Abstract
Objectives
Despite recombinant interferon-λ 4 (IFN-λ4) demonstrating anti-viral activity in vitro and the ancestral functional gene (IFNL4) being conserved in all other primates, there has been speculation that IFN-λ4 may be detrimental in humans. In light of recent rekindled interest in humoral immunity, this study aimed at evaluating the impact of baseline characteristics, including IFNL4, on antibody levels to hepatitis C virus (HCV).
Materials and methods
Pretreatment sera from 279 well-characterized North European Caucasians with chronic HCV genotype 2 or 3 infection having undergone liver biopsy were analyzed regarding IFNL4 (rs12979860) and anti-HCV antibody levels using a commercially available assay.
Results
Patients producing IFN-λ4 had higher signal to cut-off (S/CO) anti-HCV antibody ratios as compared with those lacking IFN-λ4 (IFNL4rs12979860 CT/TT versus CC, p<.0001, Mann–Whitney U-test). Additionally, in univariate analyses S/CO was significantly higher in men than women (p<.001), as well as in patients with absent/mild interface hepatitis (Ishak grade 0–2 versus 3–4, p = .009), and absent/mild steatosis (grade 0–1 versus 2–3, p = .0005). Also, an inverse correlation with HCV RNA level (rs= −0.14, p = .02) was noted. In multivariate analysis IFN-λ4, gender, steatosis and viral load remained independently associated.
Conclusions
To our knowledge, this is the first report that demonstrates that the ability to produce IFN-λ4, in addition to male gender, absent/mild steatosis, and lower viral load, augments antibody levels against HCV. This indicates that IFN-λ4 may be associated with T helper cell 2 (Th2) immune skewing, which might have clinical implications beyond HCV infection. ClinicalTrials.gov Identifier: NCT00143000
Introduction
Homozygous carriage of the C allele in rs12979860 in the proximity of the interleukin 28B gene (IL28B also known as interferon-λ 3 (IFNL3)) on chromosome 19 substantially increases the likelihood of spontaneous clearance of acute hepatitis C virus (HCV) infection [Citation1] as well as improved outcome following interferon-based HCV therapy [Citation2,Citation3]. Recently, however, this single-nucleotide polymorphism (SNP) was found to be located in intron 1 in a novel, previously overlooked gene encoding interferon-λ4 (IFNL4), and to be in strong linkage disequilibrium (LD) with an insertion/deletion polymorphism in IFNL4 exon 1 (rs368234815). Humans are polymorphic for the dinucleotide TT/ΔG allele in rs368234815 [Citation4], with the TT allele resulting in a frameshift which leads to pseudogenization of IFNL4 and nullification of IFN-λ4 production. In contrast, the ancestral wild-type allele ΔGrs368234815, which is present in all non-human primates [Citation5], allows for production of IFN-λ4.
IFN-λ4 is the most divergent member of the IFN-λ family and has only 29% amino acid identity with IFN-λ3, unlike the >80% homology observed between IFN-λ1, IFN-λ2 and IFN-λ3 [Citation4]. Recombinant IFN-λ4 signals through the IFN-λ receptor 1 [Citation6] to activate the JAK/STAT pathway, which subsequently induces expression of interferon-stimulated genes (ISGs). This in turn leads to high anti-viral activity, although the amino acid substitution K154 fixed throughout evolution in hominid lineages reduces the secretion and potency of IFN-λ4 in comparison to the ancestral E154 found in IFN-λ4 in chimpanzee and other mammals [Citation7].
IFN-λ4 expression in vitro in human hepatic cells (HepG2 and primary human hepatocytes (PHHs)) results in significant intracellular retention of IFN-λ4, reduced proliferation, increased cell death, but also in strong activation of ISGs in surrounding cells [Citation8]. Also, IFN-λ4 induction in Huh-7, HepG2 and PHHs cells leads to interferon-α unresponsiveness by upregulating levels of ISG15 and ubiquitin specific peptidase 18 (USP18) [Citation9].
