3 µg/ml dose reduction did not lead to clinical LOR. However, a small proportion of patients may need higher target TLs depending on the specific treatment goal, for example, patients with perianal fistulas. Future trials should investigate dashboard guided dosing of IFX in IBD patients during induction treatment.
Authors’ contributions
Anne Strik (AS) analysed data and wrote the manuscript. All authors contributed to study design and manuscript preparation. Geert D’Haens (GD) supervised study execution and manuscript preparation.
| Abbreviations | ||
| ADA | = | anti-drug antibodies |
| CD | = | Crohn’s disease |
| CG | = | conventional dosing group |
| CRP | = | C-reactive protein |
| ELISA | = | enzyme-linked immunosorbent assay |
| FCP | = | Fecal calprotectin |
| HBI | = | Harvey Bradshaw Index |
| IBD | = | inflammatory bowel disease |
| Infliximab | = | infliximab |
| IQR | = | interquartile range |
| LOR | = | loss of response |
| PG | = | precision group |
| PK | = | pharmacokinetic |
| PM | = | partial Mayo |
| TL | = | trough level |
| TNF | = | tumor necrosis factor |
| UC | = | ulcerative colitis |
Supplemental Material
Download MS Word (237.7 KB)Acknowledgements
The authors thank all patients who were willing to participate in this study. Moreover, the authors thank the nurses at the infusion unit of all participating centres. Special thanks to the IBD trial nurses of the Onze Lieve Vrouwe Hospital and Tergooi hospital: Toos Schakel and Sandra Moll for their involvement.
Disclosure statement
AS has no conflicts of interest.
ML has served as speaker and/or principal investigator for: Abbvie, Celgene, Covidien, Dr. Falk,
Ferring Pharmaceuticals, Gilead, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Pfizer, Protagonist therapeutics, Receptos, Takeda, Tillotts, Tramedico. He has received research grants from AbbVie, Merck Sharp & Dohme, Achmea healthcare and ZonMW.
DM is president of Projections Research Inc, a pharmaceutical consulting company.
SB has no conflicts of interest.
CP has received grant support from Takeda, Dr. Falk Pharma, speaker’s fee from Abbvie, Takeda, Ferring and Dr. Falk Pharma, consultancy from Abbvie and Takeda.
JvB has no conflicts of interest.
JJ has no conflicts of interest.
DH has no conflicts of interest.
JB has no conflicts of interest.
MD has served as advisor for Echo pharma and Robarts Clinical Trials, reports nonfinancial support from Dr. Falk Pharma, and received speaker fees from Janssen, Merck & Co., Inc., Pfizer, Takeda and Tillotts Pharma.
KG has served as speaker and/or advisor for Amgen, AbbVie, Biogen, Boehringer Ingelheim, Ferring, Hospira, Immunic AG, Janssen, MSD, Pfizer, Samsung Bioepis, Sandoz, Takeda, Tigenix and Tillotts.
AV has no conflicts of interest.
RM has received grant support from Bayer, Merck Sharp & Dohme, Roche and Shire and served as an advisor for Shire and Zeria.
GD has served as advisor for Abbvie, Ablynx, Allergan, Amakem, Amgen, AM Pharma, Arena Pharmaceuticals, AstraZeneca, Avaxia, Biogen, Bristol Meiers Squibb, Boehringer Ingelheim, Celgene/Receptos, Celltrion, Cosmo, Covidien/Medtronics, Ferring, DrFALK Pharma, Eli Lilly, Engene, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Hospira/Pfizer, Immunic, Johnson and Johnson, LGChem, Lycera, Medimetrics, Millenium/Takeda, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Nextbiotics, Novonordisk, Otsuka, Pfizer/Hospira, Photopill, Prometheus laboratories/Nestle, Progenity, Protagonist, Robarts Clinical Trials, Salix, Samsung Bioepis, Sandoz, Seres/Nestle, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant and Vifor; received speaker fees from Abbvie, Biogen, Ferring, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Millenium/Takeda, Tillotts and Vifor.
References
- Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476.
- Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–1307.e5.
- Feuerstein JD, Nguyen GC, Kupfer SS, et al.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology. 2017;153:827–834.
- Kevans D, Murthy S, Mould DR, et al. Accelerated clearance of infliximab is associated with treatment failure in patients with corticosteroid-refractory acute ulcerative colitis. J Crohn’s Colitis. 2018;12:662–669.
