Abstract
Background and aims
Patients with liver cirrhosis have high mortality, often estimated by the Child–Pugh or MELD scores. Etiologies of cirrhosis are rapidly shifting, and it is unclear if these scores perform similarly across subgroups of patients. Here, we describe the characteristics and outcomes of a large contemporary cohort of patients with cirrhosis.
Methods
This was a cohort study with retrospectively collected data. All patients with a verified diagnosis of cirrhosis during 2004–2017 at the Karolinska University Hospital, Sweden, were identified. Data at baseline to calculate Child–Pugh, MELD and confounders for mortality was collected. Competing risk regression was used to estimate risk for outcomes, adjusted for age, sex, baseline Child–Pugh score, etiology of cirrhosis and type 2 diabetes.
Results
We identified 2609 patients, with a median age of 61 years, and 68% men. Etiologies of cirrhosis shifted during the study period, with a −29% relative decrease in hepatitis C-cirrhosis and a + 154% increase in cirrhosis due to non-alcoholic fatty liver disease. The highest overall mortality was seen in patients with alcohol-related cirrhosis. MELD and Child–Pugh scores predicted 3-month and 1 to 2-year mortality reasonably well, but with a lower predictive performance in alcohol-related cirrhosis. Men were more likely than women to receive a liver transplant (sHR = 1.39, 95%CI = 1.08–1.78).
Conclusions
We confirm previous findings of a rapid shift in the etiologies of cirrhosis. Differences in sex in regard to access to liver transplantation deserve further attention.
Introduction
The landscape of cirrhosis is rapidly changing, in particular as cases caused by hepatitis C are dwindling but new cases caused by non-alcoholic fatty liver disease (NAFLD) are increasing [Citation1–7]. Therapeutic options in cirrhosis remain limited, with liver transplantation often being the only reasonable option at advanced stages. Nevertheless, mortality is high due to patients often being ineligible for liver transplantation and a scarcity of organs [Citation8]. Making a prognosis for patients with cirrhosis can be difficult. The risk of 3-month mortality can be estimated using the MELD score [Citation9,Citation10], and 1- and 2-year mortality can be estimated with the Child–Pugh score [Citation11]. These scores are commonly used to risk-stratify patients in the clinical setting. An unanswered question is if these scores perform similarly across categories of patients, such as different sex or causes of cirrhosis, in terms of prediction. In fact, due to the inclusion of creatinine (which differs between men and women), the MELD score has been suggested to lead to lower chances for women to receive a liver transplant [Citation12].
Here, we describe the characteristics and outcomes of patients with cirrhosis at a major tertiary referral centre in Sweden.
Material and methods
Study population
The Karolinska University Hospital is one of the largest in northern Europe, with around 1300 hospital beds and is a major referral centre for hepatology in the Stockholm region (population around 2.3 M in 2019). It is one of two liver transplant centres in Sweden. We first identified all patients with a diagnosis of cirrhosis ever evaluated at the hospital during the period of 1 January 2004 to 31 December 2017. Patients were identified by a search in the local electronic healthcare record (EHR) database (fully implemented as of 1 January 2004) and defined as ICD-10 codes corresponding to cirrhosis (K70.3, K74.6, B18.1G or B18.2G). All patient charts were reviewed according to a pre-specified template. The diagnosis of cirrhosis was validated and defined to be present if at least one of the following criteria were met: A liver biopsy with fibrosis stage 4; unequivocal radiological findings of cirrhosis (such as shrunken, nodular liver or presence of portal hypertension in absence of competing causes); a transient elastography of good quality with at least 15 kPa [Citation13]; a clinical diagnosis made by a specialist in gastroenterology or infectious diseases based on clinical or laboratory findings (e.g., findings of oesophageal varices or ascites).
Exclusion criteria were age <18 years, missing data to evaluate the presence of cirrhosis, not meeting our criteria for cirrhosis, and patients with existing diagnoses of cirrhosis routinely followed at the hospital at the start of the study period.
