Abstract
Objectives
Pancreatic exocrine insufficiency (PEI) is prevalent in diabetes. Pathophysiological theories imply autoimmune destruction, lack of trophic effects of insulin or impaired neuronal stimulation, but the relationship between PEI and autonomic dysfunction is largely unknown. In a pilot study, we aimed to investigate if patients with diabetes and PEI had impaired autonomic function.
Methods
We measured faecal elastase in 59 patients with type 1 or 2 diabetes, using a cut-off-value <200 μg/g to define PEI. Based on faecal elastase results, patients were stratified into matched case (n = 8) and control groups (n = 13). We used heart rate variability, baroreflex sensitivity and orthostatic hypotension tests to assess autonomic dysfunction.
Results
All baroreflex sensitivity parameters were reduced in cases with PEI compared with controls (all p < .05). The heart rate variability parameters root mean square of successive RR interval differences (p = .05) and high frequency (p = .04) were also reduced. We found no difference in orthostatic hypotension between the groups.
Conclusions
In this first-of-its-kind study, we found that diabetes patients with PEI had reduced autonomic function compared with matched controls. Although numbers are small, results support the hypothesis that autonomic dysfunction could be a contributor to PEI in diabetes.
Introduction
Diabetes mellitus is characterized by elevated blood glucose levels, attributable to dysfunction of the endocrine pancreas. Pancreatic exocrine insufficiency (PEI) is defined as insufficient secretion of pancreatic exocrine enzymes and bicarbonate-rich fluid, and may lead to clinical consequences such as fat malabsorption, steatorrhea, and malnutrition [Citation1]. This condition may appear as a late diabetes complication, where the latest studies estimate a PEI prevalence at 5%–17% of patients with type 1 or type 2 diabetes [Citation2–5].
There are several theories for the pathophysiological mechanisms leading to PEI in diabetes (). It is still unclear if the exocrine dysfunction is a primary event caused by pancreatic atrophy or glandular damage, or a secondary event, where the exocrine tissue is intact, but improperly activated by digestive reflexes [Citation6]. The autonomic nervous system is involved in both the cephalic, gastric and intestinal phases of exocrine pancreatic secretion through different vagus-mediated reflexes [Citation7–9]. Studies have suggested that enteropancreatic signaling may be responsible for as much as 50% of all post-prandial pancreatic secretion [Citation10]. Given that the pancreas is mainly innervated by small, unmyelinated nerve fibers, this autonomic neural regulation could be vulnerable for diabetes-induced damage [Citation11,Citation12]. Indeed, autonomic neuropathy is a common late complication of diabetes, yet the impact of autonomic neuronal dysfunction on the exocrine pancreas is scarcely studied in diabetes patients [Citation13,Citation14].
Figure 1. The main theories for pathophysiological mechanisms leading to pancreatic exocrine insufficiency in diabetes. The illustration is modified after Zsóri et al. [Citation1].
![Figure 1. The main theories for pathophysiological mechanisms leading to pancreatic exocrine insufficiency in diabetes. The illustration is modified after Zsóri et al. [Citation1].](/cms/asset/1a29e719-4b1c-481f-a385-14745ffcb078/igas_a_1957496_f0001_c.jpg)
Thus, in this case-control pilot study we investigated the autonomic function by state-of-the-art methods in patients with type 1 and type 2 diabetes and PEI, comparing them to a matched control group. We hypothesised that cases with PEI had impaired autonomic function, compared to diabetes patients without PEI.
Materials and methods
The study was part of a collaborative research project between Aalborg University Hospital, Aalborg, Denmark, and Haukeland University Hospital, Bergen, Norway. Main protocol and findings are presented elsewhere [Citation2]. Here, we present the analysis of autonomic nerve dysfunction in the subset of patients recruited in Bergen.
Study population
We recruited patients from the outpatient clinic at the Department of Medicine, Haukeland University Hospital. Inclusion criteria were type 1 or type 2 diabetes and age 18–75 years. Exclusion criteria were chronic pancreatitis, coeliac disease, known cardiac arrhythmias, previous upper gastrointestinal surgery, alcoholism, drug abuse and intake of orlistat or acarbose.
Upon signed inclusion, a detailed anamnesis was ascertained. Stool frequency was recorded and stool characteristics assessed using The Bristol Stool Scale. The scale describes seven types of stool, ranging from constipation (type 1 and 2) to diarrhoea (type 6 and 7) [Citation15]. Participants also delivered blood and stool samples for the measurement of glycosylated haemoglobin (HbA1c) and faecal elastase, respectively. To analyse faecal elastase, we used ScheBo® Pancreatic Elastase 1 Stool Test (Biotech AG, Giessen, Germany).
