Abstract
Objectives
To determine the predictors of difficult colorectal endoscopic submucosal dissection (ESD) and to develop a preoperative predictive model for difficult colorectal ESD procedures.
Methods
Colorectal neoplasms intended to be resected by ESD in our center between August 2013 and February 2019 were retrospectively enrolled. An ESD procedure which took more than 30 min, failed to remove the lesions en bloc or converted to surgery was defined as difficulty. Logistic regression analysis was conducted to find out the predictors of difficult ESD. A nomogram integrating independent predictors was developed and validated with respect to its discrimination, calibration and clinical application, using the receiver operating characteristic (ROC) curve, calibration plot and decision curve analysis (DCA), respectively.
Results
A total of 368 colorectal neoplasms in 355 patients were included. The independent predictors for difficult colorectal ESD were size ≥2 cm (odds ratio [OR] = 6.102, p < .001), positive non-lifting sign (OR = 6.569, p = .005), lesions located in left colon (OR = 2.475, p = .036) or rectum (OR = 2.183, p = .048), laterally spreading tumors (LSTs) (OR = 2.501, p = .008) and less colorectal ESD experience (≤20 cases) (OR = 2.3091, p = .028). The nomogram model incorporating the above predictors performed well in both of the training and validation sets (area under the cure [AUC] = 0.786 and 0.784, respectively). DCA demonstrated the clinical benefit of the nomogram was superior to that of each independent predictor alone.
Conclusions
The nomogram incorporating tumor size, location, morphology, non-lifting sign and ESD experience of operator can be conveniently used to facilitate the preoperative prediction of difficult colorectal ESD.
Introduction
Endoscopic submucosal dissection (ESD) has been used in the treatment of colorectal lesions. It has advantages over endoscopic mucosal resection (EMR) in providing a higher en bloc, complete and curative resection rate [Citation1–3]. However, ESD is a relatively more complex, technic demanding and time-consuming endoscopic procedure [Citation3]. Prolonged ESD procedure time associates with increased risk of ESD-related bleeding [Citation4,Citation5] and perforation [Citation6]. In difficult ESD procedures, the risk of anesthesia-related complications, such as deep venous thrombosis, aspiration pneumonia and cardiorespiratory distress may be increased because of the prolonged procedure time [Citation7]. Therefore, pre-ESD risk stratification for difficult procedure is of great value in arranging the operation schedule and improving the outcomes of colorectal ESD.
Previous studies had reported some predictors for difficult colorectal ESD, such as large lesion size, submucosal fibrosis, lesion located in difficult location such as the folds and flexure, and endoscopists with less experience [Citation8–13]. However, to the best of our knowledge, there is no ready to use model for predicting the procedural difficulty of colorectal ESD to date. With the ability to generate an individual numerical probability of a specific clinical event by integrating multiple variables, predictive nomogram yields a comprehensive predictor in clinical settings [Citation14]. We aimed to identify the predictors of difficult colorectal ESD procedure based on a relatively large data set and establish a nomogram for the prediction of difficult procedure. Additionally, we evaluated the predictive accuracy and clinical usefulness of the nomogram model.
Methods
Patients and lesions
Between August 2013 and February 2019, a total of 355 consecutive patients with 368 colorectal neoplasms intended to be resected by ESD at the endoscopy center of Beijing Friendship Hospital were retrospectively enrolled in this study. The demographic features of the patients and the operators’ experience were recorded. Lesions’ characteristics (size, location, endoscopic manifestations), procedure time and postoperative pathology were collected.
This study was approved by the ethics committee of Beijing Friendship Hospital in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Preoperative preparation
The colon was prepared using a polyethylene glycol electrolyte solution. Before resection, all lesions were carefully examined using video-colonoscopes (PCF-Q260JI, CF-H260AI, CF-H290I, CF-HQ290I, etc. Olympus, Tokyo, Japan) and lesion’s characteristics were recorded. The size of each lesion was determined endoscopically by comparison with the diameter of the forceps [Citation15]. Tumor location was defined as three categories: the rectum, the right colon if one lesion was located in and proximal to the splenic flexure and the left colon if it located distal to the splenic flexure. In the current study, based on the Japanese classification of colorectal carcinoma [Citation16], tumor morphology was divided into laterally spreading tumor (LST) and other types.