The baseline was defined as the first visit where a diagnosis of cirrhosis was made in a non-emergent setting. For instance, a person who survived an episode of bleeding oesophageal varices and was seen at an outpatient follow-up had a baseline defined at the later date. This was done so that biochemical parameters would not be affected by acute conditions such as sepsis or kidney injury.
Subgroups
The etiology of cirrhosis was defined as chronic viral hepatitis (B or C); alcohol-related liver disease (ALD); NAFLD; autoimmune liver disease (AIH, PBC or PSC); or other rare causes (including hereditary hemochromatosis, alpha-1-AT deficiency, cryptogenic cirrhosis, Wilsons’ disease and other causes). The definitions of these are presented in Supplementary Material eTable 1. A patient could be classified as having several aetiologies. However, in a subgroup analysis, we defined six major diagnostic groups: Alcohol-related liver disease; chronic viral hepatitis; combined viral hepatitis and alcohol-related liver disease; NAFLD; autoimmune liver disease; and other causes.
We specifically investigated differences between men and women.
Variables
Parameters extracted at baseline are presented in . These included age at baseline, sex, etiology of cirrhosis as defined above, biochemical parameters to calculate Child–Pugh and MELD scores, presence and grade of ascites and hepatic encephalopathy (HE), type 2 diabetes, height and weight. Baseline parameters are defined in Supplementary Material eTable 1.
Table 1. Baseline characteristics of the full cohort and stratified on sex.
Follow-up
Patients were followed in the electronic medical charts to ascertain events of liver transplantation and overall mortality. If patients migrated from the Stockholm region or to other caregivers in the region outside of the used EHR system, they were considered to be lost to follow-up. However, overall mortality could be ascertained in all cases for the full follow-up period as the electronic healthcare system is continuously linked to national registers on mortality [Citation14]. Follow-up was until 31 December 2018 which allowed for at least one year of possible follow-up in all study participants.
Statistical analysis
Continuous parameters are presented as medians with interquartile ranges, and categorical parameters as total numbers and percentages. Differences in groups were compared using the Mann–Whitney test, Student’s t-test, Chi-square or Fischer’s exact test as appropriate. A non-parametric test for trend was used to evaluate time trends of cirrhosis etiology during the follow-up period.
We used a competing risk regression modelling to evaluate the risk for transplant-free, overall mortality across subgroups [Citation15]. In this analysis, receiving a transplant was considered a competing risk, as this significantly alters the natural history. A separate model was used to assess the chance of receiving a liver transplant, considering overall mortality as the competing risk. These models were adjusted for a priori defined confounders, including age, sex, baseline CTP-score, type 2 diabetes and etiology of cirrhosis, unless otherwise specified below, in subgroup analyses.
Time-dependent area under the receiver operating characteristics (AUROC) curves [Citation16] were used to evaluate the performance of the MELD score to predict 3-month transplant-free overall mortality, and the Child–Pugh score to predict 1-year and 2-year transplant-free overall mortality across subgroups.
Ethical considerations
The study was approved by the regional ethics board in Stockholm, dnr 2016/1772-31 and 2018/450-32.
Results
During the inclusion period, there were 3224 diagnoses of cirrhosis made. At chart review, 211 patients (6.5%) had missing data, rendering evaluation of the presence of cirrhosis impossible. The presence of cirrhosis could not be verified in 184 cases (6.1%), corresponding to a positive predictive value of 93.9% for a presence of an ICD-10 code corresponding to cirrhosis. We further excluded 220 patients (7.7%) with the first diagnosis of cirrhosis prior to 2004, leaving 2609 patients as the final study sample (Supplementary Material eFigure 1).
