Abstract
Introduction
Gallbladder cancer (GBC) is a rare malignancy in Western population with poor prognosis. This study aimed to investigate the trends in GBC incidence, treatment pattern, and survival in Finland.
Methods
Patients diagnosed with primary GBC in a geographically defined area (Southern Finland Regional Cancer Center) during 2006–2017 were identified.
Results
Final cohort included 270 patients with GBC. The incidence was 1.32/100,000 persons, and it decreased 6.8 cases per million personyears during the study period. One hundred fifty-one (56%) patients were diagnosed at Stage IV. Fifty-one patients (19%) underwent curative-intent resection with 96% R0-resection rate. The median overall survival was 7.1 months and 5-year overall survival 11.6% for all patients, and 67.7 months and 56.8% after curative-intent resection, respectively. No improvement was noted over time in overall survival in patients with GBC, or in subgroups of different stages of GBC.
Conclusions
The incidence of GBC is slightly decreasing in Southern Finland, but survival has not improved over time.
Keywords:
Introduction
Gallbladder cancer (GBC) is the most common biliary tract cancer with commonly a poor prognosis. The GBC incidence is characterized by high geographical variability, being 0.6–4.3:100,000 in Europe, 0.14–7.4:100,000 in Asia, and 0.5–14.0:100,000 in South America [Citation1]. The incidence of GBC varies also between sexes and ethnicities being high on women compared to men and, for instance, higher among American Indians compared to non-Hispanic white persons [Citation2–4].
The incidence appears to decrease in some European countries such as in Sweden and Norway, and in the United States, but increases in Asia, especially in India [Citation3,Citation5–8]. The reason for decreasing incidence in these countries is mostly unknown, but increasing rates of cholecystectomies, lifestyle changes, and obesity-reducing factors have been proposed as potential contributors [Citation4,Citation6,Citation9]. Increase in incidence in Asia is speculated to be mainly driven by the increase of smoking, obesity and environmental pollution [Citation6,Citation10]. However, in many European countries the incidence has remained stable, for instance in the United Kingdom and Netherlands [Citation9].
Surgical treatment is the only curative treatment option for GBC. However, since only a fraction of GBCs are found at an early stage, oncologic treatments play a crucial role in lengthening survival in most patients [Citation11,Citation12]. In 2019, BILCAP trial suggested that adjuvant capecitabine could offer a survival benefit in patients with biliary tract cancers, including GBC, who had undergone curative-intent surgery [Citation13]. On the other hand, evidence for neoadjuvant therapy is still moderate, and no recommendations have been made [Citation14–17]. Although current recommendations for GBC’s surgical treatment are mostly unanimous [Citation18,Citation19], the benefits of extended cholecystectomy in stages I and IIA are still highly debated [Citation20–22]. Extended cholecystectomy comprising of liver bed resection and hilar lymphadenectomy is the cornerstone of curative-intent surgical treatment for GBC as liver-sided location and presence of lymph node metastases are the most important prognostic factors [Citation20,Citation21,Citation23]. However, recent studies have not shown any survival-benefit on routine bile duct or port-cite resections [Citation24–27].
Although most of the literature on GBC is based on single-center retrospective series, a few population-based reports are available such as from Netherlands and West Sweden [Citation28–30]. These epidemiological studies have shown a change in treatment patterns during a few last decades, but the survival remains largely unchanged [Citation28,Citation29].
This study aims to explore incidence of GBC as well as diagnostics, treatment patterns, and the survival of patients with GBC during the last decade using a population-based approach in a geographically defined area in Finland.
Methods
This was a retrospective population-based cohort study, including all patients diagnosed with GBC within the Southern Finland Regional Cancer Center (FICAN South) catchment area. The area is located in Southern Finland and has approximately 2.2 million inhabitants within 22,036 m2, being the most densely populated catchment area in Finland. During the study period, FICAN South included one university hospital acting as both secondary and tertiary referral center (Helsinki University Hospital, HUH) and two community central hospitals acting as secondary referral centers (South-Carelia Central Hospital and Kotka Central Hospital). All hospitals perform simple cholecystectomy, but surgical treatment requiring liver resection is centralized to HUH. Patients referred for surgical evaluation to HUH are discussed in a multidisciplinary team meeting with specialized radiology, hepatobiliary surgeons, oncologist, and pathologist. All hospitals provide oncological treatments including chemoradiation.