To delineate the margins of the lesions and evaluate the pit patterns, chromoendoscopy using 0.4% indigo-carmine dye and narrow band imaging with magnificence were performed. Ultrasonography was performed to better evaluate the infiltration depth of the lesions and to identify whether peripheral lymph nodes were involved, if necessary.
ESD procedure
ESD indications were in accordance with the guideline for colorectal ESD, including lesions for which en bloc resection was hard to achieve with EMR, such as large depressed tumors, large protruded lesions suspected to be malignant, non-granular LST and lesions with Vi-type pit pattern, or those with submucosal fibrosis [Citation17]. All procedures were performed under general anesthesia or deep sedation. A standard, single-channel colonoscope with a transparent cap on the tip was used. After the lesion’s margin was identified, a circumferential mucosal marker was made about 3–5 mm outside the margin using the tip of Dual-knife (KD-650U, Olympus, Tokyo, Japan). Then, a mixture of sodium hyaluronate, methylene blue and glycerol fructose was injected into the submucosal layer to make a mucosal lift. Mucosal incision and submucosal dissection were performed with Dual-knife (Olympus) step by step.
For those encountered difficulty during the ESD procedure, piecemeal resection became an alternative. As for those failed to be resected endoscopically, patients underwent surgery. Procedure time was defined as the total duration of the procedure in minutes from the first mucosal incision to the complete removal of the lesion [Citation18]. The cutoff value of prolonged procedure time was defined according to the median procedure time in our study. Procedures taking more than 30 min, those in which lesions were failed to be resected en bloc, or converted to surgery, were defined as difficult colorectal ESD procedures.
At our center, endoscopists generally perform more than 200 colorectal polypectomies previously before moving to colorectal ESD. The experience of the operator was defined as the colorectal ESD cases done before he/she performed the current procedure. We set the cutoff value of experienced endoscopists as 20 cases according to the receiver operating characteristic (ROC) curve.
Pathological evaluation
Specimens were stretched and pinned out, fixed in formalin for 24 h, cut into 2 mm slices and then stained with hematoxylin and eosin. All specimens were examined by experienced gastrointestinal pathologists. En bloc resection was defined as a specimen removed in a single piece [Citation17]. Histological diagnosis was in accordance with the Japanese classification of cancer of the colon and rectum and the Vienna classification [Citation16,Citation19].
Outcomes
The primary outcome was the identification of independent predictors of difficult colorectal ESD procedures. The secondary outcomes were to develop and validate a predictive nomogram model for difficult colorectal ESD procedures.
Development and validation of the prediction model
The whole data set was randomly partitioned into the training set and the validation set by a ratio of 2:1 in way of random numbers. In the first stage, we identified the independent risk factors for a difficult procedure using multivariate logistic regression in the training set. Then, these independent predictors were combined to develop a nomogram model. The power of each independent risk factor was weighted using the regression coefficient of the multivariate model, then a formula was built, which was then used to calculate the risk score for each patient to reflect the risk of difficult procedure. In the second stage, the performance of the inclusive nomogram was respectively evaluated in the training and validation set. ROC analyses were employed to assess the performance of the nomogram in the prediction of a difficult procedure. Furthermore, the calibration of the nomogram was assessed with the calibration curve by bootstrapping with 1000 replications in both sets. The Hosmer–Lemeshow test was used to examine the goodness-of-fit of the multivariable logistic model [Citation20]. Moreover, decision curve analysis (DCA) was conducted to estimate the clinical utility of the nomogram model by quantifying the net benefits at different threshold probabilities in the validation dataset [Citation21].
Statistical analysis
Categorical variables were summarized by absolute frequency and percentage in parentheses, and were compared by the Chi-squared or Fisher’s exact test, as appropriate. The Shapiro–Wilk test was used to test the normality of the distribution of continuous variables. Non-normally distributed continuous variables were summarized by median and interquartile range (IQR) in parentheses, and were compared by nonparametric Mann–Whitney test. Risk factors for procedure difficulty were determined using logistic regression in the training set. Then, factors with statistical significance in the univariate analysis were included in the multivariate analysis and the final model.
