Abstract
Background
EUS-guided gastroenterostomy (EUS-GE) with lumen-apposing metallic stents (LAMS) in patients with gastric outlet obstruction (GOO) has proven to be an alternative to luminal stenting in the duodenum and surgical gastroenterostomy. In severely ill patients, the method can provide improved quality of life (QoL) and symptom relief by restoration of the luminal passage of fluid and nutrients to the small intestine.
Aim
To assess the technical and clinical success and safety of EUS-GE.
Material and methods
A dual center retrospective case series of 33 consecutive patients with GOO due to malignant (n = 28) or non-malignant conditions (n = 5). The patients were treated with EUS-GE using cautery enhanced LAMS. Procedures were performed guided by EUS and fluoroscopy in general anesthesia or conscious sedation.
Results
Technical success was achieved in all patients. The median procedure time was 71 min and the median hospital stay was three days. Thirty (91%) patients were able to resume oral nutrition after the procedure. Ten patients (30%) experienced adverse events (AEs), including migration of the stent, bleeding, and infection. Four patients had fatal AEs (12%). All stent-related AEs were handled endoscopically. Five patients (15%) needed re-intervention. The median survival time for patients with malignant obstruction was 8.5 weeks (0.5–76), and 13 patients with obstructing malignancies lived 12 weeks or longer.
Conclusion
EUS-GE is a minimally invasive and efficient method for restoration of the gastrointestinal passage and may improve palliative care for patients with GOO. The method has potential hazards and should only be offered in expert centers that regularly perform the procedure.
Introduction
Upper gastrointestinal tract and pancreaticobiliary cancers and metastases may cause gastric outlet obstruction (GOO) due to either strictures or extrinsic compression of the distal part of the stomach or the duodenum. This may also be the case in benign conditions e.g., chronic pancreatitis [Citation1].
Patients with GOO have a decreased quality of life (QoL) and shortened life expectancy. They are unable to consume food and liquids, which may lead to malnutrition and dehydration [Citation2]. Frequently, hospitalization due to the need for intravenous fluids and parenteral nutrition is required. Gastroduodenal stenting with either self-expandable metal stents (SEMS) and surgical gastroenterostomy (SGE) are both effective in relieving obstruction symptoms [Citation3]. Whereas enteral stenting provides prompt symptom relief and short hospital stay, it is associated with a considerable rate of stent malfunction (i.e., migration and obstruction), which often requires endoscopic or surgical reintervention [Citation3–6]. SGE provides better long-term effects and less need for reinterventions but is associated with higher morbidity and mortality [Citation7].
In this setting, EUS-guided gastroenterostomy (EUS-GE) represents an alternative, minimally invasive procedure, which may promote adjuvant or palliative treatment. Lumen apposing metal stents (LAMS), which were initially used for drainage of pancreatic fluid collections, have also been demonstrated to be applicable to establish a permanent gastro-intestinal anastomosis in an animal study [Citation7,Citation8]. Since EUS-GEs usually are positioned far from the primary obstruction, the risk for tumoral overgrowth is reduced. Therefore, fewer reinterventions may be needed after EUS-guided gastroenterostomy compared to SEMS [Citation9,Citation10].
In this retrospective study of consecutive patients treated with EUS-GE using LAMS, we assessed the technical and clinical success as well as the safety of the procedure.
Material and method
This is a retrospective Scandinavian dual center study including consecutive patients with malignant or benign GOO treated with EUS-GE from December 2016 to August 2020. Patient data were extracted from the electronic patient records. Technical success was defined as the ability under EUS guidance to place a cautery-enhanced LAMS between the stomach and the small bowel. Clinical success was assessed by the patients’ ability to resume oral intake of fluid or solid foods. The patient’s records were followed until the time of death or up to the time of the study (1 October 2020). We used the ASGE lexicon for endoscopic adverse events to classify adverse events (AEs) [Citation11]. The study was approved by the Institutional Ethics Committees at both centers and was carried out according to the Helsinki Declaration on Research in Medicine and Biology.