Faecal elastase values were then used to group participants into cases (faecal elastase <200 µg/g) or controls (faecal elastase ≥200 µg/g). Cases and a similar-sized control group drawn from the cohort with normal faecal elastase, were examined with cardiac autonomic function tests, as described below. Controls were invited manually by attempting to match the following characteristics: sex, age, diabetes type and duration. As per clinical practice, computer tomography and/or abdominal ultrasound was performed to exclude structural changes indicative of chronic pancreatitis in patients with faecal elastase <200 µg/g.
Autonomic function tests
Cardiac autonomic function was investigated using the Heart Rhythm Scanner PE and the Biocom 5000® Bluetooth Electrocardiogram Recorder (Biocom Technologies, Poulsbo, USA). The test battery is in line with the American Association of Clinical Endocrinologists’ Position Statement of 2018, and includes both heart rate variability as well as cardiac autonomic reflex tests and orthostatic hypotension [Citation16,Citation17]. The protocol has been previously described in detail [Citation18]. All tests were performed in a fasting and relaxed state. First, we recorded resting heart rate variability with the participants in a semi-reclined position for five minutes. Results were manually edited off-line, in order to remove artefacts or erroneously detected heart beats. The software subsequently calculated the standard heart rate variability parameters as well as spectral frequency analyses. Next, we used the same equipment to investigate baroreflex sensitivity. The participants were instructed to breathe deeply at a rate of five breaths per minute. Changes in heart rate variability, as well as the expiration-inspiration ratio of the RR-intervals were calculated. Finally, we conducted orthostatic blood pressure testing, using the Welch Allyn ProBP 3400 (Welch Allyn Inc., Skaneateles Falls, USA). The blood pressure was measured while participants were lying on a couch, then after zero, one and three minutes of standing. Orthostatic hypotension was defined as a drop of ≥20 mmHg in systolic blood pressure or ≥10 mmHg in diastolic blood pressure from recumbent to standing posture [Citation19].
Ethical considerations
The study was approved by the Western Norway Regional Committee for Medical and Health Research Ethics (2013/2333). It was conducted in accordance with the Declaration of Helsinki. Prior to any study-related procedures, patients were provided with information about the project and eventual risks related to the examinations. Participants signed informed consents.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics (Ver. 26, IBM Corporation, USA). We defined p≤ .05 as the level of significance. Due to low numbers, we did not employ the Shapiro-Wilks test for normality. To maximize conservatism, we used non-parametrical testing throughout the study. Results are stated as median (interquartile range, IQR) or n (percentage, %). To investigate differences between categorical variables, we used Pearson’s chi-square test, while differences between continuous variables were determined using Mann–Whitney U-test.
Results
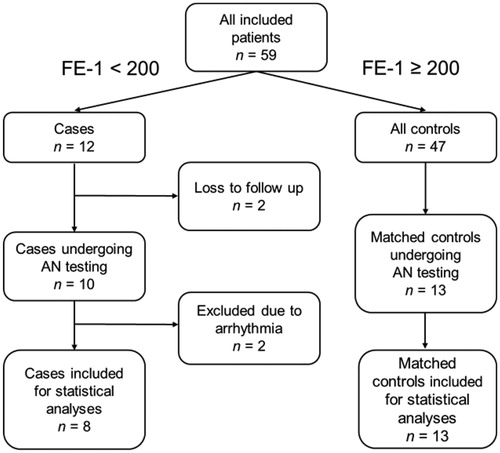
Fifty-nine patients were included in the study: 32 (54%) women, 31 (53%) type 1 diabetes with a median age of 53 (15) years. Clinical characteristics are given in . We identified 12 cases with faecal elastase <200 µg/g (five of these were <100 µg/g), giving a 20% point prevalence of PEI. Two cases withdrew from the study prior to autonomic testing, which were performed on 10 cases and 13 controls. Subsequently, two of the cases had to be excluded due to previously unknown cardiac arrhythmias: One due to frequent (>10%) premature ventricular activities, making heart rate variability analysis by our device impossible. The other due to sinus arrhythmia, with heart rate variability values in the extreme outlier range. A detailed inclusion flow chart is presented in . Use of medications was comparable in the two groups: three cases and two controls were on anti-platelets and three cases and four controls on statins. Further, one participant in each group used beta-blockers, whereas four cases and four controls were on other antihypertensives.