Patients were identified from the Finnish Cancer Registry (FCR) by including all patients with gallbladder cancer. FCR is highly accurate in comprising all patients with a solid malignancy [Citation31]. Patient medical records were obtained from all hospitals and were manually reviewed to confirm the accuracy of the diagnosis, collect data, and to exclude patients without GBC. In addition, patients’ death certificates were requested from Statistics Finland in case there was any uncertainty about the cause of death, the date of death, or the patient’s home municipality. Patient demographics, characteristics of GBC, operative, oncological, and radiological details were manually extracted from the patient records. Further, the annual population at FICAN South area for the purpose of incidence-calculations was received from Statistics Finland [Citation32].
Patients treated for primary GBC from 1 January 2006, to 31 December 2017, were included in this study. Exclusion criteria were: 1) Patient treated primarily for other biliary tract cancer, and 2) metastasis in the gallbladder. Further, if the GBC was not diagnosed until at autopsy, or patient medical records were not available (some hospitals dispose of patient records only twelve years after patients’ death), patients were excluded from detailed analysis. However, these patients were included in the annual incidence and survival analysis.
Computed tomography (CT) is routinely performed for patients diagnosed or suspected GBC in Finland, while magnetic resonance image (MRI) or positron emission tomography (PET) are used selectively. The TNM stage was based on this imaging and, if available, the histopathological examination of the removed gallbladder. Fit patients with c/pT1b-c/pT3NXM0 GBC are recommended for hepatoduodenal ligament lymphadenectomy and extended cholecystectomy (resection of gallbladder en bloc with liver parenchyma of segments 4 b and 5) or re-resection if simple cholecystectomy has been performed earlier. Patients with T4 or M1 GBC are referred to oncological treatment.
Patients undergoing surgery were categorized under either curative-intent resection or palliative/diagnostic cholecystectomy. All patients with T1b or N1 or higher GBC that underwent only simple cholecystectomy were categorized under palliative/diagnostic cholecystectomies, and all patients with T1a or peritoneal side T1b GBC undergoing simple cholecystectomy were categorized under curative-intent resection. Patients with T1b or higher, or N1 GBC, who underwent liver resection, lymfadenectomy, or bile duct resection were categorized under curative-intent resection.
Follow-up began on the day of cholecystectomy or radiological imaging, which ever led first to the diagnosis of GBC. The last follow-up date was defined as the last contact registered in the patient medical records, or death. Survival status and possible date of death were obtained from the Finnish Cancer Registry database, patient’s death certificates, or electronic patient record system, which automatically updates it from the Population Register Centre, which is an extremely reliable and up-to-date source of survival status. Patients were divided into three equal periods according to the year of diagnosis to allow comparison of the incidence and treatment of GBC: Period A, 2006–2009; Period B, 2010–2013; Period C, 2014–2017.
The institutional review board of HUH, the National Institute for Health and Welfare, and the Statistics Finland approved this study.
Statistical analysis
Incidence rates (IR) were calculated per 100,000 person years and age-standardized using the world standard population given at Nordcan project [Citation33]. For each year and period under observation, the crude IR per 100,000 person years was calculated by dividing the number of cases by person-time at risk. The absolute incidence change was calculated by subtracting the crude IRs of first and last study period. The exact 95% confidence intervals (CI) for IRs were assessed by assuming Poisson distributed rates. In addition, age-specific IR was calculated, based on which the age-standardized rates per 100,000 person years were assessed using the world standard population [Citation33]. The changes in the IR over time were evaluated by means of IR ratio (IRR). Data were aggregated on cancer cases and risk population by age (five age groups: 40–49,50–59, 60–69, 70–79, 80–) and calendar year and multiplicative Poisson regression models were applied to the number of cases with the natural logarithm of person-time as an offset term. Poisson model with calendar time was fitted as a continuous variable and for the Poisson model with calendar time as categorical variable representing three periods 2006–2009, 2010–2013, and 2014–2017, the former period being a reference. The crude and age-adjusted IRRs for calendar time (period) and their 95% CIs were assessed from the Poisson models without and with age, respectively. In addition, for interaction between age (five groups) as categorical variable and calendar time as continuous variable the Poisson regression models were tested and fitted with calendar time as categorical variable when stratifying on age (three groups <50, 50–69, ≥70 years). These analyses were conducted with the R statistical software [Citation34], including Epi and epitools packages.
