Abstract
Background
The acceptance of ABO-incompatible (ABOi) liver grafts will expand the donor pool for a patient in urgent need for a liver transplantation (LT). Here we report our results with emergency ABOi DD (deceased donor) LT using rituximab and antigen specific immunoadsorption.
Patients and Methods
2009 to 2019 we performed 20 ABOi DD LTs (adults n = 17, children n = 3) for patients in urgent need for a LT. Immunosuppression consisted of rituximab (n = 20) and basiliximab (n = 15) or anti-thymocyte globuline (n = 4), intravenous immunoglobulin (IVIG; n = 6), tacrolimus, prednisolone and mycophenolate mofetil. Fifteen patients were treated with IA (n = 14) or both IA and plasmapheresis (PP; n = 1) pre-transplant and 18 patients were treated with IA (n = 15) or both IA and PP (n = 3) post-transplant. The median pre-transplant MELD- score was 40 (range 18–40). Patient and graft survival and complications were compared to a 1:4 case matched control group of ABO-identical or compatible (ABOid/c) DDLT.
Results
The 1-, 3- and 5-year patient and graft survival rates were 85, 85 and 78% for the ABOi recipients and not significantly different compared to ABOid/c controls. Only one ABOi patient developed antibody-mediated rejection.
Conclusion
Patient and graft survival after emergency ABOi DDLT using rituximab and immunoadorption was equal to ABOid/DDLT. ABOi DD LT was a successful approach to expand the donor pool for patients in urgent need for a liver graft.
Introduction
The restricted availability of liver grafts for transplantation is of major concern worldwide. One way to increase liver graft access for the individual patients is to accept ABO-incompatible (ABOi) donors.
In East Asia there is a severe shortage of deceased donor (DD) livers and the development of ABOi living donor liver transplantation (LDLT) programmes has been unavoidable [Citation1–10]. In the early era, the outcome of ABOi LT was clearly inferior to that of ABO-identical/compatible (ABOid/c) LT due to antibody-mediated rejection (AMR), vascular and biliary complications caused by preformed anti-A/B antibodies (Ab) [Citation11–18]. However, since the introduction of rituximab induction and plasmapheresis (PP) treatment for ABOi LDLT, graft survival rates have improved dramatically. Today graft survival after ABOi LDLT are comparable to that achieved with ABOc LDLT [Citation7,Citation8,Citation19,Citation20]. However, a slightly increased risk for developing diffuse intrahepatic biliary strictures (DIHBS) after ABOi LDLT is still of concern [Citation7,Citation8].
In Scandinavia, liver grafts are almost exclusively derived from deceased donors and living donors are rarely needed. Living donation is also associated with a relatively high risk of donor morbidity [Citation21]. Therefore, for patients in urgent need for a LT, an ABOi DD graft might be lifesaving. So far, the experience with ABOi DDLT using Ab reducing immunosuppression, especially with rituximab induction, is limited [Citation22–26].
In 2009, we established a protocol for ABOi DDLT based on our experience of elective ABOi LD kidney transplantation. The protocol includes pre-transplant rituximab and antigen specific immunoadsorption (IA) with the exception that rituximab is administered just prior to the LT.
The purpose of this study was to report on the outcome of 20 ABOi DDLTs using this antibody reducing immunosuppressive protocol for patients in urgent need for a new liver. The primary endpoints were patient survival and graft survival. Furthermore, the incidence of rejection, vascular thrombosis and biliary strictures was evaluated.
Patients and methods
Patient and donor characteristics - ABOi recipients
The study population consisted of 20 emergency ABOi LTs performed between 2009 and 2019 at the Transplant Institute, Sahlgrenska University Hospital in Gothenburg, Sweden. All patients were primarily listed for a ABOid LT but were accepted for an ABOi LT as a life-saving procedure or a rescue re-transplant when their clinical status rapidly deteriorated and no ABOid/c liver was available. The indications for LT and the patient characteristics are summarized in . Seventeen recipients were adults and three were children. Seven recipients were male and 13 were female. The Model of End-Stage Liver Disease score (MELD-score) was calculated on the day of LT or the day before. Median MELD-score for the patients >12 years was 40 (range 18–40). The two patients <12 years of age had PELD scores of 21 and 8, respectively. The recipients were blood group O (n = 16), B (n = 3) and A (n = 1). Fifteen patients (75%) had a primary transplant and five (25%) had a re-transplant (four with a secondary graft and one with a tertiary graft). The total median waiting time for a LT (including waiting time for a ABOid LT) was 3 days (range 0–86).
Table 1. Patient and donor characteristics for ABO-incompatible deceased donor liver transplant recipients.
All donors were DBD (donation after brain death). There were 12 non-A2 donors (A1=8, B = 3, AB = 1) and 8 A2-donors. Eighteen LTs were performed using whole grafts and two using split grafts (for the two smallest children).
Case-control matching
The outcome was compared to a case-matched control group of ABOid/c DDLT.