Table 1. Clinical characteristics.
Group comparisons
As shown in , PEI was more frequent in patients with type 1 diabetes (p = .02). Those with PEI also trended towards having a longer diabetes duration (p = .08) and a lower Bristol Stool Scale score (p = .09). There were no differences between the groups in stool frequency, age, body mass index (BMI) and HbA1c levels. Neither did PEI patients have increased number of late complications (microangiopathies). As intended by the group stratification, there were no significant difference in clinical characteristics between the case and control groups. Plasma glucose levels at autonomic function test start did not differ between the two groups, p = .47.
Autonomic function tests
Results from the resting heart rate variability and baroreflex sensitivity tests are presented in and in and , respectively. Cases had reduced values in all baroreflex sensitivity variables: Standard deviation of heart rate (p = .03), maximal variance of heart rate (p = .01), mean variance of heart rate (p = .03) and expiration/inspiration ratio (p = .02). The resting heart rate variability parameters root mean square of successive RR interval differences (p = .05) and high frequency (p = .04) were also reduced in the PEI group.
Figure 3. Heart-rate-variability parameters in cases (left) and controls: (a) Mean HR; (b) Mean NN; (c) SDNN; (d) RMSSD; (e) Total power; (f) VLF; (g) LF; (h) HF; (i) LF norm; (j) HF norm; (k) LF/HF ratio. Statistical significance of p≤.05 are marked by *. Results are given as median (IQR). Abbreviations: HR: Heart rate; SDNN: Standard deviation of NN intervals (inter-beat intervals where artefacts are removed); RMSSD: Root mean square of successive RR interval differences; VLF: Very low frequency; LF: Low frequency; HF: High frequency; LF norm: Low frequency normalized units; HF norm: High frequency normalized units; LF/HF ratio: Low frequency/high frequency ratio; IQR: Interquartile range.
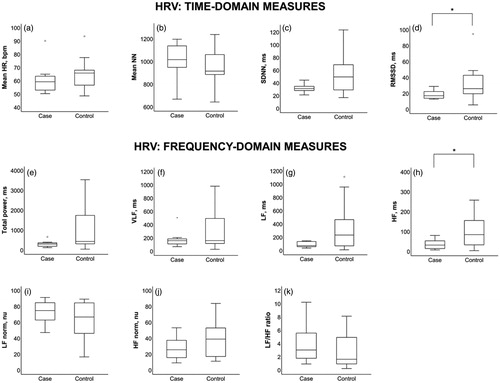
Figure 4. Baroreflex sensitivity parameters in cases (left) and controls: (a) SD of HR; (b) Maximal variance of HR; (c) Mean variance of HR; (d) E/I ratio. Statistical significance of p≤.05 are marked by *Results are given as median (IQR). SD of HR: Standard deviation of heart rate; E/I ratio: Expiration/inspiration ratio; IQR: Interquartile range.
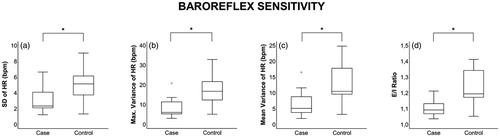
Table 2. Autonomic function tests.
Resting systolic blood pressure was 130 (23) mmHg in cases and 129 (28) mmHg in controls, p = .37. Resting diastolic blood pressure was 80 (6) mmHg in cases and 77 (9) mmHg in controls, p = .16. Resting pulse was 59 (11) beats per minute in cases and 65 (19) in controls, p = .41. The two groups showed no difference in orthostatic blood pressure or pulse values at any time point, all p> .16. Two cases (25%) and three controls (23%) had orthostatic hypotension, χ2 (1)=0.10, p = .92.
Discussion
In a case-control pilot study, we tested the hypothesis that diabetes patients with pancreatic exocrine insufficiency (PEI) had reduced autonomic function compared to patients with normal exocrine function. Measuring faecal elastase in 59 patients with type 1 and type 2 diabetes, we identified 12 (20%) with PEI. In eight of these patients and a matched control group, we investigated cardiac autonomic function. We found that patients with PEI had reduced baroreflex sensitivity and heart rate variability, but not increased frequency of orthostatic hypotension. Although cross-sectional, our findings are not contrary to the hypothesis that autonomic dysfunction is a causal factor in the development of PEI in diabetes.