Aside from impacting on HCV infection, abrogation of IFN-λ4 is also associated with more severe steatosis (Kleiner grade 3), lobular inflammation (grade 2–3), and hepatic fibrosis (stage F3–F4) in nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) especially among nonobese individuals [Citation10]. Similarly, inability to produce IFN-λ4 is associated with increased risk of some autoimmune disorders, e.g., lupus nephritis in systemic lupus erythematosus (SLE) [Citation11] and pulmonary fibrosis in systemic sclerosis (aka scleroderma) [Citation12], as well as with lower frequencies of regulatory T cells (Treg) in colonic tissue biopsies [Citation13].
Several studies have focused on IFN-λ4 and impaired adaptive and innate immunity [Citation14,Citation15], and unexpectedly there is scant in vivo evidence of potential benefits of having a functional IFNL4 gene aside from lower risk of some autoimmune diseases, despite being highly conserved in other primates. In light of this as well as renewed interest in humoral immunity, this study aimed at evaluating the impact of IFNL4 variants on anti-HCV antibody levels using a commercially available assay in a well-characterized cohort of North European Caucasian patients with chronic HCV genotype 2 or 3 infection having undergone liver biopsy.
Materials and methods
Study population
Three hundred eighty-two genotype 2 or 3 infected patients were enrolled in the NORDynamIC trial [Citation16] and previously genotyped for IFNL4rs12979860 [Citation17]. Pretreatment sera from 279 of these well-characterized patients of North European Caucasian origin (baseline characteristics summarized in ) could be retrieved and analyzed for anti-HCV antibody responses in this study.
Table 1. Baseline characteristics of the 279 patients included in the study.
Anti-HCV antibody detection
All samples were analyzed using the chemiluminescent microparticle immunoassay on the Alinity instrument (Abbott, Chicago, IL) in accordance with the manufacturer’s instructions. The assay measures the amount of anti-HCV antibodies against the recombinant HCV proteins HCr43 expressed in Escherichia coli (prepared by Chiron Corporation, Emeryville, CA), containing amino acids 1–150 in core and 1192–1457 (33c) in NS3, and c100-3 expressed in Saccharomyces cerevisiae (prepared by Chiron Corporation, Emeryville, CA), containing amino acids 1569-1931 in NS3, NS4a and NS4b. The chemiluminescent reaction is measured as relative light units (RLUs) detected by the system optics, and there is a direct relationship between the amount of anti-HCV in the sample and RLUs. A signal-to-cutoff ratio (S/CO) is determined by comparing the chemiluminescent RLUs in the reaction to the cutoff RLUs determined from an active calibration, and S/CO ≥1.0 is considered reactive. All sera were stored at −80 °C until analyzed.
IFNL4 genotyping
Rs12979860 polymorphisms in chromosome 19 were determined in serum by allelic discrimination using Taq-Man SNP Assays (Life Technologies, Carlsbad, CA) using an in-house test as described previously [Citation17].
HCV-RNA detection and genotyping
All HCV RNA samples were analyzed by Roche COBAS AmpliPrep/COBAS TaqMan HCV test as described by the manufacturer.
Liver biopsies
Liver biopsies were obtained from all patients within 12 months prior to study entry. The evaluation was performed in a blinded fashion according to the Ishak protocol [Citation18]. Additionally steatosis was graded [Citation19].
Statistical analyses
Mann–Whitney U test was used when comparing the amount of anti-HCV antibodies between groups. Logistic regression was applied after dichotomization of anti-HCV antibodies above or below study median. All parameters with a p-value above .1 in the univariate analysis was included in the multivariate analysis after checking for multicollinearity. Statistics was done in Prism (Version 9.0.1, GraphPad Software, La Jolla, CA) or SPSS (Version 27, IBM Corp, Armonk, NY) software. All reported p values are two-sided, and p values <.05 were considered significant.
