Abstract
Objective
Simulated endoscopic training can be challenging and stressful for the novice trainee. The absence of a reliable stress detection method during simulated endoscopic training makes estimating trainees’ mental stress difficult to quantify. This study concomitantly measures the responses of four saliva stress biomarkers and compares them to the video score (VS) achieved by novice endoscopists in a reproducibly stressful simulation environment.
Methods
Thirty-six male endoscopy naïve surgery residents were enrolled. After an orientation phase, a saliva specimen was collected for cortisol (sC), alpha-amylase (sAA), Chromogranin A (sCgA), and immunoglobulin A (sIgA) measurements (baseline phase, BL). Thereafter, the simulation exercise phase (E) started, practicing in the Fundamentals of Endoscopic Surgery Skills module (GI-Bronch Mentor). Immediately after, a second saliva sample for measuring the above-cited biomarkers was collected. The whole experiment was videotaped, and the VS was calculated. The percentage (E-BL)diff of each of the four saliva biomarkers was calculated and examined for correlation to VS.
Results
sCgAdiff showed the best correlation with VS, followed by sAAdiff.
Conclusions
sCgA and sAA, are saliva stress biomarkers that are easy to collect non-invasively and showed the best correlation with novice endoscopist’s performance in our simulation setting, and therefore, they could be used for monitoring stress.
Introduction
Stress is commonly associated with impaired performance, which might lead to complications and possibly disastrous effects on patient outcomes [Citation1,Citation2]. Moreover, it has been shown that novices/trainees perceive greater stress than the experienced [Citation3].
Measuring actual stress during a procedure is not always feasible because of several practical issues. Simulation is an established method for acquiring and improving both technical and non-technical skills in a controlled, reproducible, and quantitative environment that replicates real psychological challenges and mental stress [Citation4]. Therefore, the simulation milieu has been used to assess stress and develop intraoperative stress management training [Citation3,Citation5].
Stress is a psychological construct, and therefore there is an obvious difficulty to objectively measure it using physiological parameters. As there is no gold standard technique of non-invasive stress assessment, several subjective and/or objective surrogate methods have been proposed. The rationale for the objective methods lies in the fact that acute stress provokes changes in the acute autonomic nervous system (ANS). Therefore, several methods assessing ANS changes in various organ systems have been suggested as surrogate stress markers. These markers include (a) heart rhythm changes, as measured by heart rate (HR), heart rate variability (HRV), or interbeat interval (IBI); (b) electrodermal activity (EDA) levels; (c) thermal activity; and (d) saliva stress biomarkers (i.e., salivary cortisol [sC], salivary α-amylase [sAA], secretory immunoglobulin A [sIgA], and salivary Chromogranin [sCgA]. Additionally, there is an absence of a consistent methodology, leading to rather inconclusive and, in certain cases, conflicting results [Citation5].
Although the stress levels in OR teams and their impact on performance have attracted attention from long ago [Citation6], the available literature regarding non-invasive objective stress measures in the endoscopic simulation during virtual reality (VR) endoscopy training is missing, an absence also recently confirmed by others [Citation7].
Therefore, this study aimed to concomitantly measure four different stress-related salivary biomarkers (i.e., sC, sAA, sIgA, and sCgA) before and immediately after a basic simulation endoscopy task, to explore which one best correlates with performance as measured by video scoring (VS), and therefore it could potentially be used as a marker for the effectiveness of stress reduction interventions.
Methods
Participants
Thirty-six male novice residents of 23–27 years of age, were enrolled. All participants were PGY1 general surgery residents, without any previous experience in simulation or endoscopy. Written informed consent was obtained from each participant. This study was performed only on male subjects to avoid any possible confounding sex-related bias [Citation8]. The study took place at Medical Physics Lab-Simulation Center (MPLSC), Athens University Medical School, Athens, Greece, and was conducted during January-March 2019.
Ethics
The regional research ethics committee of Athens University Medical School in Athens, Greece, has approved the study (Dnr: 1718025227).
Experiment procedure
At first, all subjects responded to a baseline questionnaire, including demographic data, their prior endoscopic or simulator experience, and conformance to the following inclusion criteria: (1) abstinence of any prescribed or non-prescription medication (2) absence of flu or symptoms of upper respiratory tract infection, (3) refraining from tobacco, (4) for 12 h prior of testing refrained from alcohol and/or coffee and exercising, and (5) refrained from eating and brushing teeth for 1 h before the experiment. A post-experiment questionnaire was also completed regarding the participant’s comments on the whole procedure.
