Abstract
Background
Real-world evidence to support optimal ustekinumab dosing for refractory Crohn’s disease (CD) patients remains limited. Data from a retrospective nationwide chart review study was utilized to explore ustekinumab dosing dynamics and optimization, identify possible clinical predictors of dose intensification, and to evaluate ustekinumab trough concentrations (TCs) and concomitant medication use in Finland.
Methods
Information gathered from17 Finnish hospitals included clinical chart data from 155 adult CD patients who received intravenous ustekinumab induction during 2017–2018. Data on ustekinumab dosing and TCs, concomitant corticosteroid and immunosuppressant use, and antiustekinumab antibodies were analyzed in a two-year follow-up, subject to availability.
Results
Among 140 patients onustekinumab maintenance therapy, dose optimization was required in 55(39%) of the patients, and 41/47 dose-intensified patients (87%) persisted on ustekinumab. At baseline, dose-intensified patient group had significantly higher C-reactive protein (CRP) levels, and at week 16, significantly lower ustekinumab TCs than in patients without dose intensification. Irrespective of dose optimization, a statistically significant reduction in the use of corticosteroids was observed at both 16 weeks and one year, coupled with an increased proportion of patients on ustekinumab monotherapy. Antiustekinumab antibodies were undetectable in all 28 samples from 25 patients collected throughout the study period.
Conclusions
Nearly a third of all CD patients on ustekinumab maintenance therapy, with a history of treatment-refractory and long-standing disease, required dose intensification. These patients persisted on ustekinumab and had significant reduction of corticosteroid use. Increased baseline CRP was identified as the sole indicator of dose intensification.
Trial registration
EUPAS30920
Background
Crohn’s disease (CD) is a chronic inflammatory bowel disorder characterized by multifactorial intestinal inflammation. Several biological and nonbiological drug treatments with various modes of action are available for CD-related inflammation [Citation1]. Despite the increasing selection of available therapies, a considerable proportion of CD patients experience persisting symptomatic or nonsymptomatic inflammation that can cause disability, decreased quality of life and the need for surgical interventions [Citation2]. In the armamentarium of biologicals for treating moderately or severely active CD, the newest approved agent is ustekinumab (Stelara, Janssen-Cilag International NV,Beerse, Belgium), a fully human monoclonal IgG1k antibody targeting the p40 subunit of interleukin 12 and 23. The pivotal ustekinumab induction trials UNITI-1 and UNITI-2 showed significantly higher rates of clinical response and remission in patients receiving ustekinumab compared with patients receiving placebo [Citation3]. Responders from the two induction trials (one intravenous, weight-based induction dose) were randomized to the IM-UNITI maintenance trial that showed significantly higher efficacy in patients receiving ustekinumab compared to placebo group at 44 weeks with both subcutaneous (SC) dosing intervals: 90 mg every eight (q8w) or every 12 weeks (q12w) [Citation3]. A recent posthoc analysis of IM-UNITI long-term extension trial, trough week 152 showed that sustained corticosteroid-free remission was achieved in 53.7% of patients with q8w dosing, in 47.6% of patients with q12w dosing and in 32.4% of patients with dose adjustment between weeks eight and 32 [Citation4]. Recommended SC dosing intervals of ustekinumab as in label, are based on UNITI-1, UNITI-2, and IM-UNITI trial data [Citation5].
In a real-world setting, however, CD patients commencing ustekinumab treatment are often more treatment-refractory than the patients included in clinical trials. The patients have usually used TNF-blocking agents, which are often chosen as the primary biological treatment for CD. Furthermore, the patients have typically experienced several drug switches due to primary or secondary loss of response or intolerance [Citation2]. While ustekinumab real-world studies have shown clinical benefit even in treatment-refractory CD patient cohorts, data on dosing patterns and dose optimization remain limited [Citation6–13].
In addition to efficacy, safety and treatment persistence, concomitant medication use, and treatment immunogenicity remain as crucial factors for decisions on dose optimization. In the IM-UNITI trial, approximately one-third of the study patients received concomitant immunosuppressants [Citation3].The available data, however, do not offer clear guidance for the combination therapy with ustekinumab, although the proportion of antiustekinumab antibodies (AUAs) were equally low in patients on ustekinumab monotherapy and those on combination therapy [Citation14].The low immunogenicity and a clear exposure-response relationship in ustekinumab trough concentrations (TCs) have been reported separately in real-world and clinical studies including ulcerative colitis [Citation3,Citation4,Citation15,Citation16]. However, real-world data on ustekinumab TCs and immunogenicity are scarce among dose-optimized patients.