Autonomic dysfunction has been referred to for decades as a potential explanation for PEI in diabetes, but surprisingly little research has looked into this association [Citation14]. So far, the 1987 study by El Newihi and colleagues, is the most noteworthy investigating this issue. They assessed pancreatic exocrine function by measuring duodenal aspirate in 8 healthy volunteers and 10 patients with type 2 diabetes, chronic diarrhoea and peripheral neuropathy. Both groups had similar basal pancreatic secretion, but after administration of intrajejunal amino acids and intravenous secretin-cholecystokinin, the diabetes patients had reduced output of amylase and bicarbonate [Citation10]. As an explanation for their results, the authors proposed impairment of enteropancreatic reflexes as a likely pathophysiological mechanism [Citation10]. A few studies have also examined plasma pancreatic polypeptide responses following a meal, finding reduced levels in diabetes patients with autonomic neuropathy [Citation20–22]. However, postprandial pancreatic polypeptide has an unsatisfactory correlation with pancreatic exocrine function [Citation23].
As the first study finding an association between autonomic dysfunction and PEI in diabetes using state-of-the-art technology, our results are both novel and biologically plausible [Citation1]. Secretion of pancreatic digestive enzymes is mainly mediated by cholecystokinin, which is released upon duodenal exposure to peptides, amino acids and fatty acids [Citation7]. The presence and physiological importance of cholecystokinin A-receptors in human pancreatic acinar cells have been debated, with completely disparate findings [Citation8,Citation24,Citation25]. Instead, cholecystokinin is thought to mediate most of its stimulatory effect indirectly through vagal neurons [Citation7]. Since the vagus nerve plays such a central role in the regulation of digestive enzyme secretion, it is likely to make the pancreas susceptible to autonomic neuropathy. If vagal signalling is impaired, there might be insufficient stimulation of acinar cells, contributing to decreased enzyme secretion and the reduced faecal elastase levels seen in our case group [Citation8,Citation9].
Our findings should be investigated further in a larger follow-up study using different measures of pancreatic exocrine function. While faecal elastase is an indirect test for digestive enzyme secretion, the secretin stimulation test directly measures bicarbonate secretion into the duodenum after an intravenous secretin-infusion [Citation23,Citation26]. In contrast to cholecystokinin, secretin has a direct stimulatory effect on pancreatic ductal cells [Citation9]. If enteropancreatic signalling is impaired due to autonomic neuropathy, but pancreatic tissue otherwise remains intact, we would hypothesise to find reduced faecal elastase, but normal or near-normal bicarbonate levels in duodenal aspirates after direct stimulation by intravenous secretin.
In diabetes patients, impaired neural regulation of pancreatic exocrine function may have serious clinical consequences. Despite having a normal diet, patients may develop malabsorption, steatorrhea, weight loss and various nutritional deficiencies, including reduced levels of fat soluble vitamins [Citation27]. This may in turn increase the risk of developing sarcopenia and osteoporosis [Citation28]. Some may also experience abdominal discomfort, mild pain, flatulence and increased difficulties with glycaemic control [Citation28]. In our study, we did not find any difference in glycosylated haemoglobin (HbA1c) between patients with PEI and those with normal faecal elastase, nor any difference in BMI (). However, we found a higher frequency of PEI in type 1 diabetes and a borderline significant longer disease duration in the PEI group (22 vs. 11 years), supporting conclusions from previous studies that PEI indeed could be regarded as a diabetic late complication [Citation1,Citation2].
In addition to the association with PEI, autonomic dysfunction in diabetes is associated with other gastrointestinal complications, as well as increased cardiovascular mortality [Citation16,Citation29–31]. To assess autonomic dysfunction in this study, we used standardized methods for detecting cardiac autonomic neuropathy [Citation16]. Heart rate variability at rest is a general measure of the body’s ability to alter the heart rate to meet the physiological demand, while the baroreflex sensitivity test investigates the parasympathetic nerve fibres’ ability to adjust the heart rate in response to changes in respiration-induced blood pressure fluctuation [Citation16,Citation29]. Whereas reduced heart rate variability and impaired baroreceptor reflex may occur early in diabetes, orthostatic hypotension often develops at a later stage and might also represent sympathetic dysfunction [Citation16]. This may explain why our PEI patients had reduced heart rate variability and baroreflex sensitivity compared to controls, while we could not find any differences in orthostatic blood pressure.
