Abstract
Background and aims
Upper gastrointestinal (GI) endoscopy is frequently performed in patients with upper abdominal symptoms. Although guidelines recommend withholding an endoscopy in the absence of alarm symptoms, dyspeptic symptoms remain a predominant indication for endoscopy. We aimed to investigate the yield of upper GI endoscopy in patients with low-risk dyspeptic symptoms.
Methods
We conducted an analysis in a prospectively maintained endoscopy reporting database. We collected the results of all upper GI endoscopy procedures between 2015 and 2019 that was performed in adult patients aged <60 years with dyspeptic symptoms. Patients with documented alarm symptoms were excluded. We categorized endoscopic findings into major and minor endoscopic findings.
Results
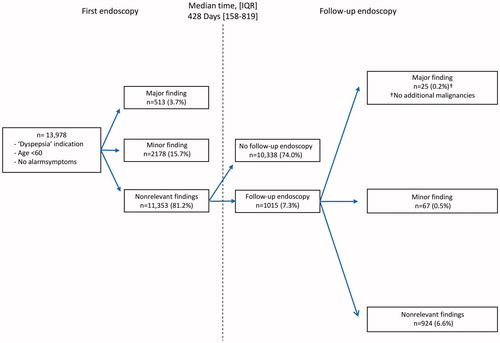
We identified 26,440 patients with dyspeptic symptoms who underwent upper GI endoscopy. A total of 13,978 patients were considered low-risk and included for analysis (median age 46 years, interquartile range (IQR) [36–53], 62% female). In 11,353 patients (81.2%), no endoscopic abnormalities were detected. Major endoscopic findings were seen in 513 patients (3.7%) and minor endoscopic findings in 2178 patients (15.6%). Endoscopic findings indicative of upper GI cancer were reported in 47 patients (0.3%), including 16 (0.1%) oesophageal, 28 (0.2%) gastric and 5 (0.04%) duodenal lesions. Despite an initial unremarkable endoscopy result, 1015 of 11,353 patients (8.9%) underwent a follow-up endoscopy after a median of 428 days [IQR 158–819]. This did not lead to the additional identification of malignancy.
Conclusions
The yield of upper GI endoscopy in low-risk (<60 years, no alarm symptoms) patients with dyspepsia is very limited. This study further supports a restrictive use of upper GI endoscopy in these patients.
Introduction
Upper gastrointestinal (GI) endoscopy plays a central role in the evaluation of upper abdominal symptoms. The current consensus is to perform upper GI endoscopy in patients presenting with alarm symptoms including dysphagia, suspicion of an upper GI bleeding or new onset symptoms above the age of 60 years [Citation1,Citation2].
Consequently, current consensus is to withhold endoscopy in patients not fulfilling these criteria. However, upper GI endoscopy remains often performed in patients with only dyspeptic symptoms [Citation3–5]. As a result, overuse of upper GI endoscopy is still a relevant concern. It is associated with patient burden, incremental healthcare expenditure and pressure on available endoscopy centre resources [Citation6].
The most common less appropriate indications for upper GI endoscopy are dyspepsia-related symptoms such as upper abdominal pain, bloating, nausea and early satiety in the absence of alarm symptoms [Citation7]. Need for reassurance of non-significance in a patient and exclusion of significant pathology are important reasons for non-adherence to guidelines [Citation8]. Previous studies have suggested that the diagnostic yield of upper GI endoscopy in these patients is low [Citation9]. However, the exact yield of upper GI endoscopy procedures in these patients including the yield of repeated upper GI endoscopy when a previous investigation did not show abnormal findings remains unclear.
The definitions of dyspepsia developed by the Rome committee are the most accepted definitions of dyspepsia [Citation10]. The Rome definitions have been developed to better differentiate between patients with gastro-esophageal reflux disease or with functional dyspepsia for inclusion in studies. There is however considerable overlap in symptom presentation in these patients, making the Rome definitions less suitable for everyday clinical practice [Citation11,Citation12]. Another, more suitable definition for clinical practice, is that of the American College of Gastroenterology (ACG) and the Canadian Association of Gastroenterology (CAG) defining dyspepsia as the presence of predominant epigastric pain lasting one month, which can be associated with upper GI symptoms such as epigastric fullness, nausea, vomiting or heartburn [Citation1].