The aim of the present study was to investigate ustekinumab dosing dynamics and dose optimization, explore possible predictors of dose intensification, ustekinumab TCs and to compare the concomitant use of corticosteroids and immunosuppressants in patients with or without dose intensification utilizing nationwide and long-term real-world data in Finnish CD patients.
Methods
Study design
The present nationwide, retrospective multicenter chart review study, FINUSTE2, assessed the persistence in addition to objective and clinical outcomes of ustekinumab treatment among CD patients at baseline, at 16 weeks, at one year, at 1.5 years and at 2 years. FINUSTE2 was an extension of the previously published FINUSTE study including 48 adult CD patients who had initiated ustekinumab treatment with IV induction during 2017 [Citation9]. In the extension study, additional follow-up data of the previously included 48 patients were obtained, and new hospitals and patients were included.
Ustekinumab therapy was initiated as a part of routine care with a weight-based intravenous (IV) induction dose between 1 January 2017 and 31 December 2018 followed by SC injections. The ustekinumab treatment had to be initiated minimum four months prior to data collection. Data from adult (≥18 years) CD patients were obtained from patient charts by local gastroenterologists and collected to electronic standardized research questionnaires. Collected data of baseline characteristics included sex, age, height, weight, year of CD diagnosis, smoking status, comorbidities and Montreal classification details (age at diagnosis, disease behavior and location and presence of perianal disease). Data on concomitant CD medication, clinical activity, serum ustekinumab TCs and AUAs, objective markers of disease activity (assessed by endoscopies and laboratory tests) were collected at baseline, 16 weeks (± 4 weeks), 1 year (± 1 month), 1.5 years (± 1 month), and 2 years (± 1 month). In addition, data on all changes to ustekinumab dosing during treatment and the reason for such changes were collected regardless of their timing.
Laboratory tests included albumin, hemoglobin, leukocytes, platelets, C-reactive protein (CRP) and fecal calprotectin (fCal). Serum ustekinumab TCs were analyzed with an enzyme-linked immunosorbent assay and serum AUAs with a radioimmunoassay (Sanquin, Amsterdam, The Netherlands). The Simple Endoscopic Score for Crohn’s Disease (SES-CD) and a modified Harvey-Bradshaw Index (mHBI, abdominal palpation omitted) were used for scoring available endoscopic findings and clinical activity, respectively [Citation17,Citation18]. CRP below 3 mg/L was considered normal.
Data were collected between May and August 2019. A more detailed description of data collection has been reported earlier [Citation19].
Statistical analysis
All analyses were performed with Stata MP 14 statistical software (StataCorp 2015, Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). Continuous variables of baseline patient’s characteristics were reported as mean and standard deviation (SD) and categorical variables as proportions. Apart for ustekinumab concentrations, which were reported as mean (±SD), laboratory measures and clinical outcomes were reported as median and interquartile range (IQR) values due to potential skewness and censoring of non-normal distributions. At all assessment timepoints, only patients with continued ustekinumab use were included in the analyses. Ustekinumab use was considered to continue if the date of ustekinumab discontinuance was less than eight weeks from the assessment timepoint. Due to the low number of patients with follow-up data beyond the one-year timepoint, statistical analyses were based on baseline, 16-week and one-year data. The significance of the change from baseline value in laboratory measures and clinical outcomes was tested with Wilcoxon matched-pairs signed-rank test. Pearson's chi-squared test for categorical variables and Wilcoxon rank-sum test for continuous variables were used to measure group differences at any timepoint in patients with and without initial ustekinumab dose intensification during maintenance treatment. For clarity, the patient group ‘without dose intensification’ also included patients whose dose interval was prolonged. Statistical significance (p) threshold was set at .05.
Results
Seventeen Finnish hospitals, including all five Finnish University hospitals, that provide specialist care participated in the study and data from 155 CD patients were collated. At baseline, which was determined by the date of first intravenous ustekinumab dose administration, only five (3.2%) patients had not received prior biologicals for CD. The patients had a long mean disease duration of 14 years and two-thirds of the patients had undergone prior CD-related surgery, indicating a highly treatment-refractory study population. At the time of study data collection, the mean duration of ustekinumab treatment was 11.8 (± 6.1) and 14.9(±6.4) months for patients without and with dose intensification, respectively. Mean follow-up time was 12.7 (±6.5) months for patients without dose intensification and 15.7(±6.0) months with at least one dose intensification. Patient characteristics are shown in . A more detailed analysis of the baseline patient and disease characteristics was previously published [Citation19].