Ethical considerations
Written informed consent was obtained from each participating patient, and ethics committees in each participating country approved the study. The study has been registered at the NIH trial registry (ClinicalTrials.gov Identifier: NCT00143000).
Results
IFNL4 and anti-HCV antibody level
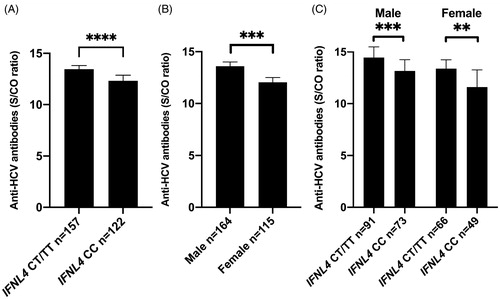
A highly significant relationship between IFNL4rs12979860 genotype and S/CO antibody level was observed (), with the lowest antibody levels (S/CO) observed in the absence of IFN-λ4 (IFNL4rs12979860 CC genotype) as compared to one (IFNL4rs12979860 CT) or 2 functional IFNL4 genes (IFNL4rs12979860 TT).
Figure 1. Signal-to-cutoff (S/CO) antibody ratio in relation to the absence of IFN-λ4 (IFNL4rs12979860 CC) or presence of IFN-λ4 (IFNL4rs12979860 CT/TT) (A), gender (B), and gender and IFN-λ4 (C). Median and interquartile range. Statistics using Mann–Whitney U test.
As IFNL4rs12979860 genotype is known to associate with interferon gamma-induced protein 10 (IP-10 aka CXCL10) [Citation20], the correlation between IP-10 and S/CO was also explored, and a non-significant trend was noted towards higher anti-HCV antibody levels when IP-10 concentrations were lower (p = .06).
As IFNL4rs12979860 genotype is known to associate with sustained virologic response (SVR) following interferon and ribavirin combination therapy for HCV, the correlation between SVR and S/CO was evaluated but was not significant.
Gender and anti-HCV antibody level
A marked gender difference in antibody levels was noted, with significantly higher S/CO in men as compared to women (p<.0001; ). For both gender the ability to produce IFN-λ4 entailed significantly higher antibody levels ().
Histopathology and anti-HCV antibody level
Significantly higher S/CO antibody levels were observed among patients with absent/mild interface hepatitis (Ishak grade 0–2 versus 3–4, p = .009; ) and absent/mild steatosis (grade 0–1 versus 2–3, p = .0005; ). As body mass index (BMI) and HCV genotype 3 infection are known to associate with steatosis [Citation19], their associations with anti-HCV S/CO were evaluated but were not significant (). A non-significant trend was noted towards lower antibody levels in patients with significant fibrosis (Ishak stage 0–2 versus 3–6 median 13.9 versus 13.0 respectively, p = .06). Please note, however, that when using univariate logistic regression this association was significant (p = .02; ).
Figure 2. Signal-to-cutoff (S/CO) antibody ratio in anti-HCV antibody assay in relation to absent/mild or moderate/severe interface hepatitis (Ishak grade 0–2 versus 3–4) (A), and absent/mild or moderate/severe steatosis (grade 0–1 versus 2–3) (B). Median and interquartile range. Statistics using Mann-Whitney U-test.
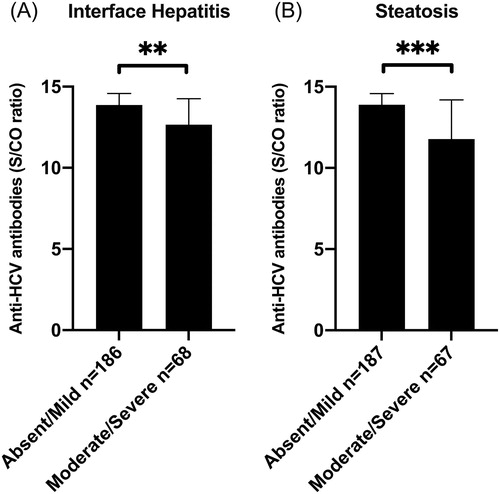
Table 2. Associations of baseline characteristics with anti-HCV S/CO ratio.