After a rest period of 30 min, a baseline (BL)-phase saliva sample was collected using an unstimulated passive drool technique. The subjects then had a 30 min initial orientation phase in Upper GI Endoscopy – Fundamental Skills module (GI-Bronch Mentor, Simbionix, Airport City, Israel). No hands-on training on endoscopy tasks was done during the orientation phase. The trainees were introduced to the basic functions of the simulator and the right posture techniques to perform an endoscopy. Then they watched a video prepared by the first author, demonstrating the five required tasks for FES hands-on component required to be proficient in flexible endoscopy. All trainees received immediate coaching during their orientation phase, and the trainer to trainee ratio was 1:1.
Then the simulation exercise (E) phase was started, with the subjects trained on the Fundamental skills module for 20 min. No trainer intervention was allowed during the exercise session. Immediately after, all participants similarly gave a second saliva specimen as before. The saliva samples were refrigerated and subsequently stored at −20 °C within 4 h of collection until assayed. The whole experiment was videotaped and stored for further analysis.
Data analysis
All the saliva samples were assessed for the four biomarkers using commercially available kits of salivary cortisol (SME-1-3002 Salivary Cortisol Research ELISA kit), α-amylase (SME-1-1902 Alpha-amylase Kinetic Reaction Kit Research), sIgA (SME-1-1602 Salivary Secretory IgA Research ELISA kit) (all from Salimetrics, Carlsbad, CA, USA; www.salimetrics.com) were used. The human Chromogranin A (CgA) was measured by an EIA Kit (Cat. No.: RSCYK070R, BioVendor GmbH Germany). The concentration of the saliva specimens was determined following the manufacturer’s instructions.
To avoid multifactorial stress bias from external factors, for each participant, the percentage difference value (Valuediff) of each parameter was calculated from its reciprocal baseline (Valuepre) and post-exercise (Valuepost) values, where Valuediff = 100·(Valuepost − Valuepre)/Valuepre. Thus, four predictor parameters to express the saliva biomarkers changes were derived.
Video scoring
Two blinded participants identity expert reviewers (NB, KG) assessed all the videos using a 4-points score for five procedure-specific items checklist () and the total score achieved was calculated by summing up their reciprocal scores.
Table 1. Objective video evaluation scoring table.
Statistical analysis
The normality of the collected data was tested using the Kolmogorov-Smirnov test. We used Cohen’s Kappa to calculate inter-rater reliability among the VS reviewers. Pearson’s correlation coefficient r between each predictor variable and video score (VS) was calculated. To check the collinearity among the predictor variables we used the Variance Inflation Factor (VIF) [Citation9]. To explore which variable (or variables) better predict the VS, we used the corrected (as our dataset was small) Akaike Information Criterion (AICc). According to this criterion, the preferred variable is the one with the smaller AICc value [Citation10]. We also used stepwise regression for this purpose. The R software version 3.5.0 was used for our statistical analysis [Citation11].
Results
All participants found that the saliva collection was problem-free and didn’t cause any distraction. All parameters in were found to be normally distributed. The VS achieved by the 36 subjects ranged from 6 to 13 (mean ± standard deviation [SD], 9.4 ± 1.8). Kappa value was 92% which means very strong reliability between the two reviewers.
Table 2. Mean ± SD and range of VS and each predictor variable, its Pearson’s correlation coefficient (r) with VS and their AICc value.
presents the mean ± SD and range of VS and each predictor variable (expressed as % difference of its reciprocal baseline to the respective post-exercise value), the Pearson’s correlation coefficient r between each variable, and the VS and AICc value of the percentage difference ((post-pre)/pre) of each variable. The VIF among the variables used was <1.5 suggesting that there is no collinearity among the VS and the predictor variables. As can be seen in and depicted in , sCgAdiff is the best parameter to predict VS as it has the highest r and the lowest AICc, followed by sAAdiff. Pearson’s correlation coefficient (r) was also calculated for every possible pair combination of the predictor parameters. Stepwise regression gave similar results with the AICc method, so we chose to present only the AICc data. presents the AICc of all possible combinations of the saliva-derived parameters. As seen, the best combination to predict VS is to use sAAdiff with sIgAdiff and sCgAdiff. When using only two parameters, sAAdiff and sCgAdiff is the best combination to predict VS as these parameters have lower AICc values than sCdiff and sIgAdiff (). sCdiff was excluded from the model as its p-value was high (0.29).