All other data were analyzed using SPSS Statistics Ver 24 (IBM, Chicago, IL, USA). Continuous variables were compared between groups using the Mann–Whitney U-test and p values <.05 were considered statistically significant. Chi-square testing was used to assess differences between study periods. Kaplan–Meier method was used to estimate the overall survival (OS) by several grouping variables, including period, cancer staging, and dissemination level. The differences in the survival between groups were inspected by plotting the estimated curves. In addition, when appropriate, log-rank test was used to check whether there were difference survival probabilities between the groups. The confidence level was set at 5%.
Results
Incidence
Three hundred ninety-six patients were identified from FCR (), out of which 294 patients had confirmed GBC during 2006–2017, giving a crude average number of 24.5 new cases per year (1.32:100,000) (). When assessed by three periods, a decrease was observed in both the crude and age-standardized IR of GBC over time (). As compared to the 2006–2009 period, the IR of GBC was lower during 2010–2013 (crude IR = 0.84) and 2014–2017 (crude IR = 0.79). After controlling for differences in the age distribution of the population at risk, even more apparent differences were observed (). The crude and age-adjusted IRRs for continuous time were 0.97 (95% CI 0.94–1.00, p = .0663) and 0.95 (95% CI 0.92–0.99, p = .0068), respectively. As a statistically significant interaction between calendar time and age was found (year*age 50–59 years p = .015, year*age 60–69 years p = .033), stratified analysis was performed and the association between calendar time and the incidence rate of GBC was studied within three age groups (). In these analyses, a decrease in the IR of GBC was observed in the age group 50–69 years but no statistical difference was noted in the IR between different time periods in age groups of 40–49 years or over 70 years. Absolute decrease in the incidence rate for population over 40 years between these periods 2006–2009 and 2014–2017 was 6.8 (95% CI −0.61 to 14.24, p = .072) GBC cases per million person-years.
Figure 1. Flow diagram for collected data. GBC: gallbladder cancer; FICAN: National Cancer Center Finland; FCR: Finnish Cancer Register.
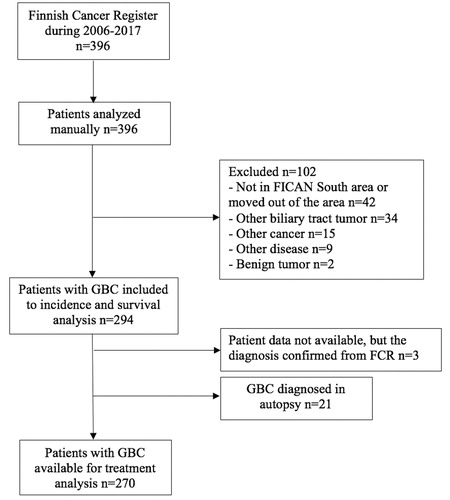
Figure 2. Crude incidence rate and number of patients with GBC in three periods per 100,000 person-years at FICAN South area during 2006–2017.
Table 1. Crude and age-specific incidence rates per 100,000 person-years and crude and age-adjusted incidence rate ratios by three periods.
Table 2. Crude incidence rate ratios by three periods by age groups under and over 70 years.