This study aimed to evaluate the yield of upper GI endoscopy in patients below the age of 60 who presented with a clinical definition of dyspepsia in the absence of alarm symptoms. Second, we determined the yield of follow-up upper GI endoscopy performed in these patients with a previous normal endoscopy.
Materials and methods
Database and data collection
For this study, we analysed data from the Trans.IT database (Rotterdam, The Netherlands). The Trans.IT database is an anonymized multicentre database that was developed in 2012 and collects GI endoscopy report data of 20 Dutch hospitals (3 academic and 17 non-academic hospitals). All participating sites use a structured reporting tool developed by Trans.IT to build up and construct all endoscopy reports. The structured reporting tool is incorporated in the report system of Endobase (Olympus Europe, Hamburg, Germany). Trans.IT developed extended ICD-10 codes for all endoscopic findings, interventions and complications allowing for systematic and uniform data collection [Citation13].
Endoscopists create a report of the endoscopic procedure in the structured reporting tool immediately following endoscopy [Citation14,Citation15]. All anonymized endoscopy reports are automatically uploaded into the database [Citation16]. Patient age, gender, indication of the procedure and endoscopic findings are extracted from each endoscopy report and automatically stored in the database.
The Trans.IT database annually collects more than 150,000 endoscopy reports, including approximately 63,000 upper GI endoscopies. Methods of data collection in this study are similar to previous research within the Trans.IT database and have been recently described [Citation17].
In this study, we analysed all upper GI endoscopies performed between January 2015 and December 2019 in patients with dyspeptic symptoms but no alarm symptoms as the indication for endoscopy. Dyspeptic symptoms included epigastric pain, upper abdominal discomfort, bloating, nausea, belching, heartburn and reflux symptoms [Citation18,Citation19]. Endoscopic reports were included if the patient was aged below 59 years. Patients below 18 years were excluded, in compliance with the Dutch general data protection regulation (GDPR) law. Endoscopic reports were excluded if one or more of the following alarm symptoms were documented: dysphagia, odynophagia, GI bleeding, unintentional weight loss, anaemia, persistent vomiting, palpable epigastric mass, family history of upper GI cancer or patients undergoing surveillance for known Barrett’s esophagus, ulcer or neoplasia.
Outcomes and definitions
Endoscopy yield was evaluated by extracting the results from individual endoscopy reports. These results are coded and were categorized into major endoscopic findings and minor endoscopic findings. A major endoscopic finding was defined as severe esophagitis (Los Angeles (LA) classification grade C and D [Citation20]), ulcer, stricture or suspicion of cancer found during upper GI endoscopy. A minor endoscopic finding was defined as mild esophagitis (LA grades A and B), suspicion of Barrett’s esophagus or gastric metaplasia found during upper GI endoscopy. Suspicion of cancer, Barretts’ esophagus and metaplasia were based upon endoscopic probable diagnoses.
Follow-up
Additionally, we performed a second search in the database to assess whether the patients in this population without abnormal upper GI endoscopy findings in our study underwent a follow-up endoscopy between January 2015 and August 2020 (follow-up at least 8 months but in the majority of patients longer). In order to correct for failed endoscopies, due to either patient- or endoscopy-related reasons, we decided that if the interval between baseline and follow-up upper GI endoscopy was 30 days or less, the endoscopic findings of the second upper GI endoscopy were considered to be part of the baseline upper GI endoscopy and consequently added the results to the baseline endoscopy. If the interval between baseline and follow-up upper GI endoscopy was more than 30 days, we regarded this as the follow-up (i.e. second) endoscopy.
Statistics
All statistical analyses were performed using IBM SPSS statistical software package version 25 (Armonk, NY). Data were exported from the Trans.IT in comma-separated files (CSV) and imported in SPSS statistical software. Categorical variables were reported as frequencies (%) and non-parametric data as medians with interquartile ranges [IQR].
Ethical considerations
Collecting patient data in the Trans.IT database has been approved by the privacy officer of the Erasmus Medical Center in accordance to the Dutch Personal Data Protection Act. All patient data is anonymously stored in a secure environment and therefore exempt from formal ethical approval. All included hospitals provided written consent for participation.