All analyses were performed using the SPSS software for Mac (Version 26.0; Chicago, IL, USA) and R software (version 4.0.1; http://www.Rproject.org). SPSS was used to generate random numbers of the cases, through which we divided the data into two parts. The performance of the nomogram model was evaluated using calibration curve and ROC curve (‘rms’ package). DCA was performed using the ‘rmda’ package. All reported p values were two sided, with statistical significance set at 0.05.
Results
Patients and neoplasm features
A total of 355 neoplasms in 368 patients considered for ESD were included. The study population and the lesion characteristics are summarized in . The median age of the patients was 64 (IQR 58–71) years, and 60.3% were men. Mucosal neoplasms and lesions with submucosal infiltration were diagnosed in 371 (86.1%) and 50 (13.6%) lesions, respectively. A total of 194 lesions (52.7%) were 2 cm or larger in diameter. There were no significant differences of baseline characteristics of the patients and lesions between the training and validation sets.
Table 1. Characteristics of patient and lesions in the total, training and validation set.
Procedural outcomes
Full ESD was achieved in 327 (88.9%) lesions, and 33 lesions (9.0%) were converted to piecemeal resection during the procedure. Eight lesions (2.2%) failed to be resected endoscopically were successfully treated by surgery then. Among the total 368 lesions, endoscopically en bloc resection was achieved in 88.9% cases with a median procedure time of 31.0 (IQR 18.0–55.0) min ().
Risk factors for difficult ESD procedures
The clinicopathological characteristics which could be achieved preoperatively were analyzed to find the risk factors for difficult ESD procedures (). Univariate analysis showed that endoscopist’s experience, lesion’s morphology, diameter, pathological type and non-lifting sign were associated with procedural difficulty. Multivariate logistic regression analysis showed that neoplasm size ≥2 cm (OR = 6.102, 95% CI 3.331–11.179, p < .001), positive non-lifting sign (OR = 6.569, 95% CI 1.747–24.696, p = .005), lesions located in left colon (versus right colon) (OR = 2.475, 95% CI 1.063–5.760, p = .036) or rectum (versus right colon) (OR= 2.183, 95% CI 1.006–4.736, p = .048), less colorectal ESD experience (≤20 cases) (OR = 2.091, 95% CI 1.083–4.038, p = .028) and LST type (OR = 2.501, 95% CI 1.268–4.933, p = .008) were independent predictors for difficult ESD procedures.
Table 2. Risk factors for difficult colorectal ESD procedures.
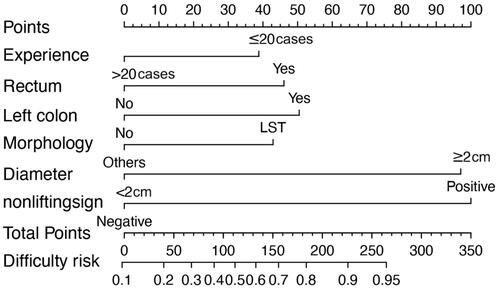
Development and validation of an individualized prediction nomogram
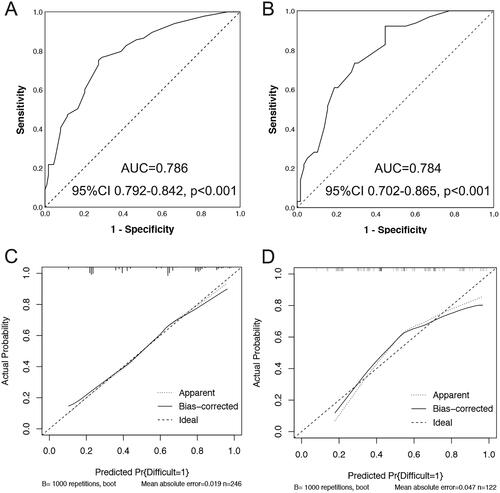
The model incorporating these independent predictors (neoplasm size ≥2 cm, positive non-lifting sign, lesions located in left colon or rectum, LST type and less colorectal ESD experience (≤20 cases)) was developed and presented as the nomogram (). The Hosmer–Lemeshow test yielded a nonsignificant statistic (p = .167), which suggested that there was no departure from perfect fit. The ROC analysis yielded an area under ROC curve (AUC) of 0.786 (95%CI 0.729–0.842, p < .001) for the training set () and 0.784 (95%CI 0.702–0.865, p < .001) for the validation set (), which implied favorable discrimination of the model. Validation by the use of bootstrap resampling, which was visualized as calibration plots, showed the bias-corrected lines lay close to the ideal lines, indicating a good agreement between prediction and observation in the training set () and validation set ().