Patients
For analyses regarding detailed patient and treatment information, 21 patients were excluded because of diagnosis on autopsy and three patients because of missing patient medical records, forming a final cohort of 270 patients. The proportion of GBCs diagnosed at autopsy was similar between the periods (Period A n = 11; 10.2% vs. Period B n = 8; 8.4% vs. Period C n = 2; 2.2%, p = .078). Baseline patient data and details on GBC are summarized in . Shortly, the female-male ratio was 2.45:1 during the study period. The mean age of patients was 73.7 (SD 11.0), and only nine (3.1%) patients were under 50 years old. The diagnosis was established by imaging in about half (51.2%, n = 149) of the patients. GBC was diagnosed from the gallbladder specimen after cholecystectomy in 104 patients (49 (47.1%) for acute cholecystitis (emergent cholecystectomy), 55 (52.9%) for symptomatic cholelithiasis (elective cholecystectomy)). For 17 patients GBC diagnosis was established in the curative-intent operation.
Table 3. Basic patient and tumor characteristics at the time of diagnosis of gallbladder cancer.
Treatment
The rate of simple cholecystectomy in patients with GBC increased during three periods (30.9% vs. 50.6% vs. 51.7%, p = .006), but the increase in the rate of curative-intent resection did not reach statistical significance (13.0% vs. 18.9% vs. 20.9%, p = .30) (). At the same time, the number of patients receiving only best supportive care decreased (44.7% vs. 29.9% vs. 27.0%, p = .039) ().
Figure 3. Flow diagram for gallbladder cancer treatment by each period for patients with available medical records. GBC: gallbladder cancer.
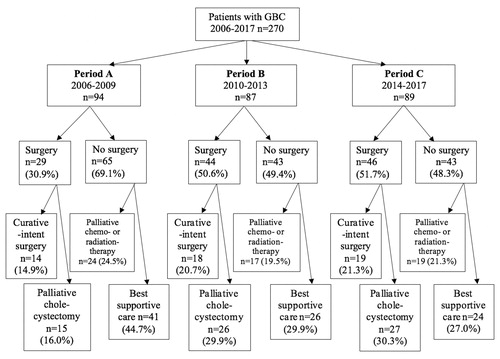
The GBC was mostly diagnosed at an advanced stage [Stage IVA, n = 25 (9.4%); Stage IVB n = 126 (47.5%)] (). The information about surgical and oncological treatments are detailed in and . Seventy-seven (28.5%) patients underwent exploration for curative-intent resection, but resection was possible only for 18.9% of patients (n = 51) (). R0-resection was achieved in 49 patients (96.1% of patients undergoing curative-intent resection), of whom four were operated with simple cholecystectomy (Stage 0–I). Median survival of these patients with curative-intent resection was 62.1 months, 67.7 months, and not reached in periods A, B, and C, respectively. There was no difference in the survival between three periods in patients undergoing curative-intent resection (p = .890) ().
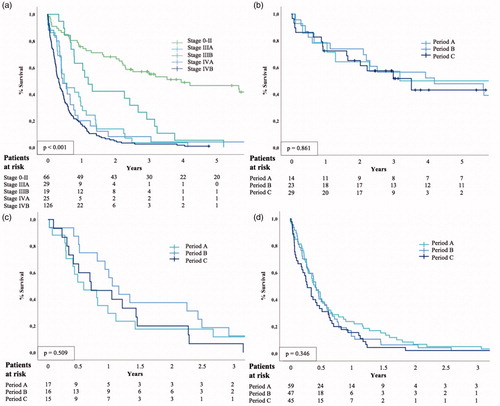
Figure 4. Kaplan–Meier curve for overall survival by three periods among (a) all patients with GBC, (b) patients with curative-intent surgery, (c) patients without systematic metastasis (M0), and (d) patients with systematic metastasis (M1) (D). The result of logrank test should be interpreted with caution, because logrank test is less likely to detect a difference between the groups in the presence of crossing survival curves.
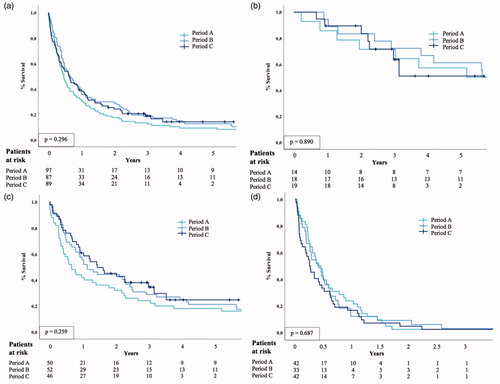
Table 4. Details of surgical operations for gallbladder cancer.