For every ABOi recipient, four matched ABOid or ABOc controls were randomly and blindly selected using the SAS for Windows version 9.4 software. Matching variables included graft number (primary, secondary or tertiary graft), MELD-score at transplantation (±5 points) and year of transplant (±3 years). For four ABOi cases we had to extend the accepted range difference of MELD-score to ±10 points (three controls) and year of transplant to ±7 years (six controls), in order to complete the number of controls for these cases. Since PELD-score is not specified in the Swedish liver transplant registry, the two patients ≤12 years of age were excluded from the matching procedure. Thus, the subsequent case-control analysis included 18 ABOi recipients.
Patient and donor characteristics - ABOid/c recipients
The control group consisted of 72 ABOid (n = 58) or ABOc (n = 14) LTs performed during the same time period at the Transplant Institute, Sahlgrenska University Hospital. Forty recipients were male and 32 were female. The median pre-transplant MELD-score was 39 (range 18–40). Median waiting time was 3 days (range 0–370 days). Fifty-six patients were primary grafted (78%) and 16 patients were re-transplanted (22%, 12 secondary grafted and four tertiary grafted). All donors were DBD.
A comparison of patient characteristics between the ABOi- and ABOic/c groups is presented in . There was no significant difference in recipient age, donor age, cold ischemia time (CIT) or MELD-score between the groups, however the percentage of patients requiring care in the intensive care unit pre-transplant was significantly higher in the ABOi DDLT group.
Table 2. Comparison of patient and donor characteristics between ABO-incompatible and ABO-identical/compatible deceased donor liver transplant recipients.
Immunosuppression - ABOi recipients
All ABOi recipients received induction therapy with rituximab (Mabthera®, Roche, Basel, Switzerland) 375 mg/m2 i.v, as a single dose day 0 prior to LT. Between 2009 and 2014 four patients received anti-thymocyte globuline (ATG®, Fresenius, Bad Homburg, Germany) 1.5 mg/kg i.v, as a single dose before reperfusion and one patient received no additional induction therapy. The remaining fifteen patients received basiliximab (Simulect®, Novartis, Basel, Switzerland) 20 mg i.v before reperfusion and on POD four. Six recipients also received intravenous immunoglobulin (IVIG; Gammagard® or Kiovig®, Takeda Pharma, Japan) 0,5 g/kg i.v, on POD 0 and one recipient with hemophagocytic lympfohistiocytosis received six doses of IVIG (day −4 until POD 5) due to severe systemic inflammation.
Maintenance immunosuppression consisted of oral tacrolimus (Prograf®, Astellas Pharma, Tokyo, Japan or Adport®, Sandoz, Holzkirchen, Germany) starting on POD 1, with an initial target trough level of 10–15 μg/l, oral mycophenolate mofetil (CellCept®, Roche, Basel, Switzerland or generic formulation) 1 g B.D, starting on POD 0 and oral prednisolone, 100 mg B.D, starting on POD 1, tapered to 5 mg by six months.
Antigen specific immunoadsorption (IA, Glycosorb®, Glycorex AB, Lund, Sweden) was performed as previously described by Norden et al. [Citation27]. Immunoadsorption was initiated as soon as the ABOi DDLT was scheduled. A total plasma volume of seven litres was circulated through a Glycosorb® column apheresis filter during approximately four hours. Post-transplant three further IA-sessions were planned at post-operative day (POD) 2, 5 and 8 irrespective of the Ab titer levels. During the first two post-operative weeks, additional IA were planned in case the Ab titer levels increased to >8 or ≥ two-fold above the lowest value post-LT. Plasmapheresis (PP) was accepted as an alternative apheresis method when IA could not be performed for logistical or technical reasons. All patients except one were treated with one or more IA-sessions pre-, and/or post-transplant. Fifteen patients (75%) were treated with IA (n = 14) or both IA and PP (n = 1) prior to the transplantation. Five patients (25%) were not treated with neither IA nor PP pre-transplant for logistical reasons or due to low anti-A/B titer. Three patients were treated with IA immediately post-transplant. Eighteen patients (90%) were treated with IA (n = 15) or both IA and PP (n = 3) post-transplant. Two patients (10%) were not treated with neither IA nor PP post-transplant due to low anti-A/B Ab titers. The number of apheresis performed for each patient is summarized in .
Table 3. Anti-A/B IgM/IgG antibody titers and HLA-antibodies for ABO-incompatible deceased donor liver transplant recipients.
Immunosuppression - ABOid/c recipients
The ABOid/c recipients received induction therapy with basiliximab 20 mg i.v before reperfusion and POD 4 and methylprednisolone (Solu-Medrol®, Pfizer, NY, USA) 1 g i.v before reperfusion. Maintenance immunosuppression consisted of delayed introduction (POD 3) of oral tacrolimus (Prograf®, Astellas Pharma, Tokyo, Japan or Adport®, Sandoz, Holzkirchen, Germany) with an initial target trough level of 5–8 µg/l and oral mycophenolate mofetil (CellCept®, Roche, Basel, Switzerland or generic), 1 g B.D starting on POD 0. Patients with autoimmune hepatitis (AIH) or primary sclerosing cholangitis (PSC) also received oral prednisolone 20 mg daily, introduced on POD1, with tapering after 10–14 days, reaching 5 mg by 3 months.