HCV RNA concentration and anti-HCV antibody level
An inverse correlation with HCV RNA level (rs= −0.14, p = .02) was noted. Although HCV RNA previously has been reported to be significantly higher in genotype 3 infected patients lacking IFN-λ4 (IFNL4rs12979860 CC genotype) as compared those with a functional gene (IFNL4rs12979860 CT/TT) enrolled in the NORDynamIC trial [Citation17], no significant differences in anti-HCV S/CO between patients infected with genotypes 2 and 3 were observed ().
Multivariate analysis and anti-HCV antibody level
Parameters associated with an anti-HCV S/CO value above or below study median were evaluated using logistic regression. IFN-λ4 genotype, gender, steatosis and viral load remained independently associated with the anti-HCV S/CO value ().
Discussion
The main findings of this study were that ability to produce IFN-λ4, in addition to male gender, absent/mild steatosis, and lower viral load, augmented antibody responses against HCV, and that highest antibody ratios were observed in carriers with two functional genes (IFNL4rs12979860 TT homozygotes) in both genders. To our knowledge, this is the first study to demonstrate that the ability to produce IFN-λ4 is associated with higher antibody levels against HCV.
Despite recombinant IFN-λ4 being highly antiviral in vitro [Citation7], and signaling through the IFN-λ receptor 1 to activate the JAK/STAT pathway and subsequently inducing expression of ISGs, the ability to produce IFN-λ4 in vivo is predictive of inferior likelihood of spontaneous resolution of acute HCV infection [Citation1] and unresponsiveness to interferon and ribavirin combination treatment [Citation2,Citation3]. Members of the IFN-λ family generally tend to favor a Th1 skewed immune response, i.e., greater activation of macrophages, cytotoxic T cells and NK cells as well as less stimulation of antibody producing B cells. However, data on IFN-λ4, which is the most divergent member of the interferon-λ family [Citation4], are scarcer in this regard [Citation21]. Interestingly, following immunization of 91 kidney, 64 lung, 24 liver and 17 heart transplant recipients on maintenance immunosuppression enrolled in a trial of intradermal versus intramuscular influenza vaccine, increased rates of seroconversion (i.e., ≥4-fold rise in titer from pre-vaccination to at least one of the three vaccine antigens) were noted in individuals capable of producing IFN-λ4 (IFNL4rs8099917 TG/GG) [Citation22], although the ethnic and racial background of the study population was not reported. Similarly, carriage of the minor G allele in rs10853727 located within IFNL4 has been associated with significantly higher antibody titers following measles vaccination in racially and ethnically diverse pediatric populations in two independent studies [Citation23,Citation24], although the impact of this particular SNP on expression or function of IFN-λ4 remains unclear. Together these previous studies support the observation noted in the present study that ability to produce of IFN-λ4 entails improved antibody responsiveness. However, the present study extends upon these findings as it excludes the potential confounding effect of ethnic and racial background, which are known to impact on many aspects of vaccine efficacy [Citation25].
The finding in this study suggesting that presence of IFN-λ4 predisposes towards a Th2-skewed immune response is intriguing and may indicate a shift from B- to T-cell responsiveness in humans following evolutionary pressure. Interestingly, the frameshift mutation in IFNL4 that rescinds IFN-λ4 production has been suggested to have appeared in homo sapiens just prior to the out-of-Africa migration, and thereafter enriched by positive selection [Citation26]. It is, thus, tempting to hypothesize that in smaller, isolated hunter–gatherer societies where exposure to epidemic pathogens is unlikely, Th2 responses may have greater importance, for example, stronger maternal antibody responses against enteric and endemic pathogenic microorganisms leading to reduce infant mortality. In contrast, after marked population growth occurred following the development of agriculture and domestication of animals in the Neolithic Revolution and subsequent urbanization, the introduction and spread of novel zoonic epidemic infectious agents was greatly facilitated, selecting for enhanced Th1 responsiveness by reducing or annulling the production of IFN-λ4.