Table 5. Oncologic treatment with or without curative-intent surgery.
Hundred fourteen (42.2%) patients were diagnosed with potentially resectable GBC (Stage 0–IIIB) ( and ), of which only 51 (45%) underwent curative-intent resection. Forty-four out of 51 with curative intention resected patients had Stage 0–IIIB GBC and other seven Stage IVA or IVB in the final histopathologic resection sample. Twenty-nine out of 51 patients (56.9%) with Stage II (T2N0M0), all 29 patients with Stage IIIA (T3N0M0), and 10 out of 19 patients (52.6%) with Stage IIIB (T1-3N1M0) did not undergo curative-intent resection (). The reason for non-operative approach was performance status unfit for surgery in seven patients and patient refusal in two patients. Forty (35.1%) patients were not referred to liver surgery unit. While for the rest, the decision not to pursue resection was made either intraoperatively (10 patients) or at multidisciplinary meeting (20 patients).
Table 6. Estimated overall survival for different stages by each research period.
About 40% (n = 21) of patients with curative-intent surgery received adjuvant therapy with gemcitabine (n = 15), capecitabine (n = 4), and 5-fluorourasil with oxaliplatin (n = 1). One patient was treated with adjuvant radiotherapy (). Most of these patients were operated at Stages III–IV (n = 14, 66.7%). Six patients with pN0 status (20% of patients with pN0) and 13 patients with pN1-2 (92.9% of patients with pN1-2) received adjuvant therapy after curative-intent surgery. The nodal status was not determined for two patients treated with adjuvant therapy. Oncologic therapy was offered for 38.8% (n = 85) of patients not undergoing a curative-intent operation. These patients were treated mainly with gemcitabine-cisplatin (n = 75) and also with capecitabine (n = 5) and a few other combination of chemotherapy (n = 5).
Survival
The estimated overall 5-year survival for GBC was 11.7% and it did not appear to change during the study period (). In patients undergoing curative-intent surgery, the estimated 1-year survival was 78.6%, 78.3%, and 72.4%, and 5-year survival was 50%, 47.8%, and 43.1% for periods A, B, and C, respectively (). No changes were observed between the periods in survival among patients with or without systemic metastases (). Survival was affected by the stage, but no stage-specific differences were noted between the periods (, ).
Figure 5. Kaplan–Meier curve for overall survival by (a) stages, and by three periods among patients with GBC (b) stage 0–II, (c) stage IIIA–B, and (d) stage IVA–B. Stage could not be determined for five patients. The result of logrank test should be interpreted with caution, because logrank test is less likely to detect a difference between the groups in the presence of crossing survival curves.
Discussion
This population-based study showed a small but significant age-adjusted decline in the GBC incidence rate during 2006–2017 in Southern Finland. Although the number of patients receiving only the best supportive care decreased, this was not reflected in overall survival improvements during the study period and the overall prognosis of GBC remain poor.
The decrease of GBC incidence in this study is similar to studies in other Western countries’ studies [Citation3,Citation7]. The reason for this decline in low-incidence countries remains unclear, but speculations about an increase in cholecystectomies or improvement in lifestyle could explain some of the change [Citation35]. According to the statistics available from the Finnish National Institute of Health and Welfare, the number of cholecystectomies has steadily increased during the study period [Citation36].On the other hand, the proportion of overweight people in the Finnish population has been growing steadily [Citation37]. Since the development of GBC takes several years, one possible explanation for the decrease in GBC incidence in 50- to 69-year-olds could be the increased rate of cholecystectomy, which prevents GBC from developing in the first place. However, with this study, this cannot be demonstrated.