Anti-a/B Ab determinations and HLA-Abs
Titrations of anti-A/B IgM and IgG Abs were performed with standard techniques using erythrocytes from panel donors, as described elsewhere [Citation27].
HLA Ab detection and specificity determination were performed using the Luminex® bead-based LABScreen TM Mixed and Single Antigen assays, respectively (One Lambda Inc, West Hills, CA, USA). In the Luminex®-based assays, a mean fluorescence intensity value (MFI) >400 (Mixed) and >1000 (Single Antigen) above the negative control was considered positive.
Histopathology and diagnosis of rejection
Liver biopsies were performed whenever clinically indicated. In addition, since 2013, a protocol biopsy was taken approximately one-week post-transplant. Histopathological evaluation was done according to the Banff criteria for T–cell mediated rejection and for AMR [Citation28,Citation29]. Immunostaining for C4d (ab 58781; Abcam, Cambridge, UK) was done using an automated procedure, the EnVision™ Flex high pH (Link) detection kit (Dako K8000). Donor biopsies from ABO-compatible grafts served as negative controls. C4d grading from 0-3 and AMR grading from 0-3 was done as described in the 2016 Banff guidelines [Citation29].
Magnetic resonance imaging
For the evaluation of biliary strictures, a magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) was performed when clinically indicated. For the ABOi recipients without clinical signs of biliary complications, the follow-up physicians were asked to perform a “protocol” MRI/MRCP. All MRI/MRCP were collectively evaluated by a radiologist with extensive experience in biliary and vascular complications following LT.
Biliary stricture was defined as a significant narrowing of the biliary tree, with or without upstream biliary dilatation. Depending on the location, a biliary stricture was classified as either anastomotic or non-anastomotic [Citation30–33]. Diffuse intrahepatic biliary strictures (DIHBS) were considered when non-anastomotic intrahepatic strictures developed without a preceding ischemic event [8].
Statistical analysis
The Graph Pad Prism Software, Version 8.4.1 (San Diego, USA) and IBM SPSS Statistics for Macintosh, Version 27.0 (Chicago, USA) was used for data analysis. Categorical variables were presented as number and percentage and quantitative variables as median, range and interquartile range. Patient survival was defined as time from LT until 31st December 2020 or death. Graft survival was defined as time from LT to 31st December 2020 until death or re-transplantation. Case control matching 1:4 was performed as described in the Patients and Methods section. Clinical characteristics were compared using Mann-Whitney U test or Fisher’s exact test. Patient and graft survival were calculated with Kaplan–Meier survival analysis and was compared with the log-rank test. Incidence of rejection, vascular thrombosis and biliary strictures were calculated as number and frequencies and compared with Fishers’ exact test. A p-value < .05 was considered statistically significant and 2-sided p-values were used.
Ethical approval
The study was conducted in accordance with the ethical principles that comply with the Declaration of Helsinki and the Declaration of Istanbul and was approved by the regional ethics committee in Gothenburg (approval number 048-13).
Results
Patient and graft survival
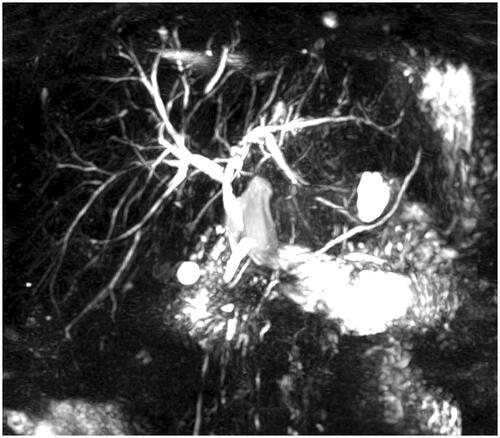
None of the ABOi recipients was retransplanted. Four ABOi recipients died, two with functioning grafts and two due to sepsis and multiorgan failure (). For all ABOi recipients (n = 20) the 1-, 3-, and 5-year patient and graft survival rates were 85, 85 and 78%. Excluding the two recipients <12 years, the 1-, 3-, and 5-year patient and graft survival were 89%, 89% and 81%. The ABOid/c matched control group (n = 72) had equivalent survival with a 1-, 3-, and 5-year patient and graft survival of 85/83%, 83/79% and 81/77%, respectively (; p = .59 and p = .43). In a subgroup analysis comparing non-A2-donor (n = 12) vs. A2-donor (n = 8) ABOi DDLT, there was no significant difference in 1-, 3-, and 5-year patient and graft survival rates (non-A2 donor 83, 83 and 83% vs A2 donor 88, 88 and 70%, p = .75).