Results
Dataset
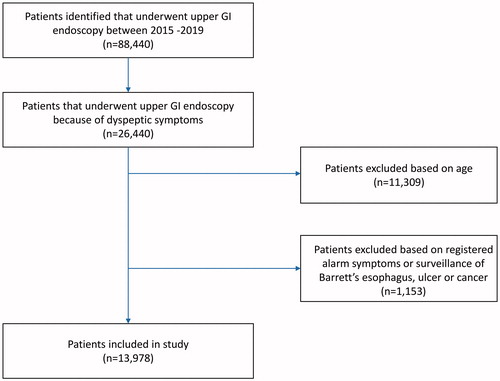
Data of 12 centres were available at the time of this study (2 academic and 10 non-academic centres). In these centres, a total of 121,406 upper GI endoscopies in 88,440 patients were performed between January 2015 and December 2019. A total of 26,440 patients underwent upper GI endoscopy because of dyspeptic symptoms. Of these, 11,309 patients were excluded because of an age older than 60 or younger than 18 years, and 1153 patients were excluded because of registered alarm symptoms or surveillance of already known Barrett’s esophagus, ulcer or cancer, resulting in the inclusion of 13,978 patients for analysis ().
Characteristics
Patient characteristics and endoscopy details are shown in . Of all patients, 8693 were female (62.2%). Median age was 46 years [IQR 36–53]. A total of 726 patients (5.2%) underwent upper GI endoscopy in an academic centre and 13,252 patients (94.8%) in a non-academic centre.
Table 1. Patient characteristics and endoscopy outcomes.
Results of upper GI endoscopy
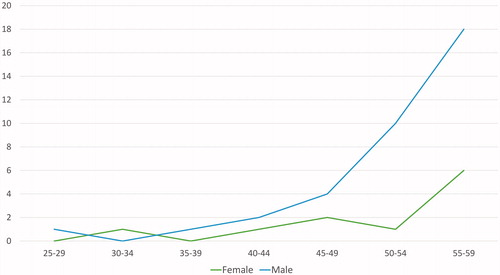
The results of upper GI endoscopy are reported in detail in . A major endoscopic finding was found in 513 patients (3.7%). Severe esophagitis was the most common major endoscopic finding, with esophagitis LA grade C seen in 187 patients (1.3%) and esophagitis LA grade D in 48 patients (0.3%). A gastric ulcer was seen in 91 patients (0.7%), a duodenal ulcer in 112 patients (0.8%) and nine patients had a gastric and duodenal ulcer (0.06%). An esophageal peptic stricture was found in 21 patients (0.2%) and a duodenal stricture related to peptic ulcer disease in six patients (0.04%). Suspicion of cancer was recorded in 47 patients (0.3%), including 16 patients (0.1%) with suspicion of oesophageal cancer, 28 patients (0.2%) with suspicion of gastric cancer and five patients (0.04%) with suspicion of duodenal cancer. Prevalence of cancer per age and gender is shown in .
A minor endoscopic finding was seen in 2178 patients (15.6%). Esophagitis LA grades A and B were the most common findings, esophagitis LA grade A seen in 1217 patients (8.7%) and grade B in 677 patients (4.8%). Suspicion of Barrett’s oesophagus was recorded in 371 patients (2.7%) and suspicion of gastric metaplasia in 18 patients (0.1%).
Follow-up endoscopy
In 11,353 patients (81.1%), initial upper GI endoscopy showed no relevant abnormalities. Of these patients, 1015 patients underwent a follow-up endoscopy (). Median time between the baseline and second endoscopy was 428 days [IQR 158–918]. During follow-up endoscopy, upper GI malignancy was not recorded (). A major endoscopic finding that was not reported during initial upper GI endoscopy was detected in 25 patients (0.2%), which included esophagitis LA grade C and grade D in eight (0.1%) and five (0.04%) patients, respectively. A gastric ulcer was seen in seven patients (0.05%) and a duodenal ulcer in four patients (0.03%).
Table 2. Endoscopic findings of the follow-up upper GI endoscopies in patients with a prior negative upper GI endoscopy.
Discussion
This study shows that the yield of upper GI endoscopy in dyspeptic patients without alarm symptoms below the age of 60 years is 3.7% for major endoscopic findings and 15.6% for minor endoscopic findings. Especially, endoscopic suspicion of an upper GI malignancy was rare. The yield of relevant findings during repeat upper GI endoscopy in a subgroup of patients with a previously unremarkable result was negligible. Most importantly, no additional cases of malignancy were detected.