Figure 1. Correlation of the response to the stress of each saliva biomarker (a: sCgAdiff, b: sAAdiff, c: sCdiff, d: sIgAdiff) with performance as measured by video score.
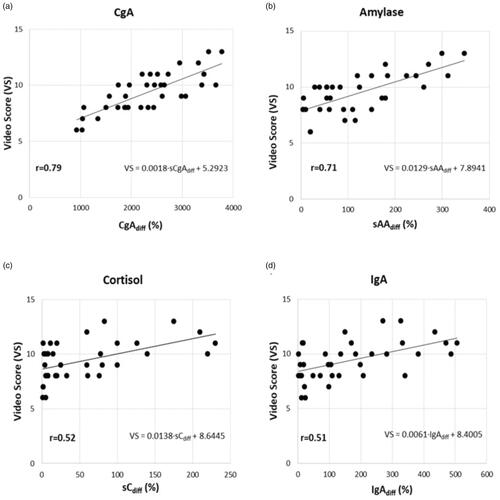
Table 3. AICc (corrected akaike information criterion) of all possible combinations of the saliva-derived parameters.
Discussion
As no data exist regarding acute mental strain measurement in the simulation mediated endoscopy field [Citation7], this study concomitantly measured the responses of salivary levels of sC, sAA, sIgA, and sCgA, all of them been previously associated with stress, and compared them with the VS of novice trainees in a reproducible stressful simulation environment [Citation12–15].
A video review offers unique advantages for assessing the subject’s performance, as their assessment follows objective, well-defined and pre-determined rules, and therefore it is less susceptible to subjective observer bias.[Citation16]. Nonetheless, a globally accepted video scoring tool for evaluation of the minimally invasive surgeon’s skills is still lacking [Citation17,Citation18].
Intubation of the esophagus, passing through the pylorus, scope navigation, and maintaining a clear endoscopic field are technically demanding, and minimal endoscopic skills are necessary for a novice to acquire them [Citation19]. Therefore, these four tasks plus the total time spent were chosen for our VS assessment score since it was expected to produce maximum stress in our simulation setting. We preferred to create our own video score and not rely on the automatically generated FES score because we wanted to consider only potentially stress-provoking parameters.
To our knowledge, this study is the first to concomitantly assess all the saliva stress-related biochemical markers in an endoscopy simulation environment. The current use of saliva specimens includes several stress biomarkers, which due to their collection simplicity, are advantageous to other invasive collecting methods [Citation4]. In our experiment, we used four stress-related saliva biomarkers (sC, sAA, sIgA, and sCgA) which were measured before as well as after a simulation endoscopic task. To exclude any other stress component not attributed to the simulation exercise, we used the percentage difference between the post- and pre-test values of each saliva biomarker. As seen in , CgA showed the highest percentage increase than any other parameter, with sCgAdiff showing the best correlation to VS (r = 0.79), whereas IgAdiff had the worst one (r = 0.51) as it showed the lowest r-value, similar to the r-value of sCdiff (r = 0.52). Additionally, as presented in , the variables with low AICc value also have a high Pearson’s correlation coefficient (r), thus indicating that the variables that better predict the VS (according to AICc) also have a high correlation with it, thereby enhancing our findings. Stepwise regression’s results were in accordance with AICc. The results of the sCdiff variable were excluded from the model because of its high p-value. The fact that the VIF of each variable was <1.5, indicates that there is not any collinearity problem, as only VIF value >5 arouses collinearity problems [Citation10].
The mental stress response to various stimuli is manifested through two main pathways: One is by the corticotropin-releasing hormone, the subsequent activation of the hypothalamic-pituitary-adrenal axis, and finally, the glucocorticoids release into the bloodstream, while the other one is accomplished by activating the ANS through catecholamines secretion.
As plasma cortisol is passively diffused to enter into saliva fluid, a stable serum/saliva ratio is maintained, and therefore its saliva levels can be used for assessing the hypothalamic-pituitary-adrenal axis stress-related changes. On the contrary, both sIgA and sAA are secreted from the salivary glands. However, their production and subsequent secretion greatly depend on several other confounding and local factors, and therefore great intra-individual variations are observed. Thus, a strict measurement protocol is needed to assess them reliably [Citation20]. Chromogranin A (CgA) is a glycoprotein, mediating the intracellular storage of catecholamines and co-released in tandem with them from the sympathetic nerves into the blood circulation [Citation21]. This close relationship of CgA with catecholamines secretion led several authors to introduce it for the assessment of ANS activity [Citation22–24]. As in our study, sCgAdiff presented the best correlation with VS among the four saliva biomarkers measured, we postulate that the mental stress produced in our simulation setting was rapidly manifested through a sympathetic (ANS) response.