Figure 1. Kaplan-Meier plot comparing patient (A) and graft survival (B) after ABO-incompatible (n = 18) and ABO-identical/compatible (n = 72) deceased donor liver transplantation (log-rank test).
Table 4. Survival and post-transplant clinical complications after ABO-incompatible deceased donor liver transplantation.
Post-transplant complications - ABOi recipients
In total, four ABOi recipients had biopsy proven T-cell mediated rejection (). Two adult patients had steroid resistant T-cell mediated rejection and both responded to anti-thymocyte globuline treatment. One patient developed AMR (Pat#12, ). This patient was retransplanted with an ABOi graft due to acute-on-chronic liver failure, in turn due to therapy resistant chronic rejection. In addition to anti-AB antibody rebound, high MFI DSA (Class II DSA, MFI 12201) was detected post-transplantation. Multiple IA-, and PP-sessions were performed but were unsuccessful in controlling the Ab rebound (). A liver biopsy at POD 12 showed histological features of AMR (AMR-score 2, ) with a strong C4d-deposition (C4d-score 3, ). Several surgical complications also complicated the clinical course. During the first post-operative weeks the patient was re-operated three times, twice for bleeding with circulatory shock and once due to bile leakage. After the second bleeding episode a partial thrombosis in the hepatic artery proper was diagnosed. Thereafter intrahepatic necrosis developed followed by multiple intrahepatic biliary strictures. Septicemia contraindicated intensified immunosuppression and re-transplantation and the patient died four months post-transplantation.
Figure 2. Plots of anti-A/B antibody titers, IgM (A) and IgG (B), pre-, and post ABO-incompatible liver transplantation (n = 20).
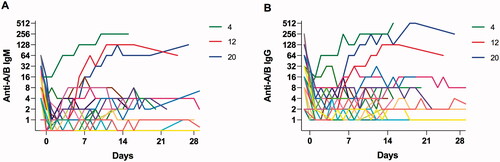
Figure 3. Light microscopy of liver biopsies 9 days (A: Pat #13) and 12 days (B and C: Pat #12) post-transplant. A. Portal area with endothelialitis and minimal microvascular inflammation possible but no definitive proof of AMR. B. Slightly dilated vessel, enlarged endothelial nucleus (arrowhead) and microvascular inflammation dominated by neutrophils (arrow) suggesting AMR grade 2. C. Immunohistochemistry for C4d is diffusely positive in this specimen.
One ABOi recipient was diagnosed with partial thrombosis at a stenosed main arterial anastomosis and at a stenosed reconstructed accessory right hepatic artery, seven weeks post-transplant. Another patient (lupus anticoagulant positive) developed portal vein thrombosis at the level of a stenosed portal vein anastomosis. The thrombosis developed when anticoagulation was paused, 13 months post-transplant and resolved some months after that the anticoagulant treatment was resumed. One patient developed an anastomotic biliary stricture, diagnosed 22 months post-transplantation (). This patient was successfully treated with multiple endoscopic dilatations and stents. Two patients developed non-anastomotic biliary strictures with clinical manifestations, both following partial arterial thrombosis (, Pat#12 and #19).
There were few opportunistic and severe infections in the ABOi group. Only one patient (Pat#12) developed a systemic fungal infection and one patient developed CMV-reactivation. There were no Pneumocystis jirovecii pneumonia infections and none of the ABOi recipients developed post-transplant lymphoproliferative disorder.
Post-transplant complications in the ABOi group that occurred within the first-year post-transplant were compared to the matched ABOid/c control group and are summarized in .
Table 5. Comparison of post-transplant complications occurring within one year after ABO-incompatible and ABO-identical/compatible deceased donor liver transplantation.
Anti-a/B Ab titers and HLA-Abs
Anti-A/B antibody titers pre-, and post-transplant are listed in . Seventeen patients (85%) had post-transplant anti-A/B titers ≤ 32. Three patients (15%) developed antibody rebound with anti-A/B titers > 32 () and were treated with repeated IA (n = 3) and PP (n = 3) and IVIG (n = 1). One of these patients developed AMR (Pat #12) as mentioned above.
Four patients (20%) in the ABOi group had detectable panel reactive HLA-antibodies (PRA) pre-transplant, but only one of them had a positive CDC-crossmatch (Pat #12). Three patients had a positive CDC-crossmatch but no HLA-antibodies. Three of the four patients with positive PRA were tested for DSA. One of them had DSA pre-transplant and one developed DSA post-transplant ().
Magnetic resonance imaging findings
Thirteen ABOi patients (65%) had a post-transplant MRI/MRCP scan performed. Apart from the biliary complications that presented clinically, the radiological analysis revealed three patients with radiological signs of non-anastomotic biliary strictures without clinical presentation (). Two of them had a single short biliary stricture in the right biliary duct. The third patient had multiple intrahepatic biliary strictures, without any vascular events and were classified as DIHBS (). All three have well-functioning grafts and have not required any interventions.
