Abstract
Background
Some 40% of colorectal cancer (CRC) patients present with anemia. Temporal trends of gradually decreasing Hb are suggested as a supplementary diagnostic tool for CRC. We set out to explore this concept in a strictly defined population.
Methods
A laboratory database identified patients ≥40 years that had ≥1 Hb test reported from primary care, Örebro county in 2000–17. Linkage to the Swedish Colorectal Cancer Registry identified patients diagnosed with CRC. Other primary care patients served as controls (1:10), matched by age and sex. Prediagnostic Hb in cases and controls were compared and temporal trajectories of Hb modelled using a nonlinear three-parameter logistic function.
Results
1,534 CRC patients and 15,333 controls were identified. The average number of reported Hb tests in primary care per year increased successively, and diagnostic delay from detection of anemia to diagnosis of CRC decreased; in 2015–17 it was median 4 (IQR 2–6) months. No association was found between last Hb and stage of right-/left-sided colon, or rectal cancer.
A statistically significantly lower Hb in CRC patients was discernable 609 days (20 months) prior to diagnosis for men and 905 days (30 months) for women, both in the range of normal Hb. The frequency of Hb testing in the general population via primary care was surprisingly low, and was ≥50% annually only in octogenarians.
Conclusion
The findings indicate a potential for Hb trends to inform the diagnostic process of CRC but whether it will translate into any clinical advantage is yet uncertain.
Introduction
Colorectal cancer (CRC) is the third most common malignancy worldwide with more than 1.8 million new cases annually, and the second leading cause of cancer mortality with 881,000 deaths in 2018 [Citation1]. In spite of screening efforts in many countries, most CRCs are detected via the clinical route based on symptoms and signs. These are, however, to a large extent also associated with benign disorders, and predictive values in a primary care population are typically low. Rectal bleeding in elderly male patients is often associated with the highest positive predictive value (PPV) above 4%, but for most CRC related symptoms, PPVs are much lower [Citation2].
The prevalence of anemia in CRC is estimated to 43–48% [Citation3–6] but again there are many other plausible and serious explanations for this condition, and PPVs of manifest anemia for CRC in primary care, for both sexes and all ages, is not estimated to more than 1–2% [Citation7,Citation8]. However, prior to manifest anemia, a decline in hemoglobin within the normal range takes place and case-control studies have identified a decline is discernable up to 4 years prior to CRC diagnosis [Citation9,Citation10]. Predictive models based on such pre-diagnostic temporal decline in hemoglobin concentration have been developed as a way of promoting early detection of CRC [Citation11,Citation12]. These findings are interesting, in particular as the concept is based on re-evaluation of already available laboratory outcomes and thereby convenient for patients.
We therefore set out to learn more about anemia in relation to diagnosis of CRC in one Swedish county, and to explore the prospects for promoting early detection of CRC by using prediagnostic time trends of Hb.
Methods
Study setting
This is a population-based case-control study involving patients from primary care in Örebro county, Sweden. No screening program for colorectal cancer was operating in the area during the study period, and primary care was almost completely managed within one public healthcare organization.
Study population
At the Department of Clinical Chemistry, Örebro university hospital, a specific database (Flexlab) holds information on the identity of patients, type of test, what department requested the test, date of analysis and outcome of all laboratory tests. This database has been in use since the late 1990s. In 2014, all primary care centres were connected to the system and from 2015 and onwards, the outcome of all point-of-care testing is also registered in this software. All patients who had at least one hemoglobin test requested in primary care during 2000–2017, Aug 22 and who were above the age of 40 at the time of testing were identified via the database. Dates of analyses and the corresponding outcome of Hb tests were retrieved.
The Swedish Colorectal Cancer Registry (SCRCR) is a nationwide cancer registry with an almost complete coverage (> 98% in 2018) [Citation13]. Patients diagnosed with colorectal cancer (C18-20) according to ICD-10 [Citation14] and reported from Örebro county between January 1st, 2000 and August 22nd, 2017 were included and data requested from the Regional Cancer Centre in Uppsala (i.e. where data is checked for completeness before transferred to the national registry SCRCR). The data obtained include date of diagnosis of CRC, tumor site and stage. In case any date of diagnosis was missing (e.g. for patients who had emergency surgery due to obstructive colon cancer), the date noted for start of treatment was used instead. Tumor site was provided as either colon or rectum for the whole study period, but since 2007 it was also reported as one of six subsites (caecum, colon ascendens, transversum, descendens, sigmoid and rectum). Stage was reported according to Dukes’ classification during 2000–03 and later according to the TNM system. In order to facilitate the analyses, all were reclassified as stage I–IV.