EUS-GE technique
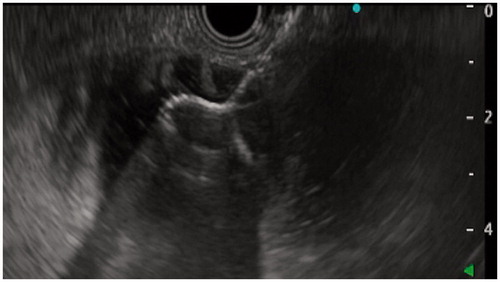
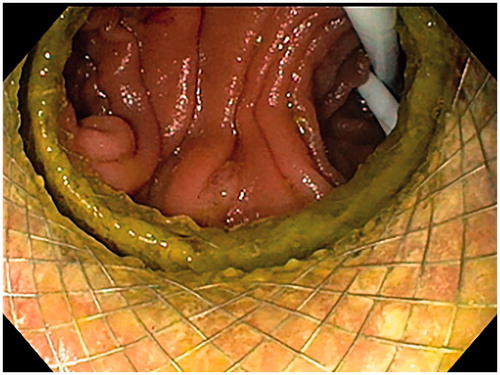
A linear echoendoscope (EG-3870 UTK, Pentax Medical, Tokyo, Japan, or GF-UCT 180, Olympus, Tokyo, Japan) with a 3.7–3.8 mm working channel was applied for the procedure. A long 0.025–0.035-inch guide wire was initially advanced through the stenosis in the upper GI-tract and into the small bowel. Over the guidewire, a 7–10 Fr naso-biliary catheter was introduced across the stenosis, and a mixture of indigo carmine, water, and contrast media (Omnipaque®, GE Healthcare, Chicago, IL) was injected through the catheter to expand the bowel segment. If we did not manage to pass a catheter through the stenosis, we performed a direct puncture of the best accessible jejunal loop from the stomach with a 19 G FNA-needle (EZ shot 3, Olympus, Tokyo, Japan). The jejunum was then expanded with the injection of contrast and saline through the FNA-needle. From the stomach, the echoendoscope was used to find a suitable location for entering the irrigated and expanded small bowel loop. We used the direct method for LAMS introduction into the small bowel, i.e., without a guidewire. We used LAMS with an electrocautery-enhanced delivery system (Hot Axios®, Boston Scientific Corp, Boston, MA) with diameters 15 or 20 mm to create the anastomosis (). After inserting the LAMS, we expanded the stent with a balloon-catheter up to 12–15 mm and endoscopically ensured the position in small bowel by observing small bowel mucosa or the reflux of indigo-carmine colored fluid through the LAMS ().
The first three patients were controlled with a barium swallow radiograph the day after the procedure. In all other patients, contrast was administered through the stent lumen at the end of the procedure under fluoroscopy to visualize a correct flow of contrast from the stomach into the small bowel. If this was confirmed, no post-procedure imaging was performed, and the patient was allowed to ingest liquids after 4 h and soft diet from the next day.
Statistical analysis
All variables are reported as numbers and/or percentages, using descriptive statistics. Normally distributed data are reported as mean and standard deviation (SD). For non-normally distributed data, median, and range are reported. Categorical data were analyzed in a cross table using the Chi-square test or Fischer’s exact test as appropriate. Statistical analysis was performed using SPSS, version 26 (IBM, Armonk, NY).
Results
A total of 33 patients (61% men; mean age 73 years (SD 13.3) underwent EUS-GE (). The GOO was due to malignant tumors or metastases in 28 (85%) and non-malignant conditions in five cases (15%) [Citation12]. An example of a LAMS stent in place in a patient with malignant duodenal obstruction is given in . The patients were generally in poor clinical condition before the EUS-GE with a median American Society of Anesthesiologists (ASA) physical status score of 3 (range 2–4).
Figure 3. CT image of EUS-guided gastroenterostomy made with a lumen apposing metal stent placed between the greater curvature of the stomach and a small bowel loop bypassing a tumor-stenosis in the hilar region of the liver.

Table 1. Demographic and procedural data.
The technical success rate was 100% (33/33) (), but in two patients two attempts were made before this was achieved. One of these patients had the first gastric fistula closed, and a second was made successfully during the same anesthesia session, while another had the gastric fistula closed with a clip, and a successful procedure with an EUS-guided GE-gastroenterostomy one week later. Thirty patients (91%) had an immediate clinical effect of the procedure, allowing them to drink and/or eat. One patient reported post-procedural aggravation of pain. The median hospitalization time was 3 days (1–12 days). The stents used had a diameter of 15 mm in 26 cases (79%) and 20 mm in 7 cases (21%). The saddle length of all the stents was 10 mm. The majority of the procedures (76%) were performed under general anesthesia, and 24% in sedation. The median procedure time was 71 (34–195) min.
Table 2. Results for 33 patients with EUS-guided gastroenterostomy.
On follow-up, 25 (89%) of patients with malignant GOO were deceased. The median survival time was 8.5 (0.5–76) weeks. Thirteen patients (46%) survived 12 weeks or longer, and only three needed endoscopic reinterventions. Two patients (one lymphoma, one duodenal cancer) were able to resume palliative chemotherapy.