Table 6. Radiological findings with MRI/MRCP after ABO-incompatible deceased donor liver transplantation.
Protocol biopsies
Protocol biopsies were performed on POD 7-26 for 14 patients (70%). Two of these showed histopathological signs of possible AMR (; ). One of these patients (Pat #12) had a concomitant rebound of anti-A/B Ab and AMR could not be ruled out clinically. The other patient (Pat #13) did not have Ab rebound, nor any clinical signs of AMR. In five additional biopsies the C4d staining was positive, but without any other signs of AMR. In the remaining seven biopsies the C4d staining was either negative (n = 5) or not done (n = 2). In five of 14 (36%) protocol biopsies, T-cell mediated rejection (RAI 3-6) was diagnosed by histopathology, but only two of these patients had clinical signs of rejection and received anti-rejection treatment.
Table 7. Protocol biopsies after ABO-incompatible deceased donor liver transplantation.
Discussion
In this study we report the outcome of 20 emergency ABOi DDLTs performed at our center between 2009 and 2019 using an Ab reducing immunosuppressive protocol containing rituximab and immunoadsorption. The 1-, 3-, and 5-year patient and graft survival rates were 85, 85 and 78% and equivalent to a matched ABOid/c control group (; p = .59 and p = .43). Eight donors (40%) in the ABOi group were subgroup A2, however there was no significant difference in survival in a subgroup analysis between A2 and non-A2 ABOi DDLT.
Our results are in line with the Asian experience of ABOi LDLT. In Japan, Korea and Taiwan, the outcome after ABOi LDLT is equivalent to that of ABOc LDLT [Citation7–9,Citation20]. Their immunosuppressive protocols are similar to ours, although rituximab is administered 1–4 weeks before the elective LDLT. Also, plasmapheresis is used instead of IA and performed earlier prior to the transplant. In 2016, Song et al. published the largest series of ABOi LDLT with 235 patients and reported a 3-year patient and graft survival of 93% and 89%, respectively [Citation7]. Their survival rate was superior to the survival in our cohort. However, the elective nature of LDLT has advantages compared to DDLT, such as extensively evaluated and healthy donors with high liver graft quality, short ischemia times and often a lower recipient MELD-score at the time of LT. Whether early pre-transplant administration of rituximab in ABOi LDLT is beneficial remains to be determined. Egawa et al. reported six cases of ABOi LDLT for fulminant hepatic failure, where rituximab was given immediately before or during the liver transplant and all six survived without AMR [Citation5].
Publications on ABOi DDLT in the modern era are scarce and there are very few reports with rituximab induction for ABOi DDLT. In 2013, Mendes reported 10 ABOi DDLT using basiliximab induction and PP (when anti-A/B titer was >16). Graft survival was only 40% with a median follow up of 19,6 months [Citation23]. In 2015, Thorsen et al. published a cohort of 61 ABOi DDLT, performed in Norway and at our center in Sweden [Citation24]. Here, less than 50% of the patients received rituximab induction and the management of anti-A/B antibody titers was variable. The 1-, 3-, and 5-year graft survival rate was 71%, 57% and 55%, respectively and significantly inferior to ABOc controls. In 2014, however, Shen et al. reported on 35 ABOi DDLTs performed for acute liver failure using rituximab induction and intravenous immunoglobulin (IVIG) for 10 consecutive days [Citation25]. Plasmapheresis was only used when AMR was suspected. In line with our outcome, they found no difference in patient and graft survival when comparing their outcome with ABOc DD LT.
Ethical dilemmas regarding liver graft allocation have probably been an obstacle for the development of ABOi DDLT programs. In large allocation systems, there are numerous critically ill patients listed for a liver transplant and the use of an ABOi DD graft will probably increase the mortality risk for potential ABOid recipients. In Scandinavia, however, organ availability is generally favourable and the waiting list mortality less than 5% [Citation34]. Organs are mainly distributed through centre allocation, but also trough a Scandinavian organ exchange program for highly urgent cases with a “pay-back” system. In Sweden, patients with chronic liver failure are normally listed when the estimated survival, without a transplant, is less than one year. The median MELD score (at the time of transplantation) in Sweden is less than 18. This means that we often have the opportunity to consider the use of an ABOc or ABOi DD liver in urgent cases, without jeopardizing the survival of the other patients on the waiting list. Also, the annual mortality rate, on the waiting list for a liver transplant in Scandinavia, has decreased during the same time-period this study was performed [Citation34].
Since the introduction of rituximab and PP in ABOi LDLT, the risk for Ab mediated graft loss has decreased [Citation5]. In our study, only one ABOi recipient developed AMR. Interestingly, this patient was highly HLA-immunized and had a positive CDC-crossmatch pre-transplant. In addition to anti-A/B antibody rebound, he also developed high MFI DSA which may have contributed to the AMR. Thus, HLA-immunization with a positive crossmatch might add immunological risk in ABOi LT and should possibly be avoided.