This is the largest Dutch study to date that systematically analysed the results of upper GI endoscopy performed in patients with low-risk dyspeptic symptoms (<60 years, no alarm symptoms). Overall, the prevalence of relevant endoscopic findings in these patients was low (3.7%, n = 517 out of n = 13,978). In contrast, a recent single centre Dutch study that analysed the outcome of 2006 unselected patients that underwent upper GI endoscopy after referral by a general practitioner (open access referral) showed higher rates of clinically relevant endoscopic findings and malignancy (19.3 vs. 3.7% in our study, and 5.2 vs. 0.3% in our study, respectively) [Citation3]. However, only 18.6% of patients in this study underwent endoscopy because of dyspeptic symptoms, were below the age of 50 and were without alarm symptoms [Citation3].
When comparing our data with a meta-analysis investigating upper GI endoscopy results in a total of 2,597 dyspeptic patients, the prevalence of significant upper GI endoscopic findings was again higher (23.6%) compared to our study (3.7%) [Citation21]. This finding could be explained by the overrepresentation of elderly patients and patients with a history of GI disease in this meta-analysis.
The low risk of relevant findings in dyspeptic patients is further illustrated by another meta-analysis that aimed to determine the predicting role of alarm symptoms for upper GI malignancy in patients with dyspepsia. This study included 57,363 patients, of which 8.669 had at least one or more alarm symptoms. Even with inclusion of these ‘high risk’ patients, only a total of 0.8% was found to have a (upper) GI cancer diagnosed [Citation9].
The current recommendation of the American College of Gastroenterology (ACG) and the Canadian Association of Gastroenterology (CAG) is to limit upper GI endoscopy to patients with dyspepsia when aged 60 years or older. In those aged below 60 years, these guidelines suggest to withhold endoscopy even if patients present with alarm symptoms due to the limited predictive value of alarm symptoms for upper GI malignancy [Citation1]. The National Institute for Health and Care Excellence (NICE) guideline suggests to perform upper GI endoscopy in patients with dysphagia or hematemesis irrespective of patient age and to withhold endoscopy in all other patients aged below 55 years [Citation2]. Our study supports withholding upper GI endoscopy in patients deemed ‘low-risk’ as only a limited number of patients included in our study had an upper GI malignancy detected (0.3%, n = 47 out of n = 13,978). This result is in line with the pooled prevalence of a recently published network meta-analysis that compared management strategies for non-investigated dyspepsia in the absence of alarm symptoms (0.4%, 20 of 5028 patients) [Citation22].
We observed that despite an initial negative upper GI endoscopy, 6.1% of patients (n = 854) underwent a follow-up endoscopy within a median time of approximately 15 months (median: 428 days; IQR: 158–819 days). In these patients, 0.2% of endoscopies resulted in an additional major finding; however, without endoscopic suspicion of malignancy. These results are in line with a retrospective study in 146 patients that underwent repeat endoscopy, also with no additional malignancies being detected, despite the presence of alarm symptoms [Citation23]. When looking from another perspective, a retrospective study concluded that upper GI malignancies could be potentially missed during initial investigation as 9.8% of patients diagnosed with upper GI malignancy had had at least one upper GI endoscopy within the preceding three years to diagnosis. In most of these cases, endoscopist errors were considered the most likely cause of failure to detect malignancy [Citation8]. In line with this observation, a case control study concluded that endoscopy programs with a larger number of procedures could be linked to missing upper GI malignancy [Citation24]. Nonetheless, in our study, in which a subgroup of symptomatic patients underwent a repeat upper GI endoscopy, no evidence of malignancy was seen, although the follow-up time may have been too short to allow detecting missed malignancies.
In order to prevent missing significant lesions, strategies are being developed to improve early detection of upper GI malignancy by implementing key performance indicators for upper GI endoscopy similar to colonoscopy, such as a thoroughly documented systematic endoscopic examination within a minimal procedure time [Citation25,Citation26]. Moreover, new techniques may help in increasing upper GI malignancy detection rates such as artificial intelligence that could potentially augment the endoscopic detection rate of relevant endoscopic findings [Citation27]. Lastly, the challenge remains to identify alternative clinical parameters for risk stratification which could help in reassuring patients and physicians and ultimately change clinical practice.