Changes in etiologies of cirrhosis during time
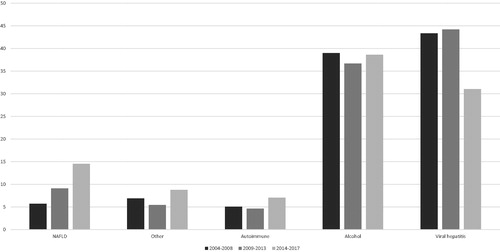
Baseline characteristics are presented in . The median age was 62 years (IQR 54–69), and 1769 (68%) were men. For the full study period, viral hepatitis (considering both viral hepatitis only and viral hepatitis with alcohol-related liver disease) contributed to most cases of cirrhosis (40.2%). However, there were considerable changes during the study period, with all viral hepatitis cases decreasing from 43.4% in the first 5 years (2004–2008) to 31.0% in the final four years (2014–2017) (ptrend < .001). Conversely, NAFLD increased from 5.7% of all cases of cirrhosis in the first five years to 14.5% in the final four years (ptrend < .001). The proportion of autoimmune liver diseases, ALD and other causes of cirrhosis were generally stable during the study period (). Absolute numbers of patients evaluated during the same period are presented in Supplementary Material eFigure 2.
Mortality and sex differences
Differences at baseline between men and women are presented in . During a median follow-up of 3.4 years (range 0–14.8, 10,137 person-years), a total of 1582 (60.6%) patients either died without transplantation (n = 1228, 54.5%) or underwent liver transplantation (n = 354, 13.6%). Transplant-free overall mortality was slightly higher in men compared with women after adjustment for baseline age, etiology of cirrhosis, Child–Pugh score and presence of type 2 diabetes (subdistribution HR [sHR] 1.13, 95%CI = 0.99–1.29).
Overall mortality was significantly different between the etiologies of cirrhosis, with the highest mortality seen in patients with ALD, viral hepatitis and ALD, and the ‘Other’ category (). Results from the adjusted competing risk regression model are presented in .
Figure 2. Cumulative incidence of overall transplant-free mortality stratified on etiology of cirrhosis during the study period, considering liver transplantation as a competing risk. Model is adjusted for age, sex, type 2 diabetes and Child–Pugh score at baseline.
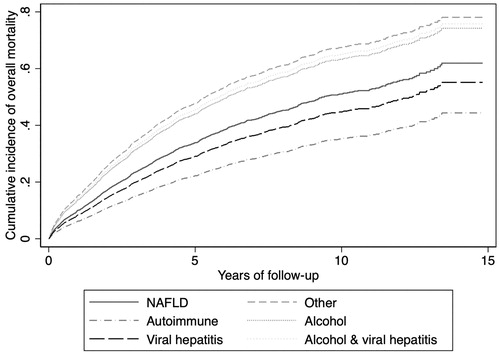
Table 2. Number of persons with different etiologies of cirrhosis, seen at Karolinska University Hospital between 2004–2017, together with number of deaths prior to liver transplantation, and performed liver transplantations.
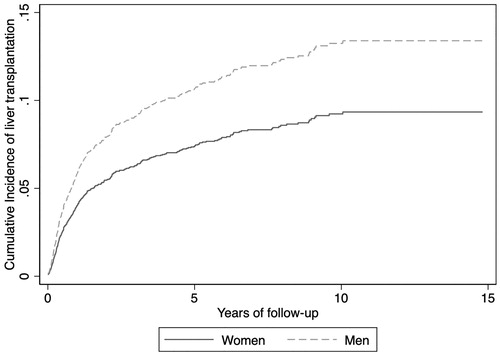
Liver transplantation was more frequently done in men (256 cases, 14.5%) than in women (98 cases, 11.7%, p = .05). After adjustment for age, Child–Pugh score, etiology of cirrhosis and type 2 diabetes, men had a higher chance of receiving a liver transplant (sHR = 1.39, 95%CI = 1.08–1.78) ().