Table 1. Clinical characteristics of CD study patients.
Dosing dynamics
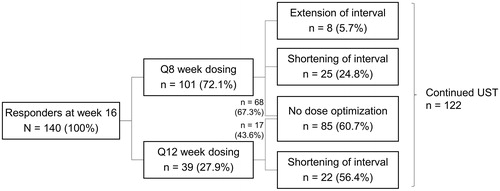
In the entire cohort, 140 (90.3%) out of 155 patients continued to ustekinumab maintenance therapy after a weight based IV induction dose as per label [Citation5]. Dosing interval at the beginning of ustekinumab maintenance therapy was q8w in 101 (72.1%) and q12w in 39 (27.9%) patients. No significant changes were detectable in the baseline characteristics between the two dosing groups (), although there was a trend towards a higher number of prior surgeries in patients assigned to q8w dosing (p = .055, ).
Fifty-five out of 140 patients (39.3%) on ustekinumab maintenance therapy underwent dose optimization (either shortening or prolongation of the dosing interval; ) and altogether 65 dose changes were carried out as eight patients experienced two or three dosing interval adjustments. In 55 patients undergoing dose optimization, the initial change was shortening of the dosing interval (dose intensification) in 47 patients (85.5%). After dose intensification, 41/47 patients (87.2%) persisted on ustekinumab therapy to the end of the follow-up period. The ustekinumab dosing interval was shortened in 24.8% of 101 patients on q8w and in 56.4% of 39 patients on q12w (). At the end of follow-up, the ustekinumab dosing interval was longer than eight weeks only in 21/122 patients (17.2%), whilst the majority (66.4%) of patients received q8w dosing. The dosing interval was shorter than eight weeks in the remaining (16.4%) of the patients the shortest interval being four weeks (q4w). Reasons for shortening of the dosing interval were insufficient response (71.2%), loss of response (13.5%), and other reasons (15.4%) including e.g., ustekinumab concentrations and disease symptoms. Furthermore, eight patients prolonged the dosing interval due to either a satisfactory clinical CD status or other reasons such as financial or compliance problems.Mean duration from the ustekinumab induction to the first dose intensification was 9.4 months (SD, 5.4; median, 8.3).
Figure 1. Dose optimization during the study. Nine out of 140 patients (6.4%) patients without dose optimization discontinued ustekinumab. Seven out of 55 patients (12.7%) with dose optimization discontinued ustekinumab. UST: ustekinumab.
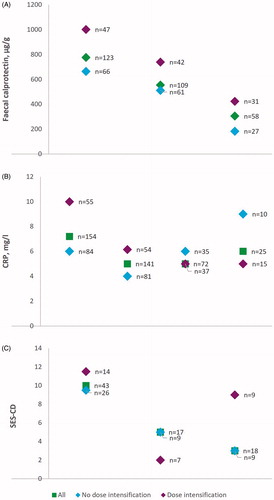
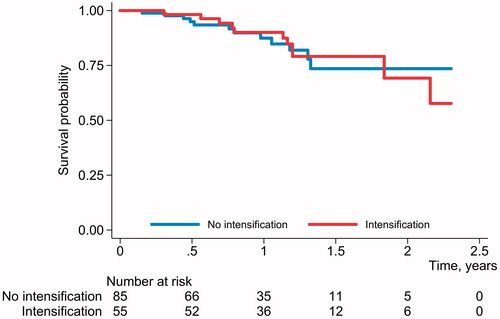
Median baseline CRP was significantly higher in dose-intensified patient group (10 mg/L; IQR, 5–18) compared to the patients without changes in dosing interval (6 mg/L; IQR <3–13; p = .027, ). When grouped according to the number of prior biological therapies (one vs. more than one prior biologicals), no statistically significant difference in the proportion of patients with ustekinumab dose intensification was detected (p = .784). Median fCal, CRP and SES-CD values over time by ustekinumab dosing interval adjustment status are shown in . The differences in the values according to the status of dosing interval adjustment were only statistically significant for the baseline CRP. No differences were detected in Kaplan-Meyer survival analysis based on dose intensification status ().
Ustekinumab trough concentrations and antiustekinumab antibodies
During the study period, a total of 61 ustekinumab TCs were measured from 52 patients (33.5% of all 155 patients) at different timepoints. Mean ustekinumab TCs at 16 weeks, one year and 1.5 years were 2.2 µg/mL (SD, 1.2; median, 2; n = 23), 2.7 µg/mL (SD, 2.6; median, 1.7; n = 25) and 2.5 µg/mL (SD, 2.1; median, 1.65; n = 8), respectively (). Reasons for measuring TCs were: insufficient response (67.2%), concentration monitoring after dose change (12.5%), routine monitoring (12.5%), and other reasons (7.8%). A low number of other ustekinumab measurements (n = 5) were measured outside of the predefined timepoints or at two years.