Figure 2. Discrimination and validation of nomogram for predicting difficult colorectal ESD. The receiver operating characteristic (ROC) curves based on the nomogram for the probability of difficult colorectal ESD in the training (A) and validation (B) sets. Calibration curves of the nomogram in the training (C) and validation (D) sets (bootstrap 1000 repetitions). Nomogram-predicted probability of difficult colorectal ESD is plotted on the x-axis and actual probability is plotted on the y-axis. The dashed 45° line represents a perfect prediction by an ideal model, and the solid line represents the performance of our nomogram, which lays closer to the dashed line, meaning a good performance of prediction. 95%CI: 95% confidence interval; AUC: Area under the curve.
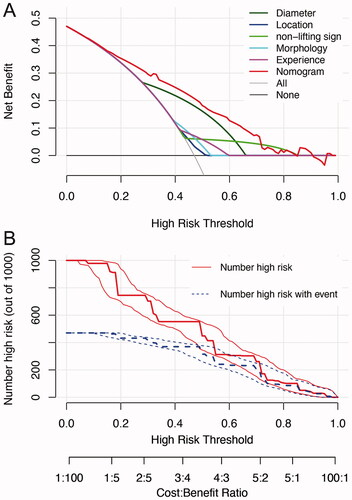
The DCA showed that if the threshold probability of difficult colorectal ESD procedure was set between 10 and 70%, the nomogram we developed showed higher net benefit than that of each independent predictor alone (). was a clinical impact plot, which illustrated the estimated number of patients who would be declared high risk for each risk threshold and visually showed the proportion of those who were true cases. In this plot, if a 40% risk threshold was used, for example, then of 1000 population screened, about 480 would be deemed high risk, with about 390 of these being true difficult procedures.
Figure 3. Decision curve analysis (DCA) and clinical impact curve for the nomogram. In the DCA (A), the y-axis represents the net benefit, and the x-axis represents the threshold probability. The gray solid line represents the hypothesis that all patients had difficulty in colorectal ESD. The black solid line represents the assumption that no patients had difficulty in colorectal ESD. The red dashed line represents the nomogram, and other colored lines represent different predictors. The decision curves showed that if the threshold probability is set between 10 and 70%, using the nomogram to predict difficulty of colorectal ESD adds more net benefit than any other predictors alone. In clinical impact curve (B) the vertical axis displays number of high risk. The two horizontal axes show the correspondence between risk threshold and cost: benefit ratio. Of 1000 patients, the heavy red solid line shows the total number who would be deemed high risk for each risk threshold. The blue dashed line shows how many of those would be true positives (cases).
Discussion
The current study demonstrated that tumor size of 20 mm or greater in diameter, positive non-lifting sign, tumor location in the left colon or rectum, LST type and less experience of the operator were independent risk factors for a difficult ESD procedure in treating colorectal neoplasms. The strength of this study was that we successfully developed the first easy-to-use nomogram model to preoperatively predict difficult ESD procedures for colorectal lesions with satisfactory predictive accuracy, and it was based on a relatively large database.
Globally, ESD has attracted more and more attention because of its advantages in curative resection of large tumors, particularly if superficial submucosal invasion is suspected. However, the difficulty of procedure, to a large extent, hinders its broader adoption [Citation22]. The difficulty of ESD procedures varies among different patients and lesions, and difficult cases always associated with prolonged procedure time [Citation7]. Accurate prediction of difficult procedures may enable better preparation for the operation, optimize procedure arrangement and resource allocation in endoscopy units, as well as be of great benefit for training programs [Citation23–25].