Predictive capacity of MELD and Child–Pugh
Within 3 months from baseline, 141 patients (5.4%) died, and 65 patients had undergone liver transplantation. The capacity of MELD to identify those that died within three months without a transplant was good (AUROC 0.832), and slightly higher for women (AUROC 0.861) compared to men (AUROC 0.815). AUROCs differed across different aetiologies of cirrhosis (0.790–0.872, ).
Table 3. Number of deaths and time-dependent area under the receiver operating characteristics curve for MELD and Child–Pugh scores to predict overall transplant-free mortality at specified timepoints considering liver transplantation as a competing risk.
In a year, 361 patients had died (13.8%), and in total 190 patients had undergone liver transplantation. The capacity of Child–Pugh to predict 1-year transplant-free overall mortality was good for the full cohort (AUROC 0.753), for both women (AUROC 0.757) and men (AUROC 0.751). A lower AUROC was observed for ALD-cirrhosis (0.691) compared to the other aetiologies (AUROCs 0.723–0.834).
Finally, at 2 years, 704 patients (27.0%) had died, and 251 persons had undergone liver transplantation. The capacity of Child–Pugh score at baseline to predict 2-year transplant-free overall mortality was lower than for the other time points for the full cohort (AUROC 0.697) and was slightly lower for women (AUROC 0.685) than in men (AUROC 0.702). Again, the lowest AUROC for the different causes of cirrhosis was found for ALD-cirrhosis (AUROC 0.641).
summarizes these time-dependent AUROCs for the different time points and subgroups.
Discussion
In this large, contemporary cohort of patients with cirrhosis, we show that NAFLD is an increasingly important contributor to cirrhosis also in Sweden, where the prevalence of obesity and type 2 diabetes are comparably low from a global perspective [Citation17–19]. The trajectories indicate that NAFLD will become a key cause of cirrhosis in the coming years, but that ALD is still the main cause of cirrhosis with no sign of a reduction in incidence. We further demonstrate a marked reduction in the proportion of cirrhosis caused by viral hepatitis. This is consistent with previous findings from our region in etiologies for the need of a liver transplant [Citation7] and indicates that this is true also for a broad population of persons with cirrhosis, as also seen elsewhere [Citation2,Citation20,Citation21].
The ability of Child–Pugh and MELD scores to predict short- and moderate-term mortality was acceptable and consistent with previous studies [Citation22]. However, the predictive power was reduced with time, as shown by the lower AUROCs for Child–Pugh at 2 years, highlighting the importance of repeated testing. Also, predictive capacity was slightly lower in patients with ALD.
We found no major differences in the predictive power for Child–Pugh or MELD between men and women, but men had a slightly higher risk for all-cause mortality. However, women were less likely to receive a liver transplant despite similar characteristics at baseline. We cannot, based on these results, know if this was because of bias in the transplant evaluation, or due to unmeasured confounding. However, the results are similar to other findings in previous publications [Citation12], and should be a topic for future, more detailed studies. Indeed, a recent study has also shown that women are at a higher risk for death while on the transplant waiting list [Citation23].
These results can be contrasted to other Swedish studies. For instance, we show a lower overall transplant-free mortality and roughly doubled transplantation rate than a recent study from southern Sweden [Citation24]. These differences could possibly be explained by selection bias in those patients with a low likelihood of compliance after transplantation are less likely to be referred to a transplant centre. NAFLD increased as a cause of cirrhosis between 2010 and 2018 also in a study from the southern Swedish region Halland, but with a lower proportion causing 5.7% of all cirrhosis cases [Citation21], compared to 9.6% seen here.
Strengths of this study include a large number of patients in different subgroups, increasing precision for the statistical analyses. Data were collected according to a structured protocol with a high bar for defining cirrhosis, increasing internal validity. We have a complete follow-up for mortality due to the high-quality national registers [Citation14].