It has been reported that sCgA is sensitive and responds rapidly to multiple mental stress stimuli, such as psychosomatic stress [Citation25], computer operation psychological stress [Citation26], and academic assessment stress [Citation27]. Additionally, it has been suggested that the rapid changes in sCgA secretion observed after a cognitive test might reveal the presence of psychological stress [Citation28]. Furthermore, it has been observed that sCgA concentrations increase during mental stress tasks but decrease during the intermissions, and therefore it is postulated that sCgA can be used for short-term estimations of mental workload [Citation29]. It has been reported that sCgA is a better stress indicator than sC as it responds more rapidly and more sensitively to psychological stressors [Citation5,Citation25]. However, others are questioning the sCgA validity to measure stress and/or the ANS activity [Citation23,Citation30].
In our analysis, sAAdiff showed the second-best correlation with VS. It has been suggested that sAA, having a good correlation with catecholamine levels in plasma, could be used as a surrogate marker of norepinephrine particularly, in a variety of stressful conditions [Citation31]. However, it has been shown that most of the stress sAA research articles do not take into account the potentially confounding effects of the salivary flow rate, neither they do not follow a standard saliva collection protocol regarding the stimulation techniques to produce saliva nor regarding the sampling’s time duration [Citation32]. Additionally, a review showed that the same precautions should apply to every saliva stress biomarker measured [Citation5]. A study assessed sAA and sC during the acute mental strain of 51 surgeons operating in the OR. Higher sAA spikes were observed in the most stressful situations [Citation33].
As it has been suggested that sC increases as a response to stress, it became the most widely used biomarker for stress studies [Citation5]. Usually, it is used together with other subjective (i.e., State-Trait Anxiety Inventory) and/or objective cardiac stress parameters (i.e., HR and HRV) [Citation34–39]. Although it was mostly reported that an increase of sC levels occurs after acute stress, some studies failed to observe such an increase [Citation34] and even diminished cortisol levels after stress have been published [Citation35]. As sC is activated through the hypothalamus-pituitary-adrenocortical axis, it responds to stress stimuli later than ANS, usually in 5 and even 10 min after cessation of the stress exposure. Therefore, it is possible that we missed to detect an sC peak in our study. It is evident that some more delayed sampling saliva collection point is necessary to be added to fully capture any stress-induced cortisol response [Citation40].
The response of sIgA levels to stress, mood, and emotionality fluctuate in a complex pattern [Citation41]. A study measured sC and sIgA in a group of surgeons engaged in operations with increasing degrees of technical difficulty. It was found that sIgA variations were not significant and did not coincide with the rest of the stress estimation variables used [Citation42]. In our analysis, sIgAdiff presented a low correlation coefficient, comparable to that of sCdiff.
Our finding that in our setting higher levels of stress correlated with higher performance is in accordance with the Yerkes-Dodson law which states that there is an optimal level of arousal that results in optimal performance [Citation43]. That optimal level of arousal differs from person to person, according to factors like the specific task, degree of skill, and confidence level [Citation44].
Limitations
Our study has several limitations. At first, this is an observational study including male novice surgery residents only, without concomitantly assigning a control group. Second, the sample size was relatively small. Therefore, our findings must be interpreted with caution. Third, we might have missed a delayed salivary cortisol response. Fourth, we preferred to use an endoscopy modified, proprietary video score formula which has been used by our group elsewhere but has not been yet externally validated [Citation44]. Furthermore, no comparison to the FES score was performed. Finally, our research focused only on a simulated training scenario of basic endoscopy skills. Hence, our findings cannot be extrapolated into real-life clinical practice.
Nevertheless, this study sheds light on the feasibility of using a non-invasive stress measurement of novice endoscopists using easy-to-collect, non-invasive saliva biomarkers. A large, controlled study including participants with different levels of clinical skills is needed to investigate further the utility of saliva stress biomarkers to evaluate and monitor simulation training.
In conclusion, the saliva biomarkers usage for assessing stress-related data was easy to implement and well-accepted by all subjects in our simulation training setting. Among the saliva biomarkers assessed before and immediately after our simulation trial, those under the control of the sympathetic ANS (sCgA and sAA) showed the best correlation with performance, as it is measured via VS. Therefore, those biomarkers can be used for monitoring stress. Further studies with a larger number of different levels of endoscopy skills are needed to exploit performance enhancement and error reduction techniques in the endoscopy training field.