We included five patients with non-malignant causes for GOO (). These patients had other serious comorbidities that made them unfit for surgical treatment or were unwilling to undergo surgery. All five patients were male, and their diagnoses, ASA score, type of anesthesia, survival time/follow-up time and status at the time of study are given in . In all patients with non-malignant causes for GOO, we experienced technical and clinical success. One of the patients experienced late stent displacement. The mean age in the group with malignant tumors causing GOO was 73.8 years (13.95) and in the group with non-malignant diagnoses it was 68.4 years (8.2) (n.s.). The median ASA score was 3 in both groups. Two of the patients lived 26 and 135 weeks with their LAMS-based gastroenterostomies. The other three patients were still alive at the time of the study.
Table 3. Patients with non-malignant indications for EUS guided gastroenterostomy.
Adverse events (AE) occurred in 10 cases (30%), of which 7 were related to the EUS-GE procedure (). According to the ASGE classification [Citation13], one AE could be classified as mild(10%), five as moderate (50%), and four as fatal (40%). The fatal AEs included two respiratory tract infections, one bleeding, and one refeeding syndrome. The fatal AEs occurred in consecutive patient number 13 in a total of 19 included in center A, and in patients 3, 4, and 7 in a total of 14 included in center B. Two of the AEs occurred during the procedure and were handled immediately, six AEs occurred within the first week after the procedure, and two AEs occurred later. The most frequent AEs were stent displacement/migration, which occurred in three cases (30%), lower airway infections in three cases (30%), and bleeding in two cases (20%). A trend of more frequent AEs were seen in patients who were treated in sedation 4/8 versus 6/25 in patients treated under general anesthesia (Fischer’s exact: n.s.). Three of the four fatal AEs happened in patients treated in general anesthesia.
Table 4. Adverse events.
Stent displacement/migration: One patient had a direct stent misplacement with the release of the distal flange into the peritoneum. This event was treated with endoscopic clipping of the gastric mucosal defect and decompression of the stomach. Two patients experienced stent migration several weeks after placing the LAMS. In one patient, the distal flange had dislodged from the small bowel and migrated into the transverse colon. The stent was removed endoscopically, the gastro-colonic fistula closed with endoscopic sutures, and a new EUS-GE was created during the same procedure [Citation13]. In the third patient with stent migration, the gastric fistula was closed with an over-the-scope clip (OTSC 12/6 GC, Ovesco®, Tübingen, Germany) using a twin grasper in the fistula, and the small bowel fistula closed spontaneously.
Bleeding: One patient experienced acute GI bleeding 1 day after the EUS-GE procedure below the gastric flange of the LAMS. The bleeding was stopped by placing an endoscopic hemoclip. The second patient experienced acute bleeding from a pancreatic tumor during the removal of an occluded duodenal stent. The stent removal was done during the same procedure as the EUS-GE. This patient subsequently died from bleeding.
Lower respiratory infections: Three patients experienced post-procedural lower respiratory infections. All were treated with intravenous antibiotics. One of the patients had peritoneal carcinomatosis with obstruction of the distal small bowel and acquired aspiration pneumonia after the procedure. Two of the patients died of lower airway infections.
Among the 27 (82%) patients who were deceased at the time of the data analysis, 20 (74%) experienced an event-free post-procedural period and died from the progression of their underlying disease. Two patients with non-malignant GOO died during the follow-up.
Discussion
In this study, we found that EUS-GE had a technical success rate of 94% in first attempt and 100% in the second. Clinical success, defined as the ability to tolerate regular intake of oral nutrients and water without vomiting, was achieved in 30 patients (91%). These numbers are similar to those previously reported [Citation14–18]. Khashab et al. compared the efficacy and safety of SGE with EUS-GE in a retrospective series including 93 patients and found that SGE had a higher technical success rate than EUS-GE (100% and 87%, respectively), but a similar clinical success rate [Citation19]. Two systematic reviews and meta-analyses from 2020, including 285 and 260 patients, respectively, with EUS-GE reported technical success of 92–93.5% and clinical success 90%. Both studies included 12 studies of GOO and/or studies using LAMS for ERCP access after gastric surgery [Citation20,Citation21].