The two datasets, the laboratory database and SCRCR, were linked together using unique Swedish personal identity numbers. Patients diagnosed with CRC, and who have had at least one hemoglobin test in primary care prior to the date of CRC diagnosis found in the laboratory database, were included and categorized as cases. Colorectal cancer patients who have had no hemoglobin test in primary care prior to diagnosis, or only had post-CRC diagnostic hemoglobin tests in primary care were not eligible for the study.
For each case with CRC, ten controls were randomly selected from the laboratory dataset of patients who have had a least one Hb test in primary care, and who had not been diagnosed with CRC since the SCRCR was initiated in 1995, and matched by sex and age. Controls were initially sought after among patients born on the same day as the case, but if there were not enough individuals born on the same day to identify ten controls, the time period was gradually expanded stepwise by one day at a time, backwards and forward and accepting up to −1/+1 of casés age, until ten controls were identified.
Definitions
Anemia was defined according to the classification from the World Health Organization (WHO) for adult population, i.e. hemoglobin less than 120 g/L for women and less than 130 g/L for men [Citation15]. Hemoglobin concentrations between 120 and 130 g/L for women and between 130 and 140 g/L for men were defined as a “low normal Hb values”. Colon cancers located from cecum to the transverse colon were defined as right-sided colon cancer (RCC), and those located in the descending and sigmoid colon as left-sided colon cancer (LCC). Information on stage (I–III) was missing for patients that had no resection of their primary cancer. Diagnostic intervals (delay), from first hemoglobin test showing anemia or low normal hemoglobin, until diagnosis of CRC were calculated.
Statistical analysis
Hemoglobin concentration was summarized as the mean value with a standard deviation (SD), and categorical and ordinal data were presented as proportions. Hemoglobin values among CRC patients refer to the last test prior to diagnosis, and corresponding for the controls prior to the “hypothetical” date of diagnosis set to the same dates as that of their matching cases. Demographics, clinical information and hemoglobin concentration of cases and controls were compared using the Chi-square test or Fisher’s exact test for categorical variables, the Mann–Whitney U test for ordinal variables and Student’s t-test for normally distributed continuous variables.
For cases there was a specific date for diagnosis of CRC and for controls, a hypothetical date of diagnosis was set to the same date as that of their matching cases. The temporal trajectories of Hb concentration prior to the date (real or hypothetical) of CRC diagnosis were modelled using the nonlinear three-parameter logistic function (NLLog3). Because the subjects may have had more than one Hb test prior to CRC diagnosis, the clustered sandwich estimator, which is used to adjust inference when errors are correlated within each subject, was used for standard error. To further examine the temporal trend of Hb, we also conducted a non-linear multi-level mixed-effects regression (NLMR) analysis using the cases and controls with 10 or more Hb tests, and subjects were included as a random effect in the NLMR model.
In order to assess the proportion of the overall population within the whole county subjected to at least on Hb test in primary care, we identified the number of patients in five age groups (40–49, 50–59, 60–69, 70–79 and 80 years and older) and per sex for each calendar year 2015–2017 who have had at least on Hb test (in the laboratory database). The denominator, i.e. the number of inhabitants in Örebro county corresponding to each of these cells (age group, sex and calendar year) was retrieved from readily available population statistics [Citation16].
A two-sided p-value < .05 was defined as statistical significance. All the statistical analyses were performed in Stata 15.1 (StataCorp, College Station, Texas, USA), and R 4.03 (R Foundation for Statistical Computing, Vienna, Austria) using packages nlme 3.1-151, lme4 1.1-26, and effects 4.2-0. The research was approved of by the Uppsala Regional Ethics Committee in August, 2018.