With regard to the risk factors for difficult colorectal ESD, large tumor size [Citation23–25], non-lifting sign [Citation25] and less-experienced endoscopist [Citation25] have been previously described. Current study further confirmed the above findings. Although rectum was considered as the easiest and safest site for ESD [Citation26], our results showed lesions located in the left colon or rectum had a higher risk of being difficult to be resected when compared to those located in the right colon, which was consistent with the results from the work of Keisuke et al. [Citation23]. One of the reasons for our finding was that, in our study, lesions located in the left colon or rectum had higher proportions of being advanced neoplasia (74.4% vs. 56.1%, p = .004) and having submucosal invasion (21.3% vs. 4.9%, p = .001) than those located in the right colon. In addition, majority (57.1%) of the lesions in our study were LSTs. Advanced lesions and submucosal infiltration were more commonly found in distally rather than in proximally located colorectal LSTs [Citation27,Citation28].
To our knowledge, this is the first study to develop a nomogram model for predicting technical difficulty of colorectal ESD procedures. All the predictors in the model can be easily obtained before ESD procedures. The utility of this model is likely to impact positively on clinical practice. Nomogram estimation of procedural difficulty may help clinicians in identifying lesions which may derive greater clinical benefit from resection by more experienced endoscopist. For example, when a LST located in the left colon with tumor size < 2 cm in diameter is encountered and the non-lifting sign is negative, and if it is operated by a less-experienced endoscopist, there is a risk of about 60% that the procedure would take more than 30 min or to be failed. If resected by experienced endoscopist, the risk could be reduced to around 40%. Therefore, neoplasms which are difficult to remove should be directed to more experienced endoscopist, and rational arrangement should be taken to guarantee favorable outcomes.
We have established a predicting model for ESD technical difficulty, combining operators’ experience with endoscopic findings of lesions. To justify the clinical usefulness, DCA was applied to assess whether the nomogram-assisted decisions would improve patient outcomes. DCA is a novel method offering insight into clinical consequences on the basis of threshold probability, from which the net benefit could be derived (Net benefit is defined as the proportion of true positives minus the proportion of false positives, weighted by the relative harm of false-positive and false-negative results [Citation14]). It showed that if the threshold probability is set between 10 and 70%, using the nomogram to predict the risk of difficult colorectal ESD adds more benefit than any other independent predictors only.
Some limitations of this study have to be addressed. First, we performed an analysis in a single tertiary center setting. Because the clinical outcomes of colorectal ESD varied according to the endoscopists’ experiences and the volume of centers, external validation is mandatory for the generalization of our findings. Second, other factors, such as tumor location in a flexure and paradoxical movement, which were identified as predictors for difficult ESD in the colorectum [Citation8], were not able to be collected and analyzed in the current study due to the retrospective design. In addition, because not all the lesions underwent endoscopic ultrasonography or magnifying endoscopy preoperatively, we did not include the preoperatively predicted invasion depth of lesion into the model. However, the model consisted of five simple and easily available factors showed good application potential.
In conclusion, tumor size ≥2 cm, positive non-lifting sign, lesions located in left colon or rectum, LST lesions and less colorectal ESD experience were independent predictors for technical difficulty in colorectal ESD. The risk-predicting model incorporating these factors can quantitatively predict the risk of being a difficult colorectal ESD procedure. Preoperative estimation of the risk using this model may assist endoscopists in better planning colorectal ESD, minimizing the risk of ESD-related complications and optimizing procedure arrangement and resource allocation.
Acknowledgements
The authors thank the medical staff and research assistants in our center for their kind help to this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, P L upon reasonable request.
Additional information
Funding
References
- De Ceglie A, Hassan C, Mangiavillano B, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: a systematic review. Crit Rev Oncol Hematol. 2016; 104:138–155.
- Dumoulin FL, Hildenbrand R. Endoscopic resection techniques for colorectal neoplasia: current developments. WJG. 2019;25(3):300–307.
- Yang D, Othman M, Draganov PV. Endoscopic mucosal resection vs endoscopic submucosal dissection for barrett's esophagus and colorectal neoplasia. Clin Gastroenterol Hepatol. 2019;17(6):1019–1028.
- Nam HS, Choi CW, Kim SJ, et al. Risk factors for delayed bleeding by onset time after endoscopic submucosal dissection for gastric neoplasm. Sci Rep. 2019;9(1):2674.