Figure 4. Box-and-whiskers plot of serum ustekinumab trough concentrations measured at different timepoints. The whiskers present 25 and 75th percentiles, respectively.
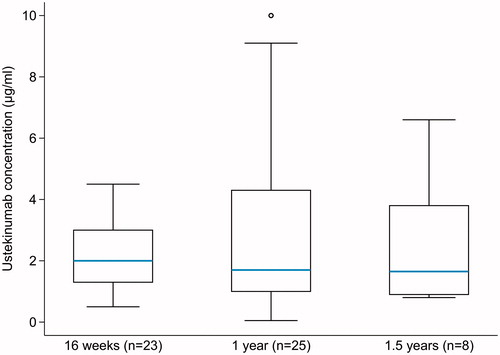
At 16 weeks, TCs were measured from 13 patients who experienced subsequent dose intensification during the maintenance treatment and eight from patients without dose intensification. There was a statistically significant difference in the mean ustekinumab TCs between the aforementioned groups, 1.6 µg/mL (SD, 0.7; median, 2) and 3.1 µg/mL (SD, 1.4; median, 3.65; p = .038), respectively. Mean ustekinumab TCs in patients grouped according to one prior biologic and two or more prior biologics appeared similar at 16 weeks and one year (data not shown). No AUAs were detected in 28 samples taken from 25 patients at different timepoints.
Concomitant medications
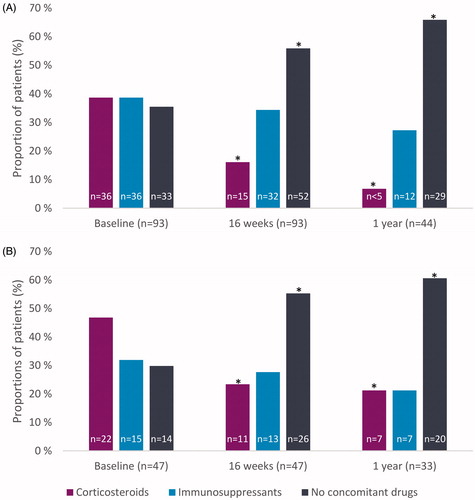
Out of 47 patients with shortened ustekinumabdosing interval, 22 (46.8%) used corticosteroids (prednisolone, prednisone, methylprednisone or budesonide) at baseline while 36/93 (38.7%) patients without dose intensification received corticosteroids at baseline (). In both groups, a statistically significant reduction in the use of corticosteroids was observed at 16 weeks and at one year (p < .05, ). Subsequently, we observed a statistically significant increase in the proportion of patients on ustekinumab monotherapy in both groups from baseline to one year (p < .05). However, there was a trend towards more prominent use of corticosteroids in the patients with dose intensification than without at one year (21 vs. 7%, respectively; p = .063). Overall, 32 and 39% of patients with or without dose intensification, respectively, used immunosuppressants, either thiopurines or methotrexate, at baseline. In both groups the results showed similar use of immunosuppressants during ustekinumab maintenance therapy at one year ().
Discussion
In the present nationwide Finnish real-world study, we evaluated ustekinumab dosing patterns, dose optimization and predictors to dose intensification such as biomarkers, ustekinumab TC and immunogenicity. The use of concomitant corticosteroids and immunosuppressants were studied based on the dose optimization status. The vast majority of the 155 CD patients included in the study started the maintenance treatment with q8w dosing. However, nearly one-third of all patients required a dose intensification, consistent with a highly refractory disease phenotype. At baseline, CRP was the only clear predictor of subsequent dose intensification. Ustekinumab treatment persistence in patients with long-standing and complicated CD remained high, allowing significant corticosteroid tapering followed by increased use of ustekinumab as a monotherapy in patients to manage CD in both patient groups, with or without dose intensification.
Ustekinumab maintenance dosing regimen differs from other biologicals as two different dosing intervals are suggested depending on the achieved induction response. Dosing q12w is appropriate after successful induction whereas q8w is suggested in the case of inadequate response [Citation20]. In the current study, most patients began their maintenance ustekinumab treatment with q8w dosing. There was a trend to a higher number of prior CD surgeries in patients with q8w dosing (p = .055). High frequency of q8w dosing regimen can be explained by the study patient’s treatment-refractory disease and long disease duration, as almost all patients had previously used biologicals. Moreover, approximately one-fourth of the patients had used three or more biologicals. These results differ markedly from the IM-UNITI trial, where approximately 40% of patients entering the ustekinumab maintenance treatment had not used prior antiTNF agents and had a shorter disease duration [Citation3].