The strength of this study lies in the fact it comprises of a large uniformly documented dataset, making it suitable to systematically analyse data of different centres and physicians. Second, all performed endoscopies are collected in the database, making it a good representation of everyday clinical practice. However, this study also comes with some limitations. First, we performed a post hoc analysis of a prospectively collected dataset which did not include a systematic registration of alarm symptoms. As a result, if alarm symptoms were not adequately documented then patients that were actually at high risk for significant pathology could wrongly have been included in our study. Nevertheless, considering the large sample size of the study and the low frequency of relevant findings, we believe the impact of this on our results would be negligible. Second, patients included in this study were not strictly patients with dyspepsia according to the Rome criteria [Citation10]. Dyspepsia is difficult to define, and the Rome criteria are the most widely accepted definitions of dyspepsia. They were developed to better differentiate gastro-esophageal reflux disease from functional dyspepsia in order to perform prospective studies with well-defined populations. The Rome criteria can however not always be used in clinical practice as there is substantial overlap in symptom presentation [Citation11,Citation12]. We believe that by including patients meeting a clinical definition of dyspepsia the results of this study are better applicable to clinical practice and could potentially help reduce overuse of upper GI endoscopy. Third, the diagnosis of upper GI malignancy was based on endoscopic appearance only. The database lacks histological confirmation, classification, and staging information of endoscopic findings. However, the availability of enhanced imaging technique on the used upper GI endoscopes, i.e. narrow-band imaging, may have significantly increased the correlation between the suspected endoscopic diagnosis of malignancy and subsequent histological confirmation [Citation28]. In addition, even if there were indeed false-positive findings based on endoscopy only, this would strengthen the overall conclusion of our study.
In conclusion, in dyspeptic patients without alarm symptoms under the age of 60 years, the yield of upper GI endoscopy is low. Our results favour strategies to further reduce performing endoscopy in these patients in order to prevent unnecessarily exposure to the risks related to endoscopy and reduce healthcare expenditure.
Disclosure statement
F. Theunissen: no conflicting interests.
M.A. Lantinga: no conflicting interests.
P.C.J. ter Borg: no conflicting interests.
R.J. Ouwendijk: Research grants from Janssen Netherlands and the Coolsingel Foundation.
M.J. Bruno: Boston Scientific; Consultant, support for industry and investigator initiated studies; Cook Medical; Consultant, support for industry and investigator initiated studies; Pentax Medical; support for investigator initiated studies; Mylan; support for investigator initiated studies; ChiRoStim; support for investigator initiated studies.
P.D. Siersema: Research grants from Norgine, Pentax, Microtech, Yakult and Motus GI (ongoing); Advisory Board of Motus GI and Boston Scientific.
References
- Moayyedi P, Lacy BE, Andrews CN, et al. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112(7):988–1013. Jul
- National Institute for Health and Care Excellence. Suspected cancer: recognition and referral (NG12). London 2017. (NICE Guideline, No. 12).
- Crouwel F, Meurs-Szojda MM, Klemt-Kropp M, et al. The diagnostic yield of open-access endoscopy of the upper gastrointestinal tract in the Netherlands. Endosc Int Open. 2018;6(4):E383–E394.
- Hassan C, Bersani G, Buri L, et al. Appropriateness of upper-GI endoscopy: an Italian survey on behalf of the Italian Society of Digestive Endoscopy. Gastrointest Endosc. 2007;65(6):767–774.
- Di Giulio E, Hassan C, Marmo R, et al. Appropriateness of the indication for upper endoscopy: a meta-analysis. Dig Liver Dis. 2010;42(2):122–126. Feb
- de Jong JJ, Lantinga MA, Drenth JP. Prevention of overuse: a view on upper gastrointestinal endoscopy. World J Gastroenterol. 2019;25(2):178–189.
- Manes G, Balzano A, Marone P, et al. Appropriateness and diagnostic yield of upper gastrointestinal endoscopy in an open-access endoscopy system: a prospective observational study based on the Maastricht guidelines. Aliment Pharmacol Ther. 2002;16(1):105–110.
- Yalamarthi S, Witherspoon P, McCole D, et al. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy. 2004;36(10):874–879.
- Vakil N, Moayyedi P, Fennerty MB, et al. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology. 2006;131(2):390–401.
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150(6):1262–1279.e2.
- Vakil N, Halling K, Ohlsson L, et al. Symptom overlap between postprandial distress and epigastric pain syndromes of the Rome III dyspepsia classification. Am J Gastroenterol. 2013;108(5):767–774.