Results
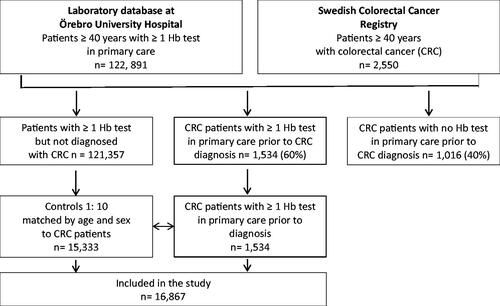
In total, 122,891 individuals aged 40 years and above had at least one Hb test analysed in primary care in Örebro county from January 1, 2000 to August 22, 2017 that was registered in the central laboratory database. During the same time period, 2,550 colorectal cancer patients of the same age group from the same geographical area were identified via the SCRCR. In all, 1,016 (39.8%) of them did not have any Hb test in primary care reported to the central database prior to their CRC diagnosis and were thus not eligible for the study. For the remaining 1,534 (60.2%) patients diagnosed with CRC, 1:10 age- and sex-matched controls (15,333 primary care patients) were identified in the laboratory database. A flowchart of the study is in .
In all, 16,867 individuals were included. Among CRC patients, the proportion of men and women was even and very few patients were below the age of 50 years (). The median age among CRC patients at last Hb test in primary care prior to CRC diagnosis was 75 years. Half of CRC patients had localized disease (stage I and II) and 2/3 had colon cancer. The annual number of Hb tests from primary care in the central laboratory database was not uniform throughout the study period, and among controls it gradually increased from 796 reported in the year 2000 to 3,293 in 2015 ().
Table 1. Basic characteristics of included patients (n = 16,867).
In all 30,797 Hb tests were identified in the data base among the controls (n = 15,333), i.e. on average 2 Hb test per control during the whole study period. For the 1,534 patients with CRC, in all 4,577 Hb tests were identified, i.e. on average 3 Hb tests per CRC patient. For CRC patients, the number of days from last Hb test to diagnosis was mean 457 (range 1–5466), median 121 (IQR 58–446) days for the whole study period 2000–17, and corresponding values for controls were mean 763 (range 1–6307), median 428 (IQR 167–1022) days.
The mean value of last Hb tests in primary care prior to diagnosis of CRC was significantly lower in CRC patients compared to in controls for both men and women, and in all age groups (there were only 3 men with CRC aged 40–49 years) (). A significantly lower hemoglobin concentration was found in colon vs rectal cancer patients and in right- vs left-sided colon cancer, for both men and women (). There was no significant difference in Hb concentration of last test prior to diagnosis by CRC stage I–IV in right-sided or left-sided colon cancer, nor for rectal cancer ().
Table 2. Mean values of last Hb test obtained in primary care prior to colorectal cancer diagnosis in cases vs controls.
Table 3. Mean values of last Hb test obtained in primary care prior to colorectal cancer diagnosis by site and stage. Values in parentheses are percentages.
For the most recent period (2015–17), all Hb tests in primary care were reported and diagnostic delay from detection of anemia to CRC diagnosis was median 4 months (128 and 133 days) in both men and women (). For the uppermost quartile, diagnostic delay from detection of anemia to CRC diagnosis was from six months up to years, but, it was much shorter compared to in the period 2000–14 ().
Table 4. Diagnostic delay (in days) from first detection of anemia or low hemoglobin in primary care to diagnosis of colorectal cancer, Örebro county per time period (2000–2014, 2015–2017 and 2000–2017).
Temporal trends of prediagnostic Hb
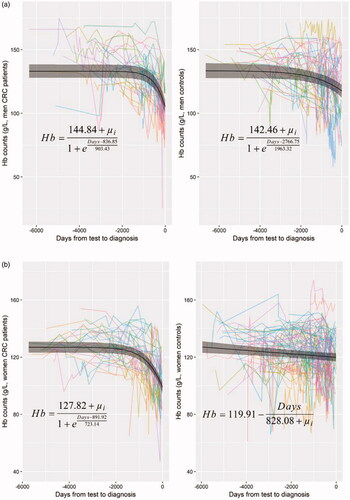
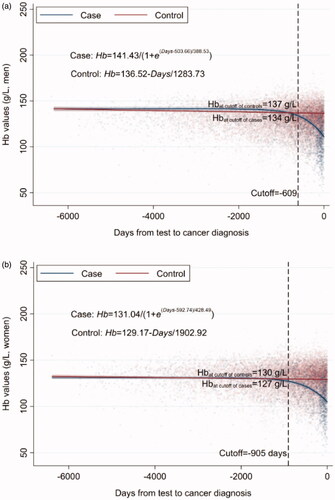
In controls, the hemoglobin concentration remained fairly stable through almost 16.5 years prior to the hypothetical CRC diagnosis and no non-linear temporal trend was found for hemoglobin in either sex. In patients with CRC on the other hand, a statistically significant nonlinear temporal trend of hemoglobin concentration was found in both sexes, and a sharp decline close to CRC diagnosis. The cutoffs indicated there was a statistically significantly lower Hb concentration 609 days (20 months) prior to CRC diagnosis in men and 905 days (30 months) in women, as compared to controls (). However, notably, the corresponding Hb levels at these two cutoffs were 134 g/L for men and 127 g/L for women, i.e. in the range of a low normal Hb. The cutoff in men was close to the mean time of first appearance of anemia prior CRC diagnosis (586 days or 19 months, ) whereas the cutoff in women was 10-month earlier than the mean time (609 days or 20 months, ) of first appearance of anemia prior to CRC diagnosis.