While the analysis of dosing patterns in IM-UNITI trial suggested regaining clinical response in 55% of patients following dose intensification from q12w to q8w [Citation3,Citation21], data on dose optimization in real-world situations remains scarce [Citation10,Citation22]. However, some recent studies report clinical outcomes derived from specific real-world cohorts consisting of only dose optimized patients [Citation11,Citation23–25]. Where reported, 22–52% of patients needed dose intensification, which appeared successful in around half of the patients [Citation10,Citation11,Citation22–25].In the present study, one-third of patients on ustekinumab maintenance therapy required dose intensification. Among patients initiating maintenance therapy with q12w dosing, two-thirds shortened the interval. This is a considerably higher proportion likely due to a more disease-refractory patient population when compared to the patients of IM-UNITI trial where only 23% needed dose adjustment from q12w to q8w [Citation21]. Our data on dosing patterns support the findings of other real-world registry studies of ustekinumab maintenance treatment where the majority or all the patients commenced their ustekinumab maintenance therapy with q8w dosing [Citation6,Citation10,Citation12,Citation13,Citation22].
Interestingly, data on recapturing or improvement of ustekinumab response in CD patients with q4w dosing is emerging [Citation11,Citation22–25]. In the present study, a sixth of patients received shorter dosing than q8wat the end of follow-up. In accordance with previous studies from Dalal et al., the most common reason for dose intensification was insufficient treatment response [Citation23]. Importantly 87.2% of patients who underwent a dose intensification persisted on ustekinumab therapy at the end of the follow-up. This is in line with the findings from previous dose-intensified patient cohorts where 61–79% of patients continued ustekinumab treatment at last follow-up [Citation11,Citation24,Citation25].
In the present study, dose-intensified patient group had significantly higher baseline CRP compared to the patients without dose intensification, with a trend towards increased fCal levels as well. Higher baseline CRP may reflect higher inflammatory burden in patients needing shortening the dose interval in agreement with a previous report where77% of dose-intensified patients had elevated CRP [Citation25]. However, in the present study, ustekinumab dose intensification resulted in CRP and fCal responses comparable to the responses of patients without dose intensification. Furthermore, no differences were detectable in endoscopic responses, although the limited number of endoscopy data available limits the interpretation of these results.
Reactive and even proactive therapeutic drug monitoring (TDM) of antiTNF-agent therapy has been suggested in several consensus statements or guidelines, but less guidance is available for ustekinumab TC measurements [Citation26–28]. Reactive ustekinumab TDM, however, has been suggested to be performed in nonresponders at the end of induction and in patients with a secondary loss of response during maintenance treatment [Citation28]. Also, three real-world studies show association between ustekinumab trough concentrations and clinical outcomes [Citation16,Citation29,Citation30]. However, the data on ustekinumab TCs related to dose intensification and their relationship to clinical outcomes is limited. In the present study, insufficient response was the main reason for reactive TDM. Week 16 is reported as the timepoint for ustekinumab concentrations reaching steady state with q8w dosing [Citation31]. In our study, at week 16 the mean ustekinumab TCs measured for patients with later dose intensification was 1.6 µg/mL and without dose intensification significantly higher, 3.1 µg/ml. Both values exceeded the level associated with maintenance of clinical remission (above 0.8–1.4 µg/mL) in the IM-UNITI trial [Citation36]. In IM-UNITI, median ustekinumab TC at 44 weeks ranged from 2.0–2.2 µg/mL in q8w dosing group and from 0.6–0.8 µg/mL in q12w dosing group [Citation32]. Our results are comparable to those reported by Verstockt et al. [Citation29], where the mean ustekinumab TC was 2.6 µg/mL at 16 weeks. A threshold of 1.9 µg/mL at 24 weeks for endoscopic response and 2.2 µg/mL for composite clinical/biochemical remission was suggested in real-world studies [Citation16,Citation29].
No AUAs were detected in 28 samples during the study, in line with three other real-world studies [Citation29,Citation33,Citation34]. In addition, low immunogenicity (4.6% of all patients) has been reported in a long-term follow-up study of IM-UNITI patients through 156 weeks [Citation4]. Immunogenicity was the lowest (2.6%) among CD patients originally randomized to q8w-dosing [Citation4].