Author contributions
NB, KG, LE, and BM: study concept. NB, KG, LE, and BM: study design. NB, KG, and TD: data acquisition. NB, KG, TD, DT, and NO: quality control of data and algorithms. NB, KG, TD, DT, NO, and LE: data analysis and interpretation. DT: statistical analysis. NB and KG: manuscript preparation. TD, NO, and LE: manuscript editing. NO, LE, and BM: manuscript review.
| Abbreviations | ||
| AICc | = | akaike information criterion |
| ANS | = | autonomic nervous system |
| BL | = | baseline phase |
| BVP | = | blood volume pressure |
| E | = | simulation exercise phase |
| EDA | = | electrodermal activity |
| FES | = | fundamentals of endoscopic surgery |
| HR | = | heart rate |
| HRV | = | heart rate variability |
| IBI | = | interbeat interval |
| OR | = | operating room |
| PPG | = | photoplethysmography |
| sAA | = | saliva α-amylase |
| sC | = | saliva cortisol |
| sCgA | = | saliva chromogranin A |
| sIgA | = | saliva secretory immunoglobulin A |
| ST | = | skin temperature |
| VIF | = | variance inflation factor |
| VS | = | video score |
Acknowledgements
Part of this work has been included in the Ph.D. thesis of one of the authors (KG, see reference [Citation4]).
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Arora S, Sevdalis N, Nestel D, et al. The impact of stress on surgical performance: a systematic review of the literature. Surgery. 2010;147(3):318–330, 330.e1–6.
- Maher Z, Milner R, Cripe J, et al. Stress training for the surgical resident. Am J Surg. 2013;205(2):169–174.
- Anton NE, Montero PN, Howley LD, et al. What stress coping strategies are surgeons relying upon during surgery? Am J Surg. 2015;210(5):846–851.
- Georgiou K. Stress indices in surgical simulation [Ph.D. thesis]; 2019. https://pergamos.lib.uoa.gr/uoa/dl/frontend/file/lib/default/data/2880271/theFile
- Georgiou K, Larentzakis A, Papavassiliou AG. Surgeons' and surgical trainees' acute stress in real operations or simulation: a systematic review. Surgeon. 2017;15(6):355–365.
- Phitayakorn R, Minehart RD, Pian-Smith MC, et al. Practicality of using galvanic skin response to measure intraoperative physiologic autonomic activation in operating room team members. Surgery. 2015;158(5):1415–1420.
- Mizota T, Anton NE, Huffman EM, et al. Development of a fundamentals of endoscopic surgery proficiency-based skills curriculum for general surgery residents. Surg Endosc. 2020;34(2):771–778.
- Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress. 2017;20(5):476–494.
- James G, Witten D, Hastie T, et al. An introduction to statistical learning: with applications in R. New York (NY): Springer; 2013.
- Burnham K, Anderson D. Multimodel interference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261–304.
- R Core Team. R: A language and environment for statistical computing. Computing. Vienna (Austria): R Foundation for Statistical Computing; 2018. Available from: https://www.R-project.org/.
- Sadi H, Finkelman M, Rosenberg M. Salivary cortisol, salivary alpha amylase, and the dental anxiety scale. Anesth Prog. 2013;60(2):46–53.
- Obayashi K. Salivary mental stress proteins. Clin Chim Acta. 2013;425:196–201.
- Takatsuji K, Sugimoto Y, Ishizaki S, et al. The effects of examination stress on salivary cortisol, immunoglobulin A, and chromogranin a in nursing students. Biomed Res. 2008;29(4):221–224.
- Reshma AP, Arunachalam R, Pillai JK, et al. Chromogranin A: Novel biomarker between periodontal disease and psychosocial stress. J Indian Soc Periodontol. 2013;17(2):214–218.
- Mellinger JD, Williams RG, Sanfey H, et al. Teaching and assessing operative skills: from theory to practice. Curr Probl Surg. 2017;54(2):44–81.
- Oussi N, Loukas C, Kjellin A, et al. Video analysis in basic skills training: a way to expand the value and use of BlackBox training? Surg Endosc. 2018;32(1):87–95.
- Sleiman Z, Tanos V, Van Belle Y, et al. The European academy laparoscopic “suturing training and testing” (SUTT) significantly improves surgeons’ performance. Facts Views Vis Obgyn. 2015;7(3):153–160.