We report an AE rate of 30% (). This rate is higher than reported in most other studies [Citation1,Citation10,Citation19,Citation20]. Seven AEs were attributable to the procedure or presence of the LAMS. A retrospective multicenter study compared 52 patients with endoscopic luminal stenting and 30 EUS-GE and found AE rates of 11.5% for luminal stenting and 16.7% for EUS-GE. The EUS-GE group, however, had a significantly lower need for re-intervention compared to the luminal stenting group, 4% versus 28.5%, respectively [Citation9,Citation22]. In a study comparing direct versus balloon-assisted EUS-GE in 77 patients, Chen et al. reported an AE rate of 6.5% for both methods combined, and only one AE was categorized as severe [Citation1]. Khashab et al. compared the safety of SGE (n = 63) and EUS-GE (n = 30) in a retrospective study and found an AE rate of 25% in SGE and 16% in EUS-GE, but due to small numbers, the difference was insignificant. Sixty percent of AEs in our study occurred within 2 weeks after the procedure. Unlike other reports, we experienced four fatal events. Lower respiratory tract infections occurred in three patients and were fatal in two. Both these patients had been intubated during the endoscopic procedure, so the airways had been protected perioperatively. We saw a trend in AEs of 4/8 (50%) treated in sedation, only one of these was fatal (bleeding). In patients treated under general anesthesia, 6/25 (24%) patients had an AE, and three of these were fatal (two lower respiratory infections and one refeeding syndrome). The numbers are very limited and the difference was not significant. However, we recommend to use general anesthesia, because it renders the operator more freedom to perform corrective procedures during the session. A recent European multicenter study of EUS-GE in malignant GOO from seven centers reported a similar rate of AEs, 26.7% in a cohort of 46 patients. Five patients (11.1%) had fatal AEs, four from stent misplacements leading to perforation and abdominal sepsis, and one due to postprocedural bleeding [Citation23]. Patients with malignant GOO often have advanced malignant disease, are fragile, and lower airway infections may be fatal. The GOO condition increases the risk of aspiration of bowel contents, and a EUS-GE may reduce this risk. In one of our patients with carcinomatosis, however, a more distal intestinal obstruction was present, and EUS-GE led to fatal aspiration, probably because of regurgitation of intestinal contents through the LAMS. One patient experienced fatal bleeding from pancreatic cancer after the removal of an occluded duodenal stent. This AE was not directly related to the LAMS or the EUS-GE itself. The last fatal AE happened in a severely malnourished patient with metastatic lung cancer who developed refeeding syndrome leading to death. In center B, all three fatal AEs occurred in the first half of the included patients, and none in the last half. Endoscopic reintervention was needed in five patients (15%), which is comparable to other reports. This includes the change of LAMS in one patient after 30 months using the established fistula. In a systematic review and meta-analysis, EUS-GE with LAMS had a frequency of AEs of 12% and a need for unplanned reintervention in 9%, but many included studies had a limited observation time [Citation9,Citation20]. The EUS-GE procedure is complex and technically challenging and requires precision and timing. In this material the very first cases of EUS-GE performed at each center are included, which may explain the higher AE rate.
Patients with malignant GOO have a poor prognosis due to their advanced malignant disease and tumor-directed therapy is often discontinued before EUS-GE is considered. The survival time is dependent on the stage of the disease at the time of intervention. In this study, we have not registered the stage of the disease at the time of EUS-GE. As EUS-GE becomes more established as a treatment modality for this severely ill group of patients, earlier referral in the course of the disease may further improve clinical success. EUS-GE was well tolerated also by very ill patients with a median ASA score of 3 at the time of the procedure. We found that these patients with malignant GOO had a median lifespan of 8.5 (0.5–76) weeks after the procedure. This indicates that the treatment is efficient in restoring the ability to absorb water and nutrients from the small bowel and may improve the QoL substantially in the course of the disease. Other studies report a longer survival time of 116 and 103 days, equivalent to 14.7–16.7 weeks, perhaps indicating that EUS-GE was performed at an earlier stage in many cases [Citation1,Citation9,Citation19)]. However, three of the patients with malignant conditions were still alive at follow-up, and 13 (46%) patients with malignancy survived 12 weeks or more.
Using EUS-GE for benign GOO is less studied, but good results are also reported for this group that needs a minimally invasive but long-lasting solution [Citation14]. In the present study, all five patients with benign GOO had technical- and clinical success with a median follow-up time of 26 weeks (20–135) with one case of late stent displacement, which was handled endoscopically.
The primary limitations of this study are its retrospective design and the limited sample size. In the future, prospective randomized studies comparing intraluminal stenting, EUS-GE and SGE are warranted with the main focus on QoL.