- Van den Houte K, Carbone F, Goelen N, et al. Effects of Rome IV definitions of functional dyspepsia subgroups in secondary care. Clin Gastroenterol Hepatol. 2020;S1542-3565(20)30906-X. doi:10.1016/j.cgh.2020.06.043.
- Groenen MJ, Hirs W, Becker H, et al. Gastrointestinal endoscopic terminology coding (GET-C): a WHO-approved extension of the ICD-10. Dig Dis Sci. 2007;52(4):1004–1008.
- Groenen MJ, Kuipers EJ, van Berge Henegouwen GP, et al. Computerisation of endoscopy reports using standard reports and text blocks. Neth J Med. 2006;64(3):78–83.
- Soekhoe JK, Groenen MJ, van Ginneken AM, et al. Computerized endoscopic reporting is no more time-consuming than reporting with conventional methods. Eur J Intern Med. 2007;18(4):321–325.
- Groenen MJ, van Buuren HR, van Berge Henegouwen GP, et al. Validation study of automatically generated codes in colonoscopy using the endoscopic report system endobase. Scand J Gastroenterol. 2010;45(9):1121–1126.
- Lantinga MA, Theunissen F, Ter Borg PCJ, on behalf of the Trans.IT foundation study group, et al. Impact of the COVID-19 pandemic on gastrointestinal endoscopy in the Netherlands: analysis of a prospective endoscopy database. Endoscopy. 2021;53(02):166–170.
- Dent J. Definitions of reflux disease and its separation from dyspepsia. Gut. 2002;50 Suppl 4 (Suppl 4):iv17–20.
- Gerson LB, Kahrilas PJ, Fass R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin Gastroenterol Hepatol. 2011;9(10):824–833.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–180.
- Ford AC, Marwaha A, Lim A, et al. What is the prevalence of clinically significant endoscopic findings in subjects with dyspepsia? Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8(10):830–837.
- Eusebi LH, Black CJ, Howden CW, et al. Effectiveness of management strategies for uninvestigated dyspepsia: systematic review and network meta-analysis. BMJ. 2019;367:l6483.
- Pongprasobchai S, Asanaleykha N, Tantayakom P. Repeat upper gastrointestinal endoscopy in patients with functional dyspepsia: yield, findings, and predictors of positive findings. Gastroenterol Res Pract. 2015;2015:904683.
- Tai FWD, Wray N, Sidhu R, et al. Factors associated with oesophagogastric cancers missed by gastroscopy: a case-control study. Frontline Gastroenterol. 2020;11(3):194–201.
- Veitch AM, Uedo N, Yao K, et al. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat Rev Gastroenterol Hepatol. 2015;12(11):660–667.
- Dekker E, Houwen B, Puig I, et al. Curriculum for optical diagnosis training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy. 2020;52(10):899–923.
- Luo H, Xu G, Li C, et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20(12):1645–1654.
- Hoffman A, Manner H, Rey JW, et al. A guide to multimodal endoscopy imaging for gastrointestinal malignancy - an early indicator. Nat Rev Gastroenterol Hepatol. 2017;14(7):421–434.
Appendix
M.E. van Leerdam (Antoni van Leeuwenhoek, Amsterdam and Leiden University Medical Center, Leiden); F.J.G.M. Kubben (Maasstad Ziekenhuis, Rotterdam); M. Rasica (Bravis Ziekenhuis, Roosendaal); J.J. Uil (Ziekenhuis Gelderse Vallei, Ede); M. Kerkhof (Groene Hart Ziekenhuis, Gouda); R.M.E. Slangen (HagaZiekenhuis, Den Haag), P.J. Bus (Laurentius Ziekenhuis, Roermond); W.J. Thijs (Martini Ziekenhuis, Groningen); M.J.M. Groenen (Rijnstate Ziekenhuis, Arnhem), E. van Hoboken (Rode Kruis Ziekenhuis, Beverwijk), W. Bruins Slot (Spaarne Gasthuis, Haarlem), L. Wormmeester (Treant Zorggroep, Hoogeveen), F. Vleggaar (UMC Utrecht, Utrecht), L.A. Noach (Ziekenhuis Amstelland, Amstelveen), E.J. Kuipers (Erasmus Medical Center, Rotterdam).