We observed a change in the GBC diagnosis, with a reduction in diagnostics based on only imaging. This change directly results from an increased number of cholecystectomies and exploratory procedures, which include a histopathologic sample in suspected cases. However, the rate of curative-intent surgery did not change during the study period, similarly as reported in a Swedish population-based study [Citation29]. Patients’ overall rate of proceeding to curative-intent surgery in our study was 19%, which is lower than the resection rate reported in West Sweden (37%), Netherlands (36%) or in Ontario (46%) [Citation29,Citation30,Citation38]. Differences in curative-intent resection rates might reflect an actual difference in aggressiveness to surgical treatment but may also be explained by differences in defining curative-intent resection. For example, 45% with Stage II and IIIA GBC that were classified as curative-intent resection in West Sweden were actually treated with simple cholecystectomy only. In our study, such patients were classified as having undergone palliative/diagnostic cholecystectomy. By excluding these patients, the curative-intent resection rate declines to 32.4%, which is still significantly higher compared to ours. 55% of patients with potentially resectable GBC (Stages 0–IIIb) did not undergo curative-intent resection, and only 16% of those were due to patient refusal or unfitness for surgery.
The ultimate goal of curative-intent surgery is R0-resection, and the rate of R0-resection in the whole cohort of patients with GBC can be compared between populations in different studies. The R0-resection was obtained in 96% of patients who underwent curative-intent resection, leading to a 18% R0-resection rate in all patients with GBC. Corresponding R0-resection rate among the whole population with GBC was 35% in West Sweden and 23% in Netherlands. The differences in R0-resection rates may stem from the differences in the stages of GBC at diagnosis. In our study, 56%of patients were diagnosed at an advanced stage (Stage IV) and thus not eligible for curative-intent surgery. However, the proportion of these patients with Stage IV was almost equal in the other studies, 44.3% in Ontario and 61.2% in West Sweden. In Netherlands, 45.3% of patients had metastatic disease, but the proportion of stage IVA patients was not reported.
As surgery is the only potential for curation in GBC, the above mentioned differences in resections rates may reflect overall survival. The 5-year overall survival was 12% in our study, which is similar to West Sweden (approximately 11%) and Netherlands (13%) albeit them having a higher curative-intent resection rates [Citation29,Citation30]. On the other hand, the 5-year overall survival was clearly higher in Ontario (25%), which also had very high curative-intent resection rate [Citation38].
Adjuvant therapy has shown a survival benefit (in prespecified sensitivity and per-protocol analyses) for patients with the locally advanced or nodal-positive disease after R0 resection and is therefore recommended in current guidelines [Citation18,Citation23,Citation39]. In this study, all but one patient with nodal-positive status after curative-intent resection were treated with adjuvant therapy. The estimated median survival, 35 months, on these patients was significantly worse than patients with negative nodes, but almost fivefold better compared to nodal-positive patients without curative-intent resection (7.5 months). Recently, based on the BILCAP trial results, capecitabine has been recommended for adjuvant therapy [Citation13,Citation19,Citation40]. In our study, patients were treated before the publication of BILCAP trial results and capecitabine was not widely used.
Some limitations need to be mentioned. First, as a retrospective study itself has restrictions, such as an absence of treatment standardization, data availability, and treatment protocol variations between different periods. Second, despite the high accuracy of FCR, biliary cancers as a group may be prone to inaccuracy and underreporting [Citation41]. However, patient medical records were manually extracted to confirm the accuracy of the diagnosis. It is still possible that some patients with GBC are missing from the FCR, and thus missing from this study. Third, the number of patients with GBC in this study was relatively low, which might lead to type 2 error.
In conclusion, for unknown reasons, the incidence of GBCs seems to be declining in the Southern Finland region, especially among those under 70 years of age. There was no change in overall survival over the 12 years of the study. Wider use of neoadjuvant and adjuvant therapy along with increasing the rate of curative-intent resection may help in improving outcomes of GBC.