- Ueki N, Futagami S, Akimoto T, et al. Effect of antithrombotic therapy and long endoscopic submucosal dissection procedure time on early and delayed postoperative bleeding. Digestion. 2017;96(1):21–28.
- Yamamoto K, Shimoda R, Ogata S, et al. Perforation and postoperative bleeding associated with endoscopic submucosal dissection in colorectal tumors: an analysis of 398 lesions treated in Saga, Japan. Intern Med. 2018;57(15):2115–2122.
- Goto O, Fujishiro M, Kodashima S, et al. Is it possible to predict the procedural time of endoscopic submucosal dissection for early gastric cancer? J Gastroenterol Hepatol. 2009;24(3):379–383.
- He Y, Wang X, Du Y, et al. Predictive factors for technically difficult endoscopic submucosal dissection in large colorectal tumors. Turk J Gastroenterol. 2016;27(6):541–546.
- Iacopini F, Saito Y, Bella A, et al. Colorectal endoscopic submucosal dissection: predictors and neoplasm-related gradients of difficulty. Endosc Int Open. 2017;05(09):E839–E846.
- Imai K, Hotta K, Ito S, et al. A risk-prediction model for en bloc resection failure or perforation during endoscopic submucosal dissection of colorectal neoplasms. Dig Endosc. 2020;32:932–939.
- Imai K, Hotta K, Yamaguchi Y, et al. Preoperative indicators of failure of en bloc resection or perforation in colorectal endoscopic submucosal dissection: implications for lesion stratification by technical difficulties during stepwise training. Gastrointest Endosc. 2016;83(5):954–962.
- Matsumoto S, Uehara T, Mashima H. Construction of a preoperative scoring system to predict the difficulty level of colorectal endoscopic submucosal dissection. PLoS One. 2019;14(6):e0219096.
- Perez-Cuadrado-Robles E, Snauwaert C, Moreels TG, et al. Risk factors for conversion to snare resection during colorectal endoscopic submucosal dissection in an expert Western center. Endoscopy. 2019;51(2):152–160.
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–e180.
- Kim JH, Park SJ, Lee JH, et al. Is forceps more useful than visualization for measurement of Colon polyp size? WJG. 2016;22(11):3220–3226.
- Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3d English edition [secondary publication]. J Anus Rectum Colon. 2019;3:175–195.
- Tanaka S, Kashida H, Saito Y, et al. Japan gastroenterological endoscopy society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2020;32(2):219–239.
- Kanzaki H, Ishihara R, Ohta T, et al. Randomized study of two endo-knives for endoscopic submucosal dissection of esophageal cancer. Am J Gastroenterol. 2013;108(8):1293–1298.
- Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51(1):130–131.
- Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32(1):67–80.
- Kerr KF, Brown MD, Zhu K, et al. Assessing the clinical impact of risk prediction models with decision curves: guidance for correct interpretation and appropriate use. J Clin Oncol. 2016;34(21):2534–2540.
- Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointestinal Endoscopy. 2017;86:74–86.e17.
- Hori K, Uraoka T, Harada K, et al. Predictive factors for technically difficult endoscopic submucosal dissection in the colorectum. Endoscopy. 2014;46(10):862–870.
- Sato K, Ito S, Kitagawa T, et al. Factors affecting the technical difficulty and clinical outcome of endoscopic submucosal dissection for colorectal tumors. Surg Endosc. 2014;28(10):2959–2965.
- Takeuchi Y, Iishi H, Tanaka S, et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int J Colorectal Dis. 2014;29(10):1275–1284.
- Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection: is it suitable in Western countries? J Gastroenterol Hepatol. 2013;28(3):406–414.
- Bogie RMM, Veldman MHJ, Snijders L, et al. Endoscopic subtypes of colorectal laterally spreading tumors (LSTs) and the risk of submucosal invasion: a meta-analysis. Endoscopy. 2018;50(3):263–282.
- Myung DS, Kweon SS, Lee J, et al. Clinicopathological features of laterally spreading colorectal tumors and their association with advanced histology and invasiveness: an experience from Honam province of South Korea: a Honam association for the study of intestinal diseases (HASID). PLoS One. 2017;12(10):e0184205.