Figure 2. (a) Temporal trend of Hb concentration prior to CRC diagnosis in men. (b) Temporal trend of Hb concentration prior to CRC diagnosis in women.
NLMR analysis with ten or more Hb tests prior to CRC diagnosis during 16.5 years, which were available for, in total, 31 men and 37 women, confirmed the nonlinear temporal trend of Hb levels with a sharp drop near diagnosis among CRC patients ( for men and for women, left panel). The difference of time trend between CRC and control was distinct. There was no nonlinear trend among the controls, only a slight linear decline ( for women, right panel).
Frequency of Hb tests in primary care – CRC patients, controls, general population
The number of hemoglobin tests in primary care per two month-period prior to diagnosis was more frequent in CRC patients compared to controls during 4 to 6 months prior to diagnosis (). This finding was similar for the whole study period 2000–2017 (data not shown).
Table 5. Median (mean) number of Hb tests in primary care per two months prior to diagnosis of CRC in CRC patients and age- and sex-matched controls during 2015–2017.
The proportion of all inhabitants in Örebro county subjected to at least one Hb test in primary care annually during 2015–2017 are provided in . Among individuals aged 80 years and above, a good half of the population of both men and women, are reported to have one Hb test annually whereas for inhabitants aged 60–69 years, less than a third had a Hb test every year.
Table 6. Proportion of inhabitants (%) in Örebro county who had at least one Hb test in primary care by age group, sex and calendar year.
Discussion
In this study of one Swedish county, available data on Hb tests reported from primary care were not uniform and gradually increased during the period 2000–2017 but even so, a statistically significant lower Hb concentration in patients diagnosed with CRC compared to age- and sex-matched controls was discernable 20 and 30 months prior to CRC diagnosis for men and women, respectively, for the whole study period. During most recent years, 75% of CRC patients with anemia detected in primary care are diagnosed within 6 months.
We had the advantage of accessing a population-based laboratory database and a high coverage cancer registry for this investigation. Our results are strengthened by the fact that other primary care patients, and not healthy individuals, served as controls. The study reflects a real-life situation in which patients may present in primary care with a subnormal Hb value for many other reasons than CRC. To the best of our knowledge, this is also the first study directly to compare temporal trends of hemoglobin between CRC patients and other primary care attenders over 18 calendar years.
There are also several limitations to our study, such as the question of generalizability of data from one single county. It follows from the relatively low number of CRC patients that it did not allow for e.g. Receiver Operating Characteristic (ROC) analyses at various cutoffs of prediagnostic time trends of Hb, nor any in-depth analysis of data from most recent and thereby relevant years. We chose to focus on Hb and did not include or tried to assess the importance of other complementary laboratory findings, e.g. complete blood counts, iron status or markers of inflammation. We chose to focus on Hb findings in primary care specifically as this is where most CRC patients present initially, but to get a full understanding of prediagnostic Hb in CRC in general, CRC patients managed in other settings should also be included (outpatient clinics, in-hospital, etc.). As in the future, EHRs for e.g. primary and secondary care will use the same laboratory module, this will be even more relevant.