We observed a significant reduction in corticosteroid use within first year of ustekinumab maintenance treatment irrespective of ustekinumab dose intensification. Kopylov et al. [Citation25] also report discontinuation of systemic corticosteroids in 67.6% of patients at week 16 after dose intensification to q4w.On the contrary, Haider et al. [Citation22] reported that a higher number of dose-intensified patients received steroids at the end of follow-up, although this study included low patient numbers. In our study, 21% of the patients who underwent a dose intensification were still on corticosteroid treatment at one year, probably due their more refractory CD also indicated by the higher CRP levels at baseline.
Evidence of the evolution of immunosuppressant use, such as thiopurines or methotrexate, during ustekinumab maintenance therapy is limited. Ustekinumab was not included in a large TruveMarketScan database study on combination therapy with immunosuppressants stating that combination therapy may increase the persistence on anti-TNF agents and vedolizumab [Citation35]. Unlike the antiTNF agents, however, a combination of ustekinumab with immunosuppressants does not affect ustekinumab TCs or efficacy [Citation15,Citation31,Citation36]. While real-world studies have reported the baseline use of concomitant immunosuppressants, reports on the evolution of their use during the ustekinumab maintenance or after dose intensification are scarce.
In our study over a third of patients were on immunosuppressants at baseline (). This proportion is in line with several cohorts where 36 to 52% of patients used immunosuppressants at baseline [Citation10,Citation11,Citation13, , Citation33], but somewhat higher than 5 to 22% range observed in other studies [Citation6,Citation8,Citation9]. The significant increase of patients on ustekinumab monotherapy resulted mostly from reduction in the use of corticosteroids in both patient groups, with or without dose intensification. Out of 77 patients on ustekinumab maintenance therapy at one year, 21% with dose intensification and 27% without dose intensification used immunosuppressants. These proportions are slightly less compared to the proportion reported in IM-UNITI long-term extension where 35.4% of patients on q8w dosing were taking concomitant immunosuppressants through 92 weeks [Citation37]. Iborra et al. report that 28% of the patients who were using concomitant immunosuppressants at baseline were able to discontinue them completely during the follow-up, although these data were not stratified based on dose intensification status [Citation10]. It is important also in refractory CD patients to achieve comparable treatment efficacy with biological monotherapies as with combination therapies because potential adverse effects of thiopurines are well known. The use of thiopurines increases the risk of opportunistic infection up to 2–3 fold [Citation38]. Additionally, the use of thiopurines increases the risk of non-Hodgkin lymphoma, and especially Epstein Barr virus seronegative young males are at risk of severe postmononucleosis lymphoma [Citation37]. Additionally, the use of thiopurines increases the risk of urinary tract cancers in elderly men [Citation39].
The retrospective design and setting of our nationwide study resulted in incomplete data collection for some clinical and laboratory variables. Data on ustekinumab dose adjustments were collected regardless of their timing whereas data on clinical outcomes and laboratory variables, including ustekinumab TCs, were collected according to study protocol timepoints, subject to availability. Due to the variation in the data collection timepoints, we could not confidently assess the association between ustekinumab dose adjustments and efficacy outcomes. Limited endoscopic data availability may be partly due to the lack of national guidelines for follow-up of endoscopic response and varying local endoscopic resources between different IBD units. Despite these limitations, the current study adds valuable knowledge in real-world setting on individualized treatment regimens, related concomitant medication, TCs and low immunogenicity of ustekinumab among CD patients.
Conclusion
In this real-world study of CD patients in Finland, including two-year follow-up, most patients commenced ustekinumab maintenance treatment with a q8w dosing interval, whilst nearly a third of all patients required shortening of the dosing interval, suggesting a highly refractory disease phenotype. High baseline CRP was found as a sole indicator for dose intensification. Majority of patients, irrespectively of dose optimization, persisted on ustekinumab at one year and showed significantly reduced concomitant corticosteroid use. Ustekinumab mean TCs were significantly lower in patients with subsequent dose intensification at week 16. No antibodies to ustekinumab were detected, which indicated low immunogenicity.
Ethical approval
The ethics committee of Tampere University Hospital reviewed the amended study protocol (R18055), followed by approvals from the local register holders. The European Union electronic Register of Post-Authorization Studies (EUPAS Register) has registered the study extension (EUPAS30920).