Our study has some limitations. Being a pilot, the main limitation is the small size of the material, limiting our possibility to perform multivariate analyses. We tried to compensate by matching case and control groups by age, sex, diabetes type and duration, however, good matching is hard to achieve when numbers are small. Importantly, the groups were similar in terms of cardiovascular disease, which might have an impact on cardiac autonomic function. Despite the numbers, our results showed a clear difference in all baroreflex sensitivity parameters and two heart rate variability parameters, increasing the robustness of our findings. To determine pancreatic exocrine function, we used faecal elastase instead of direct hormone-stimulated tests, which is considered gold standard. Faecal elastase has high diagnostic accuracy for detection of moderate to severe PEI, but performs worse in identifying mild PEI [Citation23,Citation32]. The fact that we only used one test could lead to overestimation of the true prevalence of PEI [Citation33]. Faecal elastase is also prone to false positive test results in patients with watery diarrhoea [Citation34]. As both cases and controls had normal stool consistency, this was most likely not an issue in our study. Since patients were allowed to continue their regular drugs, we are unable to rule out an eventual drug influence on autonomic test results. However, most drugs increase the heart rate variability, and resting heart rates were similar in cases and controls, both supporting the notion that drugs did not impact the results [Citation35]. Finally, as we did not perform additional radiological testing or had information about antibody status, some patients may have been misclassified as type 1 or type 2 diabetes instead of pancreatogenic diabetes (type 3c diabetes) [Citation36].
Conclusions
In this first-of-its-kind pilot study we found that diabetes patients with low faecal elastase had signs of impaired autonomic nerve function compared to matched controls. Although not excluding other factors, these results support the theory that autonomic dysfunction is a contributor to PEI in type 1 and type 2 diabetes. There is a paucity of studies in this field, and we would encourage larger studies, also utilizing direct tests for pancreatic exocrine function and more extensive autonomic function mapping.
| Abbreviations | ||
| ELISA | = | enzyme-linked immunosorbent assay |
| FE-1 | = | faecal elastase-1 |
| HF | = | high frequency |
| HRV | = | heart-rate-variability |
| PEI | = | pancreatic exocrine insufficiency |
| RMSSD | = | root mean square of the standard deviation |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Zsóri G, Illés D, Terzin V, et al. Exocrine pancreatic insufficiency in type 1 and type 2 diabetes mellitus: do we need to treat it? A systematic review. Pancreatology. 2018;18(5):559–565.
- Søfteland E, Poulsen JL, Starup-Linde J, et al. Pancreatic exocrine insufficiency in diabetes mellitus - prevalence and characteristics. Eur J Intern Med [Internet]. 2019;68:18–22. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0953620519302547
- Terzin V, Várkonyi T, Szabolcs A, et al. Prevalence of exocrine pancreatic insufficiency in type 2 diabetes mellitus with poor glycemic control. Pancreatology. 2014;14(5):356–360.
- Demir K, Karaca C, Ahishali E, et al. A cross-sectional study to assess the prevalence of pancreatic exocrine insufficiency among diabetes mellitus patients in Turkey. Pancreas. 2016;45(7):e39–e40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27400162
- Vujasinovic M, Zaletel J, Tepes B, et al. Low prevalence of exocrine pancreatic insufficiency in patients with diabetes mellitus. Pancreatol. 2013;13(4):343–346.
- Campbell-Thompson M, Rodriguez-Calvo T, Battaglia M. Abnormalities of the exocrine pancreas in type 1 diabetes. Curr Diab Rep. 2015;15(10):75.
- Pandol SJ. Pancreatic secretion. In: Feldman M, Friedman L, Brandt L, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 10th ed. Philadelphia (PA): Elsevier Saunders; 2016. p. 934–943.
- Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127(3):957–969.
- Chey WY, Chang T-M. Secretin: historical perspective and current status. Pancreas. 2014;43(2):162–182.
- el Newihi H, Dooley CP, Saad C, et al. Impaired exocrine pancreatic function in diabetics with diarrhea and peripheral neuropathy. Dig Dis Sci. [Internet]. 1988;33(6):705–710. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2897272
- Chey WY, Chang T. Neural hormonal regulation of exocrine pancreatic secretion. Pancreatology. 2001;1(4):320–335.
- Chien H-J, Chiang T-C, Peng S-J, et al. Human pancreatic afferent and efferent nerves: mapping and 3-D illustration of exocrine, endocrine, and adipose innervation. Am J Physiol Gastrointest Liver Physiol. 2019;317(5):G694–706.