In general, Hb and anemia are intriguing aspects of diagnostic work-up for CRC. Interestingly, a recent study reported normocytic anemia was more frequent, in around 30%, compared to microcytic anemia that was estimated to be present in merely up to 14% of CRC patients [Citation6]. Anemia is, not surprisingly, reported as an important missed diagnostic opportunity (MDO) and is estimated to be associated with the longest diagnostic delay for CRC [Citation17,Citation18]. Our findings indicate there has been a reduction of diagnostic delay for CRC patients presenting with anemia in the study population, and it might be even more pronounced than the figures indicate since there was incomplete reporting of Hb tests from primary care until 2015, i.e. point-of-care tests that indicated anemia in the first place were not reported.
For iron deficiency anemia (IDA) specifically, PPVs for CRC are estimated to be in the range of 3–10%, and for male patients exclusively even up to 18% [Citation4,Citation6,Citation19]. But studies on the management of IDA in primary care report only a limited proportion of patients, in the range of 30–55%, are referred for early endoscopic evaluation [Citation19–22]. Male patients and patients with more severe laboratory findings seem to be more likely to be referred for investigation [Citation19,Citation22]. On the other hand, no explanation or IDA-related cause will be found even after long-term follow-up for many patients, and even up to 60% of patients with IDA in primary care [Citation22]. It illustrates the complexity of clinical decision making.
The lack of an association between oncological stage and Hb concentration through all subsites (right- and left-sided colon and rectal cancer) () was of interest from this perspective. Both a Norwegian and a Finnish study have reported a similar lack of association between anemia and N- and M-stage of CRC [Citation3,Citation6]. It means the severity of anemia, among patients presenting in primary care, will not per se reflect the severity (oncological spread) of CRC. It adds to the clinical challenges for GPs as even a mild anemia could indicate serious disease. Further studies must verify the finding but the implications are interesting insofar as CRC-related anemia seems to call less on urgency (preventing spread) but rather on careful attention (not missing any patient).
The previously mentioned case-control study from Israel was based on 1074 patients diagnosed with CRC and normal Hb values, and controls were randomly selected from the same healthcare organization as cases [Citation10]. It was found that Hb levels started to decline up to even 4 years prior to CRC diagnosis, in a way that was not discernable among controls. The frequency of testing was on average one Hb per 8 months and 38% of the patients were tested on average twice a year. Another case-control study included blood donors in 1968–2002 from Sweden and Denmark [Citation9], and evaluated declining hemoglobin concentration before diagnosis of a range of cancers. For colon cancer, a statistically significant decline was discernable 2 years prior to diagnosis and odds ratios of detecting right-sided cancer per on SD departure from population average was estimated to 1.37 (95% CI 1.08–1.73) and 1.14 (95% CI 1.01–1.28) for left-sided colon cancer. The study included in all 882 patients with colon cancer, and the median number of Hb concentration measurements was 10 but, notably, this study was based on a healthy population of blood donors and the median age was as low as 52.
This study demonstrated that declining Hb trends are discernable long before a clinical diagnosis of CRC is established, also when other primary care patients serve as controls and in particular for female patients. Future studies will determine whether Hb trends can be used to inform the diagnostic process and identify patients at increased risk of CRC. It is well worth noting that the frequency of Hb testing in age groups of the population generally recommended screening due to an increased at risk of CRC (60–74 years in Sweden) was surprisingly low in our setting and even in recent years, less than a third of all inhabitants in this age group had an annual Hb test in primary care. Given the resources involved in traditional screening programs, we find this intriguing, in particular as right-sided colon cancer often present with silent anemia and FOBT is less sensitive in this group [Citation23]. Trend analyses of Hb might be a beneficial alternative for these patients.
In our understanding of the data, it seems as if anemia detected in primary care is managed in a judicious way for the majority of patients when it comes to the risk of CRC. For some patients the response to anemia might be inadequate causing a very long diagnostic delay but to get a full understanding, this group of patients must be identified and described more in detail. This is important for clinical work and as an ethical imperative but as an overall strategy it may be less promising. The time might have come to focus also on a gradually declining Hb concentration within the normal range, and whether CRC stage distribution could be affected by the use of such specific algorithms.
Conclusion
The unspecific symptoms of colorectal cancer continue to elude clinicians. The readiness to investigate manifest anemia as a possible sign of colorectal cancer seems to have improved, and further in-depth analyses on the potential utility of pre-diagnostic time trends of Hb in CRC seem justified.
Acknowledgments
We thank Carina Martinsson, Department of Clinical Chemistry, Örebro University Hospital for help in retrieving the data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Hamilton W, Lancashire R, Sharp D, et al. The risk of colorectal cancer with symptoms at different ages and between the sexes: a case-control study. BMC Med. 2009;7:17.