Recent publications do not report a significantly increased risk for HAT following ABOi LDLT [Citation7,Citation35]. In our cohort, none of the ABOi recipients developed complete HAT. However, two patients developed partial arterial thrombosis, both with a potential predisposing cause (circulatory shock and technical failure with the arterial anastomosis). Diffuse intrahepatic biliary strictures (DIHBS) following ABOi LT however still seem to be of concern. Song et al found that the incidence of DIHBS was approximately 7% and appeared on average 2.7 months post-transplantation without preceding HAT [Citation8]. They also reported that in more than half of the cases, DIHBS occurred without prior clinical suspicion and without association to anti-A/B titer levels [Citation8]. In our study, only one patient in the ABOi group developed DIHBS which was discovered on a protocol MRCP. This patient did not have any clinical manifestations or antibody rebound and the graft is still functioning well.
In most ABOi LDLT programmes, the aim is to reduce the anti-A/B Ab titer to ≤8 to 64 pre-transplantation [Citation8,Citation19,Citation20]. Egawa et al. found that an anti-A/B IgG titer ≥16 at transplant and an anti-A/B IgG and IgM titer ≥64 post-transplant were significant risk factors for AMR [Citation5]. In 2018, Yamamoto et al. and Lee et al. published successful ABOi LDLT using rituximab without plasmapheresis for ABOi LDLT [Citation36,Citation37]. None of the patients in these studies had post-transplant Ab titer levels higher than 64. In our study, all patients except one (95%) had an anti-A/B Ab titer level ≤32 pre-transplant and for 17 of 20 patients (85%), we managed to keep the anti-A/B Ab titers ≤32 post-transplant. In three cases (15%) we could not prevent Ab rebound and one of these patients developed AMR.
Since we have a long experience and tradition of using IA for ABOi kidney transplantation at our centre, we preferred to use IA over PP. Immunoadsorption is a well-tolerated and effective method to selectively remove only anti-A/B antibodies [Citation27,Citation38]. Immunoadsorption, as opposed to PP, does not affect coagulation nor rituximab pharmacokinetics [Citation38–39]. However, since PP sometimes is used for the treatment of acute and acute-on-chronic liver failure, by removing toxins and other drivers of systemic inflammation [Citation40], PP might be beneficial in ABOi DDLT, at least pre-transplant, provided that fresh frozen plasma is used as replacement fluid (to avoid reduction of coagulation factors).
The expression level and distribution of A-, and B-antigen within the liver vasculature and bile ducts, is also likely to be of importance in ABOi LT. In our experience, A2-donor ABOi DDLT has been performed without rituximab and anti-A Ab reduction with superior survival rates compared to A1- and B-donor ABOi DDLT [Citation41]. Also, Kluger et al. have reported that A2-to-O ABOi LT had equivalent survival compared to ABOid LT [Citation42]. Likewise, Thorsen et al. concluded that A2 donor ABOi DDLT is a safe option in urgent cases. Thus, A2-donor liver grafts seem to be less immunogenic compared to A1-, and B-donor grafts. However, blood group related differences in A/B-antigen expression have mainly been studied in kidney biopsies and are poorly characterized in liver tissue [Citation43–44]. Therefore, in this study we chose to use the same immunosuppressive protocol for both A2-, and non-A2 donor ABOi LT.
The limitations of this study included the relatively small size of the ABOi DDLT cohort and the slight variance in induction therapy chosen for some of the ABOi recipients. Also, the prospective close follow-up of the ABOi cohort versus the retrospective selection and follow up of the control group made it hard to accurately compare post-transplant complications between the groups. Since it was difficult to get reliable follow-up information regarding post-transplant infection for the ABOid/c control group, post-transplant infections could unfortunately not be compared in the study. Lastly, the limited number of protocol biopsies made it hard to draw any conclusions about the value of protocol biopsies in ABOi DDLT.
In conclusion, emergency ABOi DDLT, using an anti-A/B Ab reducing protocol with rituximab and IA, resulted in equivalent patient and graft survival compared to ABOid/c DDLT with acceptable risks. Therefore, the utilization of ABOi DD liver grafts provides an opportunity to expand the donor pool for patients in urgent need for a LT, increasing their chance of survival.