Disclosure statement
Dr. Hanna Koppatz reports grants from Mary and Georg Ehnrooth’s Foundation during the conduct of the study; grants from Vatsatautien tutkimussäätiö foundation and grants from Martti I. Turunen Foundation outside the submitted work. Dr. Ville Sallinen reports grants from Vatsatautien Tutkimussäätiö Foundation, Mary and Georg Ehrnrooth’s Foundation and Helsinki University Hospital research funds during the conduct of the study; and grants from Finska Läkaresällskapet, Academy of Finland, Finnish Cancer Foundation (Syöpäsäätiö) and personal fees from Finnish Gastroenterological Society, City of Vantaa, outside the submitted work. Dr. Katriina Peltola reports personal fees as following: consulting/advisory role: Orion Pharma, BMS, MSD, Novartis, Pfizer, Ipsen, Roche, Varian; stockholder: Faron Pharmaceuticals; speakers’ bureau: BMS, Pfizer, MSD; expert testimony: Ipsen; travel/accommodations/expenses: Roche, BMS; Institutional research funding: AbbVie, Bayer, BMS, MSD, Roche, Exelixis, Orion Pharma, Eisai, Novartis.
Med cand. Sini Takala, Drs. Heikki Mäkisalo, and Arno Nordin, and statistician Anna But have no conflicts of interest or financial ties to disclose.
Additional information
Funding
References
- World Health Organization. Cancer WIAfRo. global cancer observatory: cancer today. 2018 [cited 2019 Jun 26]. Available from: http://gco.iarc.fr/
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
- Henley SJ, Weir HK, Jim MA, et al. Gallbladder cancer incidence and mortality, United States 1999-2011. Cancer Epidemiol Biomarkers Prev. 2015; 24:1319–1326.
- Jaruvongvanich V, Yang JD, Peeraphatdit T, et al. The incidence rates and survival of gallbladder cancer in the USA. Eur J Cancer Prev. 2019; 28:1–9.
- Rahman R, Simoes EJ, Schmaltz C, et al. Trend analysis and survival of primary gallbladder cancer in the United States: A 1973-2009 population-based study. Cancer Med. 2017; 6:874–880.
- Malhotra RK, Manoharan N, Shukla NK, et al. Gallbladder cancer incidence in Delhi urban: A 25-year trend analysis. Indian J Cancer. 2017; 54:673–677.
- Kilander C, Lagergren J, Ljung R, et al. The population-based incidence and mortality of biliary tract cancer in Sweden. Cancer Epidemiol. 2018; 56:14–20.
- Bjerregaard JK, Mortensen MB, Pfeiffer P, Academy of Geriatric Cancer Research (AgeCare). Trends in cancer of the liver, gall bladder, bile duct, and pancreas in elderly in denmark, 1980-2012. Acta Oncol. 2016; 55 Suppl 1:40–45.
- Torre LA, Siegel RL, Islami F, et al. Worldwide burden of and trends in mortality from gallbladder and other biliary tract cancers. Clin Gastroenterol Hepatol. 2018; 16:427–437.
- Murphy BL, Ubl DS, Zhang J, et al. Trends of inguinal hernia repairs performed for recurrence in the United States. Surgery. 2018;163:343–350.
- Bergquist JR, Shah HN, Habermann EB, et al. Adjuvant systemic therapy after resection of node positive gallbladder cancer: time for a well-designed trial? (Results of a US-national retrospective cohort study). Int J Surg. 2018; 52:171–179.
- Hickman L, Contreras C. Gallbladder cancer: diagnosis, surgical management, and adjuvant therapies. Surg Clin North Am. 2019; 99:337–355.
- Primrose JN, Fox RP, Palmer DH, et al., BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673.
- Agrawal S, Mohan L, Mourya C, et al. Radiological downstaging with neoadjuvant therapy in unresectable gall bladder cancer cases. Asian Pac J Cancer Prev. 2016; 17:2137–2140.
- Creasy JM, Goldman DA, Dudeja V, et al. Systemic chemotherapy combined with resection for locally advanced gallbladder carcinoma: surgical and survival outcomes. J Am Coll Surg. 2017; 224:906–916.
- Chaudhari VA, Ostwal V, Patkar S, et al. Outcome of neoadjuvant chemotherapy in “locally advanced/borderline resectable” gallbladder cancer: the need to define indications. HPB. 2018; 20:841–847.
- Hakeem AR, Papulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer - a systematic review. Eur J Oncol Surg. 2019;45:83–91.