- Papamichael K, Van Stappen T, Vande Casteele N, et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:543–549.
- Ungar B, Mazor Y, Weisshof R, et al. Induction infliximab levels among patients with acute severe ulcerative colitis compared with patients with moderately severe ulcerative colitis. Aliment Pharmacol Ther. 2016;43:1293–1299.
- Dreesen E, D’Haens G, Baert F, et al. DOP047 Infliximab exposure predicts superior endoscopic outcomes in patients with active Crohn’s disease: pharmacokinetic–pharmacodynamic analysis of TAILORIX. J Crohn’s Colitis. 2018;12:S063–S064.
- Brandse JF, Mathôt RA, van der Kleij D, et al. Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:251–258.e1-e2.
- Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254.
- Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54.
- Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT i trial. Gut. 2014;63:1721–1727.
- Levesque BG, Greenberg GR, Zou G, et al. A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn’s disease. Aliment Pharmacol Ther. 2014;39:1126–1135.
- Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J Crohn’s Colitis. 2013;7:736–743.
- Lowenberg M, Brandse J, Vos L, et al. G. High infliximab trough levels are associated with impaired quality of life in IBD patients in clinical and biochemical remission on maintenance IFX therapy. J Crohn’s Colitis. 2014;8:S262–S263.
- Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–1329.e3.
- D’Haens G, Vermeire S, Lambrecht G, et al. Increasing infliximab dose based on symptoms, biomarkers, and serum drug concentrations does not increase clinical, endoscopic, and corticosteroid-free remission in patients with active luminal Crohn’s disease. Gastroenterology. 2018;154:1343–1351.e1.
- Brandse JF, Mould D, Smeekes O, et al. A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:650–660.
- Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259.
- Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62:e02042-17.
- Felton TW, Roberts JA, Lodise TP, et al. Individualization of piperacillin dosing for critically ill patients: dosing software to optimize antimicrobial therapy. Antimicrob Agents Chemother. 2014;58:4094–4102.
- Sheiner LB, Beal S, Rosenberg B, et al. Forecasting individual pharmacokinetics. Clin Pharmacol Ther. 1979;26:294–305.
- Sheiner LB, Beal SL. Evaluation of methods for estimating population pharmacokinetic parameters. I. Michaelis-menten model: Routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1980;8:553–571.
- Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:711–715.
- Vande Casteele N, Buurman DJ, Sturkenboom MGG, et al. Detection of infliximab levels and anti-infliximab antibodies: a comparison of three different assays. Aliment Pharmacol Ther. 2012;36:765–771.
- Mould DR, Upton RN, Wojciechowski J. Dashboard systems: implementing pharmacometrics from bench to bedside. Aaps J. 2014;16:925–937.
- Xu Z, Mould D, Hu C, et al. Population pharmacokinetic analysis of infliximab in pediatrics using integrated data from six clinical trials abstract 139760. Clin Pharmacol Drug Dev. 2012;1:203.
- Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33(9):987–995.
- Yarur AJ, Kanagala V, Stein DJ, et al. Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther. 2017;45:933–940.
- Davidov Y, Ungar B, Bar-Yoseph H, et al. Association of induction infliximab levels with clinical response in perianal Crohn's disease. J Crohns Colitis. 2017;11:549–555.
- Strik AS, Löwenberg M, Buskens CJ, et al. Higher anti-TNF serum levels are associated with perianal fistula closure in Crohn’s disease patients. J Crohn’s Colitis. 2018;54:453–458.
- Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17:1655–1668.e3.
- Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017;15:1580–1588.e3.
- Fernandes SR, Bernardo S, Simões C, et al. Proactive infliximab drug monitoring is superior to conventional management in inflammatory bowel disease. Inflamm Bowel Dis. 2020;26:263–270.
- Papamichael K, Juncadella A, Wong D, et al. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared with standard of care in patients with inflammatory bowel disease. J Crohn’s Colitis. 2019;13:976–981.
- Dubinsky MC, Phan BL, Singh N, et al. Pharmacokinetic dashboard-recommended dosing is different than standard of care dosing in infliximab-treated pediatric IBD patients. AAPS J. 2017;19:215–222.
- Van Lent-Evers NAEM, Mathôt RAA, Geus WP, et al. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit. 1999;21:63–73.
- D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224.
- Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2018;67:818–826.
- Ungar B, Levy I, Yavne Y, et al. Optimizing anti-TNF-alpha therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:550–557.e2.
- Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:330–335.e1.