Author contributions
Study design and idea: AB, TH, MRK, TS, ES and CW; Data acquisition: C-GaB, AE, MH, MiH, EH, TI, MK, RK, CN, HN, TS, U-MS and JT; Analysis of data: C-GaB, AB, TH, MRK, TS, ES and CW; Writing of manuscript: C-GaB, TH, MRK, TS, ES and CW; Revision of manuscript: C-GaB, AB, AE, TH, MH, MiH, EH, TI, MRK, RK, CN, HN, TS, ES, U-MS, JT and CW. All authors have read and approved the manuscript in the current state.
Acknowledgements
The authors thank Petri Mankinen for his expertise in the study management, Kai Kysenius for language editing and the members of FINUSTE study group for data collection: Kalle Hakala, Eija Hirsi, Urpo Nieminen, Airi Jussila, Inka Koskinen, Veikko Moilanen, Karri Utriainen, and Ilkka Vihriälä.
Disclosure statement
TS has received speaker or consultant fees from Abbvie, Ferring, Janssen-Cilag, Pfizer, Takeda and Tillotts Pharma in addition to research grants from Janssen-Cilag and Takeda.
C-GaB has received consultant, lecture or congress fees from Abbvie, Meda, MSD, Mylan, Ferring, Janssen-Cilag, Pfizer, Ratiopharm, Takeda and Tillotts Pharma.
TI has received consultant, lecture or congress fees from AbbVie, Ferring, Janssen-Cilag, MSD, Pfizer, Takeda and Tillotts Pharma.
TH and ES are partners and employees of ESiOR Oy and report grants and nonfinancial support to ESiOR Oy from Janssen-Cilag during the conduct of the study.
AE has received research grant from Janssen-Cilag and consultant fees from MSD, Pfizer, Takeda and Janssen-Cilag.
MH has received lecture or congress fees from Abbvie, MSD, Tillots Pharma and Takeda.
MK has received consultant fees from Takeda.
RK has received consultant, lecture or congress fees from Ferring, Janssen-Cilag, MSD, Pfizer, Takeda, Tillotts Pharma and Vifor Pharma.
CN has received congress fees from Pfizer and Vifor Pharma.
HN has received lecture fees from Abbvie, Ferring, Janssen-Cilag, MSD, Pfizer, Sanofi and Takeda.
MiH has received consultant or congress fees from Janssen-Cilag, Ferring and Tillotts Pharma.
U-MS declares no conflicts of interest.
JT has received lecture or consultant fees from Janssen-Cilag and Pfizer.
CW, AB, and MRK. are all employees of Janssen-Cilag.
Additional information
Funding
References
- Hindryckx P, Vande Casteele N, Novak G, et al. The expanding therapeutic armamentarium for inflammatory bowel disease: how to choose the right drug[s] for our patients? J Crohns Colitis. 2018;12(1):105–119.
- Danese S, Bonovas S, Peyrin-Biroulet L. Positioning ustekinumab in Crohn's disease: from clinical evidence to clinical practice. J Crohns Colitis. 2017;11(10):1258–1266.
- Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–1960.
- Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: Three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn's disease. J Crohns Colitis. 2020;14(1):23–32.
- stelara-epar-product-information_en.pdf. [cited 2020 Dec 7]. https://www.ema.europa.eu/en/documents/product-information/stelara-epar-product-information_en.pdf.
- Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, et al. Ustekinumab for Crohn's disease: results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J Crohns Colitis. 2020;14(1):33–45.
- Eberl A, Hallinen T, Af Björkesten C-G, et al. Ustekinumab for Crohn's disease: a nationwide real-life cohort study from Finland (FINUSTE) ). Scand J Gastroenterol. 2019;54(6):718–725.
- Liefferinckx C, Verstockt B, Gils A, et al. Long-term clinical effectiveness of ustekinumab in patients with Crohn's disease who failed biologic therapies: a national cohort study. J Crohns Colitis. 2019;13(11):1401–1409.
- Hoffmann P, Krisam J, Wehling C, et al. Ustekinumab: “real-world” outcomes and potential predictors of nonresponse in treatment-refractory Crohn's disease. World J Gastroenterol. 2019;25(31):4481–4492.
- Iborra M, Beltrán B, Fernández-Clotet A, et al. Real-world long-term effectiveness of ustekinumab in Crohn's disease: results from the ENEIDA registry. Aliment Pharmacol Ther. 2020;52(6):1017–1030.
- Ollech JE, Normatov I, Peleg N, et al. Effectiveness of ustekinumab dose escalation in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2021;19(1) :104–110.
- Bar-Gil Shitrit A, Ben-Ya’acov A, Siterman M, et al. Safety and effectiveness of ustekinumab for induction of remission in patients with Crohn’s disease: a multicenter Israeli study. United European Gastroenterol J. 2020;8(4):418–424.