The observation in the present study that antibody ratios were significantly higher in men than in women is in line with well-documented gender differences in immune responses observed in numerous vaccine trials [Citation27], as well as following SARS-CoV-2 infection [Citation28].
The impact of hepatic histopathology on antibody levels is noteworthy, and, to our knowledge, the highly significant association with steatosis has not previously been reported. In univariate analysis, interface hepatitis was also significant, but in multivariate analysis, steatosis grade was the only aspect of liver histopathology that remained independent associated with antibody levels, with more pronounced steatosis being linked with significantly lower antibody levels. This was somewhat surprising as neither body mass index (BMI) nor HCV genotype 3 infection, which both are known to associate with steatosis [Citation19], were not significantly associated with anti-HCV antibody levels. Interestingly, obesity is reported to be associated with a heightened state of immune activation as exemplified by elevated antibody titers to heat-shock protein-27 [Citation29], which additionally controverts the notion that higher BMI was driving the association between steatosis and antibody levels observed in this study.
The inverse correlation between anti-HCV antibody ratios and HCV RNA level was expected as baseline HCV RNA levels are reportedly elevated in the absence of IFN-λ4 (IFNL4rs12979860 CC) [Citation17]. However, in the multivariate analysis, both viral load and IFNL4 genotype were independently associated with anti-HCV antibody levels.
In conclusion, the present study demonstrated that IFNL4 genetic variants resulting in ability to produce IFN-λ4 are linked with elevated anti-HCV antibody levels. This finding implies that IFN-λ4 may impact on host antibody responses and warrants further investigation as this likely has clinical implications beyond HCV, e.g., levels of antibody against other viruses.
Author contributions
Je.Wa.: concept and design, experiments and procedures, and writing of article; K.H.: experiments and procedures, and writing of article; Jo.We.: concept and design, and writing of article; S.N.: experiments and procedures, and writing of article; P.C.: concept and design, and writing of article; M.F.: concept and design, and writing of article; K.M.: concept and design, and writing of article; N.L.: concept and design, and writing of article; G.N.: concept and design, and writing of article; M.L.: concept and design, experiments and procedures, and writing of article.
Acknowledgements
The authors thank Vilma Molnegren, Ludmila Adamek, Marliesa Griffin Wahlberg, and Anette Roth for technical assistance.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798–801.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401.
- Bochud PY, Bibert S, Negro F, et al. IL28B polymorphisms predict reduction of HCV RNA from the first day of therapy in chronic hepatitis C. J Hepatol. 2011;55(5):980–988.
- Prokunina-Olsson L, Muchmore B, Tang W, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45(2):164–171.
- Prokunina-Olsson L. Genetics of the human interferon Lambda region. J Interferon Cytokine Res. 2019;39(10):599–608.
- Hamming OJ, Terczynska-Dyla E, Vieyres G, et al. Interferon Lambda 4 signals via the IFNlambda receptor to regulate antiviral activity against HCV and coronaviruses. Embo J. 2013;32(23):3055–3065.
- Bamford CGG, Aranday-Cortes E, Filipe IC, et al. A polymorphic residue that attenuates the antiviral potential of interferon lambda 4 in hominid lineages. PLoS Pathog. 2018;14(10):e1007307.
- Onabajo OO, Porter-Gill P, Paquin A, et al. Expression of interferon Lambda 4 is associated with reduced proliferation and increased cell death in human hepatic cells. J Interferon Cytokine Res. 2015;35(11):888–900.