- Tesfaye S, Toronto Diabetic Neuropathy Expert Group, Boulton AJM, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care [Care]. 2010;33(10):2285–2293. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2945176&tool=pmcentrez&rendertype=abstract
- Hardt PD, Ewald N. Exocrine pancreatic insufficiency in diabetes mellitus: a complication of diabetic neuropathy or a different type of diabetes? Exp Diabetes Res. 2011;2011:761950.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol stool form scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(7):693–703.
- Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Vol. 43Diabetes Diabetes Metab J. 2019;43(1):3–30.
- Vinik AI, Camacho PM, Davidson JA, Handelsman Y, Lando HM, Leddy AL, et al. American association of clinical endocrinologists and American college of endocrinology position statement on testing for autonomic and somatic nerve dysfunction. Endocr Pract. 2017;23(12):1472–1478.
- Sangnes DA, Søfteland E, Bekkelund M, et al. Wireless motility capsule compared with scintigraphy in the assessment of diabetic gastroparesis. Neurogastroenterol Motil [Internet]. 2019;32(4):e13771. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/nmo.13771.
- Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation [Internet]. 2007;115(3):387–397. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17242296
- Lugari R, Gnudi A, Dall'Argine P, et al. Diabetic autonomic neuropathy and impaired human pancreatic polypeptide secretion in response to food. J Clin Endocrinol Metab. 1987;64(2):279–282.
- Hossdorf T, Wagner M, Hoppe HW. Pancreatic polypeptide (PP) response to food in type I diabetics with and without diabetic autonomic neuropathy. Hepatogastroenterology. 1988;35(5):238–241.
- Glasbrenner B, Dominguez-Munoz E, Riepl RL, et al. Cholecystokinin and pancreatic polypeptide release in diabetic patients with and without autonomic neuropathy. Digest Dis Sci. 1995;40(2):406–411.
- Chowdhury RS, Forsmark CE. Review article: pancreatic function testing. Aliment Pharmacol Ther. 2003;17(6):733–750.
- Murphy JA, Criddle DN, Sherwood M, et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterol. 2008;135(2):632–641.
- Liang T, Dolai S, Xie L, et al. Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J Biol Chem. 2017;292(14):5957–5969.
- Erchinger F, Engjom T, Tjora E, et al. Quantification of pancreatic function using a clinically feasible short endoscopic secretin test. Pancreas [Internet]. 2013;42(7):1101–1106. Available from: https://insights.ovid.com/crossref?an=00006676-201310000-00008
- Lindkvist B, Nilsson C, Kvarnström M, et al. Importance of pancreatic exocrine dysfunction in patients with type 2 diabetes: a randomized crossover study. Pancreatology. 2018;18(5):550–558.
- Radlinger B, Ramoser G, Kaser S. Exocrine pancreatic insufficiency in type 1 and type 2 diabetes. Curr Diab Rep. 2020;20(6):18.
- Vinik AI, Casellini C, Parson HK, et al. Cardiac autonomic neuropathy in diabetes: a predictor of cardiometabolic events. Front Front Neurosci. 2018;12:591.
- Brock C, Søfteland E, Gunterberg V, et al. Diabetic autonomic neuropathy affects symptom generation and brain-gut axis. Diabetes Care [Internet]. 2013;36(11):3698–3705. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24026548
- Wegeberg AML, Brock C, Ejskjaer N, et al. Gastrointestinal symptoms and cardiac vagal tone in type 1 diabetes correlates with gut transit times and motility index. Neurogastroenterol Motil. 2020;33(1):e13885.
- Leeds JS, Oppong K, Sanders DS. The role of fecal elastase-1 in detecting exocrine pancreatic disease. Nat Rev Gastroenterol Hepatol. 2011;8(7):405–415.
- Vanga RR, Tansel A, Sidiq S, et al. Diagnostic performance of measurement of fecal elastase-1 in detection of exocrine pancreatic insufficiency: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(8):1220–1228.e4.
- Engjom T, Erchinger F, Laerum BN, et al. Diagnostic accuracy of a short endoscopic secretin test in patients with cystic fibrosis. Pancreas [Internet]. 2015;44(8):1266–1272. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006676-201511000-00014
- Schönauer M, Thomas A, Morbach S, et al. Cardiac autonomic diabetic neuropathy. Diab Vasc Dis Res. 2008;5(4):336–344. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18958844
- Ewald N, Bretzel RG. Diabetes mellitus secondary to pancreatic diseases (Type 3c)-are we neglecting an important disease? Eur J Intern Med. 2013;24(3):203–206.