- Vassiliou MC, Kaneva PA, Poulose BK, et al. Global assessment of gastrointestinal endoscopic skills (GAGES): a valid measurement tool for technical skills in flexible endoscopy. Surg Endosc. 2010;24(8):1834–1841.
- Georgiou K, Larentzakis AV, Khamis NN, et al. Can wearable devices accurately measure heart rate variability? A systematic review. Folia Med. 2018;60(1):7–20.
- Winkler H, Fischer-Colbrie R. The chromogranins a and B: the first 25 years and future perspectives. Neuroscience. 1992;49(3):497–528.
- Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348(12):1134–1149.
- Noto Y, Sato T, Kudo M. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth Analg. 2005;101(6):1873–1876.
- Obara S, Iwama H. Assessment of psychological tension after premedication by measurement of salivary chromogranin A. J Clin Anesth. 2005;17(7):554–557.
- Nakane H, Asami O, Yamada Y, et al. Salivary chromogranin a as an index of psychosomatic stress response. Biomed Res. 1998;19(6):401–406.
- Nakane H, Asami O, Yamada Y, et al. Effect of negative air ions on computer operation, anxiety and salivary chromogranin A-like immunoreactivity. Int J Psychophysiol. 2002;46(1):85–89.
- Ng V, Koh D, Mok BYY, et al. Salivary biomarkers associated with academic assessment stress among dental undergraduates. J Dent Educ. 2003;67(10):1091–1094.
- Kanamaru Y, Kikukawa A, Shimamura K. Salivary chromogranin-A as a marker of psychological stress during a cognitive test battery in humans. Stress. 2006;9(3):127–131.
- Nomura S, Mizuno T, Nozawa A, et al. Characteristics of salivary chromogranin a as a short-term mental stress biomarker. Trans Jpn Soc Med Biol Eng. 2010;48(2):207–212.
- Dimsdale JE, O'Connor D, Ziegler M, et al. Does chromogranin a respond to short-term mild physiologic challenge? Neuropsychopharmacology. 1989;2(3):237–240.
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34(4):486–496.
- Bosch JA, Veerman ECI, de Geus EJ, et al. α-Amylase as a reliable and convenient measure of sympathetic activity: don't start salivating just yet! Psychoneuroendocrinology. 2011;36(4):449–453.
- Engelmann C, Schneider M, Kirschbaum C, et al. Effects of intraoperative breaks on mental and somatic operator fatigue: a randomized clinical trial. Surg Endosc. 2011;25(4):1245–1250.
- Alobid I, de Pablo J, Mullol J, et al. Increased cardiovascular and anxiety outcomes but not endocrine biomarkers of stress during performance of endoscopic sinus surgery: a pilot study among novice surgeons. Arch Otolaryngol Head Neck Surg. 2011;137(5):487–492.
- Wetzel CM, Black SA, Hanna GB, et al. The effects of stress and coping on surgical performance during simulations. Ann Surg. 2010;251(1):171–176.
- Arora S, Sevdalis N, Aggarwal R, et al. Stress impairs psychomotor performance in novice laparoscopic surgeons. Surg Endosc. 2010;24(10):2588–2593.
- Arora S, Aggarwal R, Moran A, et al. Mental practice: effective stress management training for novice surgeons. J Am Coll Surg. 2011;212(2):225–233.
- Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons-a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488–494.
- Ghazali DA, Faure JP, Breque C, et al. Evaluation of stress patterns during simulated laparoscopy in residency. Minerva Chir. 2016;71(4):252–261.
- Allen AP, Kennedy PJ, Cryan JF, et al. Biological and psychological markers of stress in humans: focus on the trier social stress test. Neurosci Biobehav Rev. 2014;38:94–124.
- Bosch JA, Ring C, de Geus EJC, et al. Stress and secretory immunity. Int Rev Neurobiol. 2002;52:213–253.
- Marrelli M, Gentile S, Palmieri F, et al. Correlation between Surgeon's experience, surgery complexity and the alteration of stress related physiological parameters. PLOS One. 2014;9(11):e112444.
- Nakayama N, Arakawa N, Ejiri H, et al. Heart rate variability can clarify students' level of stress during nursing simulation. PLOS One. 2018;13(4):e0195280.
- Oussi N, Georgiou K, Larentzakis A, et al. Validation of a novel needle holder to train advanced laparoscopy skills to novices in a simulator environment. Surg Innov. 2020;27(2):211–219.