Conclusion
EUS-GE is an efficient and minimally invasive treatment option in the management of both malignant and benign GOO. It combines early symptom relief and a short hospital stay. It is a good option for treatment GOO caused by both malignant and non-malignant conditions. EUS-GE, however, remains a technically challenging procedure with a risk of serious AEs and should therefore be performed only in expert centers. There is a need for randomized controlled studies on the treatment options in GOO related to QoL outcomes.
Acknowledgments
The authors thank the dedicated endoscopy nurses and anesthesiology teams at Haukeland University Hospital, Bergen, Norway and Hvidovre Hospital, Copenhagen University Hospital, Copenhagen, Denmark for their cooperation, skills, and positive attitude for minimally invasive endoscopic procedures.
Disclosure statement
Pham K. D. C. is a consultant for Boston-Scientific, Olympus, Ambu and has received lecture fees from Taewoong Medical and Cook Medical. The other authors declare no conflict of interest.
Additional information
Funding
References
- Chen YI, Kunda R, Storm AC, et al. EUS-guided gastroenterostomy: a multicenter study comparing the direct and balloon-assisted techniques. Gastrointest Endosc. 2018;87(5).
- Perinel J, Adham M. Palliative therapy in pancreatic cancer-palliative surgery. Transl Gastroenterol Hepatol. 2019;4:28.
- Jeurnink SM, van Eijck CH, Steyerberg EW, et al. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7(1):18.
- Khashab M, Alawad AS, Shin EJ, et al. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg Endosc. 2013;27(6):2068–2075.
- Park JH, Song HY, Yun SC, et al. Gastroduodenal stent placement versus surgical gastrojejunostomy for the palliation of gastric outlet obstructions in patients with unresectable gastric cancer: a propensity score-matched analysis. Eur Radiol. 2016;26(8):2436–2445.
- van Halsema EE, Rauws EA, Fockens P, et al. Self-expandable metal stents for malignant gastric outlet obstruction: a pooled analysis of prospective literature. WJG. 2015;21(43):12468–12481.
- Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy. 2011;43(4):337–342.
- Binmoeller KF, Shah JN. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: a porcine study. Endoscopy. 2012;44(5):499–503.
- Chen YI, Itoi T, Baron TH, et al. EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017;31(7):2946–2952.
- Perez-Miranda M, Tyberg A, Poletto D, Toscano E, et al. EUS-guided gastrojejunostomy versus laparoscopic gastrojejunostomy: an International Collaborative Study. J Clin Gastroenterol. 2017;51:896–899.
- Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71(3):446–454.
- Pham KD, Havre RF. Treatment of aortoduodenal syndrome with endoscopic ultrasound-guided gastroenterostomy. Endoscopy. 2020;52(7):E227–E8.
- Pham KD, Havre RF. Endoscopic management of gastrocolic fistula after endoscopic ultrasound-guided gastrojejunostomy (EUS-GJ). Endoscopy. 2019;51(7):E169.
- Chen YI, James TW, Agarwal A, et al. EUS-guided gastroenterostomy in management of benign gastric outlet obstruction. Endosc Int Open. 2018;6(3):E363–E8.
- Ge PS, Young JY, Dong W, et al. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019;33(10):3404–3411.
- Itoi T, Tsuchiya T, Tonozuka R, et al. Novel EUS-guided double-balloon-occluded gastrojejunostomy bypass. Gastrointest Endosc. 2016;83(2):461–462.
- Kerdsirichairat T, Irani S, Yang J, et al. Durability and long-term outcomes of direct EUS-guided gastroenterostomy using lumen-apposing metal stents for gastric outlet obstruction. Endosc Int Open. 2019;7(2):E144–E50.
- Tyberg A, Perez-Miranda M, Sanchez-Ocana R, et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: a multicenter, international experience. Endosc Int Open. 2016;4(3):E276–81.
- Khashab MA, Bukhari M, Baron TH, et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017;05(04):E275–E81.
- Iqbal U, Khara HS, Hu Y, et al. EUS-guided gastroenterostomy for the management of gastric outlet obstruction: a systematic review and meta-analysis. Endosc Ultrasound. 2020;9(1):16–23.
- Antonelli G, Kovacevic B, Karstensen JG, et al. Endoscopic ultrasound-guided gastro-enteric anastomosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(11):1294–1301.
- Chen YI, Itoi T, Baron TH, et al. Erratum to: EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017;31(9):3765.
- Kastelijn JB, Moons LMG, Garcia-Alonso FJ, et al. Patency of endoscopic ultrasound-guided gastroenterostomy in the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2020;8(9):E1194–E201.