- Edna TH, Karlsen V, Jullumstro E, et al. Prevalence of anaemia at diagnosis of colorectal cancer: assessment of associated risk factors. Hepatogastroenterology. 2012;59(115):713–716.
- Kwon YH, Lim HK, Kim MJ, et al. Impacts of anemia and transfusion on oncologic outcomes in patients undergoing surgery for colorectal cancer. Int J Colorectal Dis. 2020;35(7):1311–1320.
- Stapley S, Peters TJ, Sharp D, et al. The mortality of colorectal cancer in relation to the initial symptom at presentation to primary care and to the duration of symptoms: a cohort study using medical records. Br J Cancer. 2006;95(10):1321–1325.
- Vayrynen JP, Tuomisto A, Vayrynen SA, et al. Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival. Sci Rep. 2018;8(1):1126.
- Ewing M, Naredi P, Zhang C, et al. Identification of patients with non-metastatic colorectal cancer in primary care: a case-control study. Br J Gen Pract. 2016;66(653):e880–e886.
- Hamilton W, Lancashire R, Sharp D, et al. The importance of anaemia in diagnosing colorectal cancer: a case-control study using electronic primary care records. Br J Cancer. 2008;98(2):323–327.
- Edgren G, Bagnardi V, Bellocco R, et al. Pattern of declining hemoglobin concentration before cancer diagnosis. Int J Cancer. 2010;127(6):1429–1436.
- Goldshtein I, Neeman U, Chodick G, et al. Variations in hemoglobin before colorectal cancer diagnosis. Eur J Cancer Prev. 2010;19(5):342–344.
- Kinar Y, Akiva P, Choman E, et al. Performance analysis of a machine learning flagging system used to identify a group of individuals at a high risk for colorectal cancer. PLoS One. 2017;12(2):e0171759.
- Kinar Y, Kalkstein N, Akiva P, et al. Development and validation of a predictive model for detection of colorectal cancer in primary care by analysis of complete blood counts: a binational retrospective study. J Am Med Inform Assoc. 2016;23(5):879–890.
- Svenska Kolorektalcancerregistret: Tarmcancerrapport 2018. Regionalt cancercentrum Norr Norrlands universitetssjukhus SE-901 85 UMEÅ: Svenska Kolorektalcancerregistret; 2019. p. 20.
- International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)-WHO Version for; 2016 WHO; 2016 [updated 2018 Jan; cited 2019]. Available from: https://icd.who.int/browse10/2016/en
- WHO VMNIS: Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. [Internet]. Evidence and Programme Guidance Unit Nutrition for Health and Development. World Health Organization. [cited 2019 Jan 30]. Available from: https://www.who.int/vmnis/indicators/haemoglobin.pdf
- Statistics Sweden; 2020 [cited 2021 Jan 21]. Available from: https://www.scb.se/en/
- Siminoff LA, Rogers HL, Harris-Haywood S. Missed opportunities for the diagnosis of colorectal cancer. Biomed Res Int. 2015;2015:1–9.
- Singh H, Daci K, Petersen LA, et al. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am J Gastroenterol. 2009;104(10):2543–2554.
- Yates JM, Logan EC, Stewart RM. Iron deficiency anaemia in general practice: clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad Med J. 2004;80(945):405–410.
- Damery S, Ryan R, Wilson S, Improving Colorectal Outcomes Group, et al. Improving Colorectal Outcomes G. Iron deficiency anaemia and delayed diagnosis of colorectal cancer: a retrospective cohort study. Colorectal Dis. 2011;13(4):e53–e60.
- Droogendijk J, Beukers R, Berendes PB, et al. Screening for gastrointestinal malignancy in patients with iron deficiency anemia by general practitioners: an observational study. Scand J Gastroenterol. 2011;46(9):1105–1110.
- Schop A, Stouten K, Riedl J, et al. Long-term outcomes in patients newly diagnosed with iron deficiency anaemia in general practice: a retrospective cohort study. BMJ Open. 2019;9(11):e032930.
- Haug U, Kuntz KM, Knudsen AB, et al. Sensitivity of immunochemical faecal occult blood testing for detecting left- vs right-sided colorectal neoplasia. Br J Cancer. 2011;104(11):1779–1785.