- Sung PS, Hong SH, Chung JH, et al. IFN-lambda4 potently blocks IFN-alpha signalling by ISG15 and USP18 in hepatitis C virus infection. Sci Rep. 2017;7(1):3821.
- Petta S, Valenti L, Tuttolomondo A, et al. Interferon lambda 4 rs368234815 TT > deltaG variant is associated with liver damage in patients with nonalcoholic fatty liver disease. Hepatology. 2017;66(6):1885–1893.
- Chen JY, Wang CM, Chen TD, et al. Interferon-lambda3/4 genetic variants and interferon-lambda3 serum levels are biomarkers of lupus nephritis and disease activity in Taiwanese. Arthritis Res Ther. 2018;20(1):193.
- Metwally M, Thabet K, Bayoumi A, et al. IFNL3 genotype is associated with pulmonary fibrosis in patients with systemic sclerosis. Sci Rep. 2019;9(1):14834.
- Mehta M, Hetta HF, Abdel-Hameed EA, et al. Association between IL28B rs12979860 single nucleotide polymorphism and the frequency of colonic Treg in chronically HCV-infected patients. Arch Virol. 2016;161(11):3161–3169.
- Obajemu AA, Rao N, Dilley KA, et al. IFN-lambda4 attenuates antiviral responses by enhancing negative regulation of IFN signaling. JI. 2017;199(11):3808–3820.
- O'Brien TR, Prokunina-Olsson L, Donnelly RP. IFN-lambda4: the paradoxical new member of the interferon lambda family. J Interferon Cytokine Res. 2014;34(11):829–838.
- Lagging M, Langeland N, Pedersen C, NORDynamIC Study Group, et al. Randomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology. 2008;47(6):1837–1845.
- Rembeck K, Alsio A, Christensen PB, et al. Impact of IL28B-related single nucleotide polymorphisms on liver histopathology in chronic hepatitis C genotype 2 and 3. PLoS One. 2012;7(1):e29370.
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699.
- Westin J, Nordlinder H, Lagging M, et al. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002;37(6):837–842.
- Lagging M, Askarieh G, Negro F, for the DITTO-HCV Study Group, et al. Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One. 2011;6(2):e17232.
- Egli A, Santer DM, O'Shea D, et al. The impact of the interferon-lambda family on the innate and adaptive immune response to viral infections. Emerg Microbes Infect. 2014;3(7):e51.
- Egli A, Santer DM, O'Shea D, et al. IL-28B is a key regulator of B- and T-cell vaccine responses against influenza. PLoS Pathog. 2014;10(12):e1004556.
- Haralambieva IH, Ovsyannikova IG, Kennedy RB, et al. Associations between single nucleotide polymorphisms and haplotypes in cytokine and cytokine receptor genes and immunity to measles vaccination. Vaccine. 2011;29(45):7883–7895.
- Dhiman N, Ovsyannikova IG, Cunningham JM, et al. Associations between measles vaccine immunity and single-nucleotide polymorphisms in cytokine and cytokine receptor genes. J Infect Dis. 2007;195(1):21–29.
- Al Rifai M, Khalid U, Misra A, et al. Racial and geographic disparities in influenza vaccination in the U.S. among individuals with atherosclerotic cardiovascular disease: Renewed importance in the setting of COVID-19. Am J Prev Cardiol. 2021;5:100150.
- Key FM, Peter B, Dennis MY, et al. Selection on a variant associated with improved viral clearance drives local, adaptive pseudogenization of interferon lambda 4 (IFNL4). PLoS Genet. 2014;10(10):e1004681.
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–349.
- Korte W, Buljan M, Rosslein M, et al. SARS-CoV-2 IgG and IgA antibody response is gender dependent; and IgG antibodies rapidly decline early on. J Infect. 2021;82(1):e11–e14.
- Tavallaie S, Rahsepar AA, Abdi H, et al. Association between indices of body mass and antibody titers to heat-shock protein-27 in healthy subjects. Clin Biochem. 2012;45(1-2):144–147.