- Harris RJ, McDonnell M, Young D, et al. Early real-world effectiveness of ustekinumab for Crohn's disease. Frontline Gastroenterol. 2020;11(2):111–116.
- Bots S, Gecse K, Barclay M, et al. Combination Immunosuppression in IBD. Inflamm Bowel Dis. 2018;24(3):539–545.
- Adedokun OJ, Xu Z, Marano C, et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(10):2244–2255.e9.
- Gómez Espín R, Nicolás De Prado I, Gil Candel M, et al. Association between ustekinumab trough concentrations and biochemical outcomes in patients with Crohn’s disease. A real life study. Rev Esp Enferm Di. 2021;113(2):110–115.
- Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60(4):505–512.
- Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet Lond Engl. 1980;315(8167):514.
- Af Björkesten C-G, Ilus T, Hallinen T, et al. Objectively assessed disease activity and drug persistence during ustekinumab treatment in a nationwide real-world Crohn’s disease cohort. Eur J Gastroenterol Hepatol. 2020;32(12):1507–1513.
- Samaan M, Campbell S, Cunningham G, et al. Biologic therapies for Crohn’s disease: optimising the old and maximising the new. F1000Res. 2019;8:1210.
- Sands B, Gasink C, Jacobstein D, et al. OC-045 Efficacy & safety of dose adjustment & delayed response to ustekinumab in moderate-severe Crohn’s disease patients: results from IM-UNITI maintenance study. Gut. 2017;66(Suppl 2):A23.
- Haider SA, Yadav A, Perry C, et al. Ustekinumab dose escalation improves clinical responses in refractory Crohn’s disease. Therap Adv Gastroenterol. 2020;13:175628482095924.
- Dalal RS, Njie C, Marcus J, et al. Predictors of ustekinumab failure in Crohn’s disease after dose intensification. Inflamm Bowel Dis. 2020. DOI:10.1093/ibd/izaa282
- Fumery M, Peyrin-Biroulet L, Nancey S, et al. Effectiveness and safety of ustekinumab intensification at 90 mg every four weeks in Crohn’s disease: a multicenter study. J Crohns Colitis. 2021;15(2):222–227.
- Kopylov U, Hanzel J, Liefferinckx C, et al. Effectiveness of ustekinumab dose escalation in Crohn's disease patients with insufficient response to standard-dose subcutaneous maintenance therapy . Aliment Pharmacol Ther. 2020;52(1):135–142.
- Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153(3):827–834.
- Mitrev N, Vande Casteele N, Seow CH, et al. Consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46(11–12):1037–1053.
- Papamichael K, Cheifetz AS, Melmed GY, et al. Appropriate therapeutic drug monitoring of biologic agents for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019;17(9):1655–1668.e3.
- Verstockt B, Dreesen E, Noman M, et al. Ustekinumab exposure-outcome analysis in Crohn's disease only in part explains limited endoscopic remission rates. J Crohns Colitis. 2019;13(7):864–872.
- Soufflet N, Boschetti G, Roblin X, et al. Concentrations of ustekinumab during induction therapy associate with remission in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2019;17(12):2610–2612.
- Adedokun OJ, Xu Z, Gasink C, et al. Pharmacokinetics and exposure response relationships of ustekinumab in patients with Crohn's disease. Gastroenterology. 2018;154(6):1660–1671.
- Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy. Aliment Pharmacol Ther. 2018;48(1):65–77.
- Painchart C, Brabant S, Duveau N, et al. Ustekinumab serum trough levels may identify suboptimal responders to ustekinumab in Crohn's disease. Dig Dis Sci. 2020;65(5):1445–1452.
- Battat R, Kopylov U, Bessissow T, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2017;15(9):1427–1434.e2.
- Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis. 2019;25(8):1417–1427.
- Restellini S, Khanna R, Afif W. Therapeutic drug monitoring with ustekinumab and vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(10):2165–2172.
- Ghosh S, Kramer BC, Gasink C, et al. P680 Long-term efficacy of ustekinumab with and without concomitant immunosuppressants for Crohn’s disease: results from IM-UNITI long-term extension through 2 years. J Crohns Colitis. 2019;13(Supplement_1):S459–S460.
- Axelrad JE, Roy A, Lawlor G, et al. Thiopurines and inflammatory bowel disease: current evidence and a historical perspective. World J Gastroenterol. 2016;22(46):10103–10117.
- Bourrier A, Carrat F, Colombel J-F, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther. 2016;43(2):252–261.