Limitations include a risk for selection bias as the hospital is a regional referral centre for patients with advanced cirrhosis and for transplant evaluations. We did not include diagnoses of decompensated cirrhosis to define the presence of cirrhosis, which might have led to missing such cases, but we believe that doing this would risk even more selection bias in including patients with more severe disease. As data were collected retrospectively, patients were not followed in a protocol-mandated approach with some resulting missing data. In many instances, however, missing data could be collected from other caregivers thanks to the same electronic healthcare chart system being in place for most caregivers in the Stockholm region. Theoretically, liver transplants could have been performed in the only other liver transplant centre in Sweden (Gothenburg), but we find that such rare cases would be unlikely to affect our findings.
Conclusions
In this large, contemporary cohort of patients with a verified diagnosis of cirrhosis, NAFLD increased by 154% as a cause of cirrhosis, suggesting that healthcare systems will need to adapt to this new situation. Mortality and access to liver transplantation differed significantly between etiologies. We further found that men were more likely to receive a liver transplant than women, in spite of slightly higher mortality, which should be further addressed.
Author contributions
Study conception and design: HH; Acquisition of data: HH, AL; Statistical analysis: HH; Analysis and interpretation of data: All; Drafting of manuscript: HH; Critical revision: All; Guarantor of article: HH. All authors approved the final version of the article, including the authorship list.
Supplemental Material
Download MS Word (42.8 KB)Acknowledgements
The authors thank medical students Heval Batti, Mariana Maksi Schao, Nelly Pater, Julie Wulff and Hanne Åström for assistance in data collection.
Disclosure statement
HH has received research grants to his institution from Astra Zeneca, EchoSens, Gilead, Intercept and Pfizer. He is a Board advisory member for Bristol Myers-Squibb and Gilead. These are all outside of the submitted work.
References
- Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896–904.
- Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735.
- Liu ZQ, Jiang YF, Yuan HB, et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70(4):674–683.
- Sepanlou SG, Safiri S, Bisignano C, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(3):245–266.
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171.
- Belli LS, Perricone G, Adam R, et al. Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69(4):810–817.
- Holmer M, Melum E, Isoniemi H, et al. Nonalcoholic fatty liver disease is an increasing indication for liver transplantation in the Nordic countries. Liver Int. 2018;38(11):2082–2090.
- Toniutto P, Zanetto A, Ferrarese A, et al. Current challenges and future directions for liver transplantation. Liver Int. 2017;37(3):317–327.
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31(4):864–871.
- Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96.
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85.
- Mindikoglu AL, Regev A, Seliger SL, et al. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16(10):1147–1157.
- Lim JK, Flamm SL, Singh S, et al. American Gastroenterological Association Institute Guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology. 2017;152(6):1536–1543.
- Brooke HL, Talback M, Hornblad J, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
- Cattaneo MM, Spinelli D. Estimating receiver operative characteristic curves for time-dependent outcomes: the stroccurve package. The Stata J. 2017;4:1015–1023.
- Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. New Eng J Med. 2017;377(1):13–27.
- Ezzati M, Bentham J, Di Cesare M, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642.
- Ogurtsova K, Fernandes J, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
- Enomoto H, Ueno Y, Hiasa Y, et al. Transition in the etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol. 2020;55(3):353–362.
- Vaz J, Eriksson B, Strömberg U, et al. Incidence, aetiology and related comorbidities of cirrhosis: a Swedish population-based cohort study. BMC Gastroenterol. 2020;20(1):84.
- Peng Y, Qi XS, Guo XZ. Child–Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: a systematic review and meta-analysis of observational studies. Medicine. 2016;95(8):e2877.
- Locke JE, Shelton BA, Olthoff KM, et al. Quantifying sex-based disparities in liver allocation. JAMA Surg. 2020;155(7):e201129.
- Nilsson E, Anderson H, Sargenti K, et al. Clinical course and mortality by etiology of liver cirrhosis in Sweden: a population based, long-term follow-up study of 1317 patients. Aliment Pharmacol Ther. 2019;49(11):1421–1430.