- Lee SE, Kim KS, Kim WB, et al., Korean Association of Hepato-Biliary and Pancreas Surgery. Practical guidelines for the surgical treatment of gallbladder cancer. J Korean Med Sci. 2014;29:1333–1340.
- Benson AB, D'Angelica MI, Abbot DE, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. Version 5. 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary_blocks.pdf
- Shindoh J, de Aretxabala X, Aloia TA, et al. Tumor location is a strong predictor of tumor progression and survival in T2 gallbladder cancer: an international multicenter study. Ann Surg. 2015; 261:733–739.
- Lee H, Choi DW, Park JY, et al. Surgical strategy for T2 gallbladder cancer according to tumor location. Ann Surg Oncol. 2015; 22:2779–2786.
- Lee SE, Jang JY, Lim CS, et al. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol. 2011;17:174–180.
- Aloia TA, Jarufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. HPB. 2015;17:681–690.
- Nigri G, Berardi G, Mattana C, et al. Routine extra-hepatic bile duct resection in gallbladder cancer patients without bile duct infiltration: a systematic review. Surgeon. 2016; 14:337–344.
- Ethun CG, Postlewait LM, Le N, et al. Routine port-site excision in incidentally discovered gallbladder cancer is not associated with improved survival: a multi-institution analysis from the US extrahepatic biliary malignancy consortium. J Surg Oncol. 2017; 115:805–811.
- Fuks D, Regimbeau JM, Le Treut YP, et al. Incidental gallbladder cancer by the AFC-GBC-2009 study group. World J Surg. 2011; 35:1887–1897.
- Fuks D, Regimbeau JM, Pessaux P, et al. Is port-site resection necessary in the surgical management of gallbladder cancer? J Visc Surg. 2013; 150:277–284.
- Witjes CD, van den Akker SA, Visser O, et al. Gallbladder cancer in the netherlands: incidence, treatment and survival patterns since 1989. Dig Surg. 2012; 29:92–98.
- Lindner P, Holmberg E, Hafstrom L. Gallbladder cancer - no improvement in survival over time in a Swedish population. Acta Oncol. 2018; 57:1482–1489.
- de Savornin Lohman E, de Bitter T, Verhoeven R, et al. Trends in treatment and survival of gallbladder cancer in the Netherlands; identifying gaps and opportunities from a nation-wide cohort. Cancers. 2020;12:918.
- Leinonen MK, Miettinen J, Heikkinen S, et al. Quality measures of the population-based Finnish cancer registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017; 77:31–39.
- Finnish National Institute of Health and Welfare. Somatic health care 2018. Database report 51/2019 [cited 2019 Dec 19]. Available from: http://www.julkari.fi/handle/10024/139004
- Danckert B, Ferlay J, Engholm G, et al. NORDCAN: cancer incidence, mortality and survival in the nordic countries, Version 8.2. 2019 Mar 26 [cited 2020 Jun 30].
- R Core Team. R: a language and environment for statistical computing. Vol. 2019. Vienna (Austria): R foundation for statistical computing; 2017. Available from: https://www.R-project.org/
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006; 118:1591–1602.
- Finnish National Institute of Health and Welfare. Annual number of operations. Database report. 2019 [cited 2021 Feb 20]. Available from: https://sampo.thl.fi/pivot/prod/fi/thil
- World Health Organization. Nutrition, physical activity and obesity. Finland; [cited 2021 Feb 26]. Available from: https://www.euro.who.int/__data/assets/pdf_file/0008/243296/Finland-WHO-Country-Profile.pdf
- Akhtar-Danesh N, Akhtar-Danseh GG, Seow H, et al. Treatment modality and trends in survival for gallbladder cancer: a population-based study. J Gastrointest Cancer. 2021; 52:256–262.
- Valle JW, Borbath I, Khan SA, et al., ESMO Guidelines Committee. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28–v37.
- Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019; 37:1015–1027.
- Kilander C, Mattsson F, Ljung R, et al. Systematic underreporting of the population-based incidence of pancreatic and biliary tract cancers. Acta Oncol. 2014; 53:822–829.