Author contributions
USD: Research design, performance of the research, data analysis and drafted the manuscript. GH: Research design, data analysis and reviewed the paper. BG: Research design, data analysis and reviewed the paper. JM: Performance of the research, data analysis and reviewed the paper. LR: Performance of the research, data analysis and reviewed the paper. AS: Performance of the research, data analysis and reviewed the paper. WB: Research design, performance of the research, data analysis and drafted the manuscript.
| Abbreviations | ||
| AAT | = | α1-antitrypsin deficiency |
| Ab | = | antibody |
| ABOc | = | ABO-compatible |
| ABOi | = | ABO-incompatible |
| ABOid | = | ABO-identical |
| ACLF | = | acute-on-chronic liver failure |
| AIH | = | autoimmune hepatitis |
| ALC | = | alcohol |
| ALF | = | acute liver failure |
| AMR | = | antibody-mediated rejection |
| AS | = | anastomotic stricture |
| CCC | = | cholangiocarcinoma |
| CDC | = | complement-dependent cytotoxicity |
| CIT | = | cold ischemia time |
| CT | = | computed tomography |
| DBD | = | donation after brain death |
| DD | = | deceased donor |
| DIHBS | = | diffuse intrahepatic biliary strictures |
| DSA | = | donor specific antibodies |
| GS | = | graft survival |
| HAT | = | hepatic artery thrombosis |
| HBV | = | hepatitis B virus |
| HCC | = | hepatocellular carcinoma |
| HCV | = | hepatitis C virus |
| HLH | = | hemophagocytic lymphohistiocytosis |
| IA | = | antigen specific immunoadsorption |
| ICU | = | intensive care unit |
| IVIG | = | intravenous immunoglobulin |
| LD | = | living donor |
| LDLT | = | living donor liver transplantation |
| LT | = | liver transplantation |
| MELD | = | model for end-stage liver disease |
| MFI | = | mean fluorescence intensity |
| MRCP | = | magnetic resonance cholangiopancreatography |
| NAS | = | non-anastomotic strictures |
| Pat | = | patient |
| PBC | = | primary biliary cirrhosis |
| PELD | = | pediatric end stage liver disease |
| POD | = | post-operative day |
| PP | = | plasmapheresis |
| PLD | = | polycystic liver disease |
| Pre-tx | = | pre-transplant |
| PS | = | patient survival |
| PSC | = | primary sclerosing cholangitis |
| PVT | = | portal vein thrombosis |
| RAI | = | rejection activity index |
| Retx | = | re-transplantation |
| TCMR | = | T-cell mediated rejection |
Disclosure statement
The authors declare no conflicts of interest and have no financial disclosures.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Tanabe M, Shimazu M, Wakabayashi G, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation. 2002;73(12):1959–1961.
- Nakamura Y, Matsuno N, Iwamoto H, et al. Successful case of adult ABO-incompatible liver transplantation: beneficial effects of intrahepatic artery infusion therapy: a case report. Transplant Proc. 2004;36(8):2269–2273.
- Usuda M, Fujimori K, Koyamada N, et al. Successful use of anti-CD20 monoclonal antibody (rituximab) for ABO-incompatible living-related liver transplantation. Transplantation. 2005;79(1):12–16.
- Egawa H, Teramukai S, Haga H, et al. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008;47(1):143–152.
- Egawa H, Teramukai S, Haga A, et al. Impact of rituximab desentitization on blood-type-incompatible adult living donor liver transplantation: a japanese multicenter study. Am J Transplant. 2014;14(1):102–114.
- Kawagishi N, Ohuchi N, Satomi S. Current aspects of ABO-incompatible liver transplantation. Clin Case Rep and Rev. 2016;2(4):375–379.
- Song G-W, Lee S-G, Hwang S, et al. ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab. Am J Transplant. 2016;16(1):157–170.
- Song G-W, Lee S-G, Hwang S, et al. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 2014;61(3):575–582.
- Ikegami T, Yoshizumi T, Soejima Y, et al. Feasible usage of ABO incompatible grafts in living donor liver transplantation. Hepatobiliary Surg Nutr. 2016;5(2):91–97.
- Park G-C, Song G-W, Moon D-B, et al. A review of current status of living donor liver transplantation. Hepatobiliary Surg Nutr. 2016;5(2):107–117.
- Starzl TE, Koep LJ, Halgrimson CG, et al. Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77(2):375–388.
- Raut V, Uemoto S. Management of ABO-incompatible living-donor liver transplantation: past and present trends. Surg Today. 2011;41(3):317–322.
- Farges O, Kalil AN, Samuel D, et al. The use of ABO-incompatible grafts in liver transplantation: a life saving procedure in highly selected patients. Transplantation. 1995;59(8):1124–1133.
- Lo C-M, Shaked A, Busuttil RW. Risk factors for liver transplantation across the ABO barrier. Transplantation. 1994;58(5):543–547.
- Gugenheim J, Samuel D, Reynes M, et al. Liver transplantation across ABO blood group barriers. Lancet. 1990;336(8714):519–523.
- Gordon RD, Iwatsuki S, Esquivel O, et al. Liver transplantation across ABO blood groups. Surgery. 1986;100(2):342–348.
- Demetris AJ, Jaffe R, Tzakis A, et al. Antibody-mediated rejection of human orthotopic liver allografts. Am J Pathol. 1988;132(3):489–502.
- Reding R, Veyckemans F, de Ville de Goyet J, et al. ABO-incompatible orthotopic liver allografting in urgent indications. Surg Gynecol Obstet. 1992;174(1):59–63.
- Lee C-F, Cheng C-H, Wang Y-C, et al. Adult living donor liver transplantation across ABO-incompatibility. Medicine (Baltimore)). 2015;94(42):e1796.
- Kim JM, Kwon CHD, Joh J-W, et al. Case-matched comparison of ABO-incompatible and ABO-compatible living donor liver transplantation. Br J Surg. 2016;103(3):276–283.
- Ghobrial RM, Freise CE, Trotter JF, A2ALL Study Group, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135(2):468–476.
- Hanto DW, Fecteau AH, Alonso MH, et al. ABO-incompatible liver transplantation with no immunological graft losses using total plasma exchange, splenectomy, and quadruple immunosuppression: evidence for accommodation. Liver Transpl. 2003;9(1):22–30.
- Mendes M, Ferreira AC, Ferreira A, et al. ABO-incompatible liver transplantation in acute liver failure: a single portuguese center study. Transplant Proc. 2013;45(3):1110–1115.
- Thorsen T, Dahlgren US, Aandahl EM, et al. Liver transplantation with deceased ABO-incompatible donors is life-saving but associated with increased risk of rejection and post-transplant complications. Transpl Int. 2015;28(7):800–812.
- Shen T, Lin B-Y, Jia J-J, et al. A modified protocol with rituximab and intravenous immunoglobulin in emergent ABO-incompatible liver transplantation for acute liver failure. Hepatobiliary Pancreat Dis Int. 2014;13(4):395–401.
- Boberg KM, Foss A, Midtvedt K, et al. ABO-incompatible deceased donor liver transplantation with the use of antigen-specific immunoadsorption and anti-CD20 monoclonal antibody. Clin Transplant. 2006;20(2):265–268.
- Nordén G, Briggs D, Cockwell P, et al. ABO-incompatible live donor renal transplantation using blood group a/B carbohydrate antigen immunoadsorption and anti-CD20 antibody treatment. Xenotransplantation. 2006;13(2):148–153.
- Demetris A, Adams D, Bellamy C, et al. Update of the international banff schema for liver allograft rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An international panel. Hepatology. 2000;31(3):792–799.
- Demetris A, Bellamy C, Hübscher SG, et al. 2016 Comprehensive update of the banff working group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant. 2016;16(10):2816–2835.
- Roos FJM, Poley J-W, Polak WG, al.et al. Biliary complications after liver transplantation; recent developments in etiology, diagnosis and endoscopic treatment. Best Pract Res Clin Gastroenterol. 2017;31(2):227–235.
- Villa NA, Harrison E. Management of biliary strictures after liver transplantation. Gastroenterol Hepatol (N Y). 2015;11(5):316–328.
- Lee HW, Suh K-S, Shin WS, et al. Classification and prognosis of intrahepatic biliary stricture after liver transplantation. Liver Transpl. 2007;13(12):1736–1742.
- Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut Liver. 2011;5(2):133–142.
- The Nordic Liver Transplant Registry (NLTR) annual report 2019. 2020. www.scandiatransplant.org.
- Kim SH, Park J, Park SJ. Impact of ABO-incompatibility on hepatic artery thrombosis in living donor liver transplantation. Ann Transl Med. 2019;7(22):625.
- Yamamoto H, Uchida K, Kawabata S, et al. Feasibility of monotherapy by rituximab without additional desensitization in ABO-incompatible Living-Donor Liver Transplantation. Transplantation. 2018;102(1):97–104.
- Lee EC, Kim SH, Shim JR, et al. A comparison of desensitization methods: Rituximab with/without plasmapheresis in ABO-incompatible living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2018;17(2):119–126.
- Tydén G, Kumlien G, Genberg H, et al. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5(1):145–148.
- Puisset F, White-Koning M, Kamar N, et al. Population pharmacokinetics of rituximab with or without plasmapheresis in kidney patients with antibody-mediated disease. Br J Clin Pharmacol. 2013;76(5):734–740.
- Tan EXX, Wang MX, Pang J, et al. Plasma exchange in patients with acute and acute-on-chronic liver failure: a systematic review. WJG. 2020;26(2):219–245.
- Skogsberg U, Breimer ME, Friman S, et al. Successful ABO-incompatible liver transplantation using A2 donors. Transplant Proc. 2006;38(8):2667–2670.
- Kluger MD, Guarrera JV, Olsen SK, et al. Safety of blood group A2-to-O liver transplantation: an analysis of the united network of organ Sharing database. Transplantation. 2012;94(5):526–531.
- Breimer M, Samuelsson BE. The specific distribution of glycolipid-based blood group a antigens in human kidney related to A1/A2, Lewis, and secretor status of single individuals. A possible molecular explanation for the successful transplantation of A2 kidneys into O recipients. Transplantation. 1986;42(1):88–91.
- Breimer ME, Mölne J, Nordén G, et al. Blood group a and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and secretor status. Transplantation. 2006;82(4):479–485.
- Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that developed in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426–1437.