Abstract
Objectives
We evaluated the relationship between serum concentration and efficacy of adalimumab (ADA), an anti-tumor necrosis factor-alpha agent, in pediatric patients with inflammatory bowel disease (PIBD).
Materials and methods
This retrospective cross-sectional study traced 75 patients with PIBD (Crohn’s disease, n = 57) treated with ADA at two tertiary centers in Finland in 2012–2018. Drug levels and drug antibody titers were chart-reviewed, and the treatment continuation rate of ADA therapy was evaluated. We also assessed the impact of trough levels in the first 3 months on the continuation of ADA within one year of therapy.
Results
ADA was introduced at a median age of 13.4 years, and the median disease duration was 2.7 years. During the first year, 22 patients (29%) discontinued ADA due to either loss of response (20%, n = 15) or anti-drug antibody formation (5.3%, n = 4). Regarding trough levels in the first 3 months, 9/16 patients (56%) with trough levels <5 mg/L and 12/20 (60%) with trough levels <7.5 mg/L at 3 months discontinued the therapy by the end of the first year. In comparison, only 8/32 patients (25%) with trough levels >7.5 mg/L at 3 months discontinued treatment during the first year (p = .005). At the last follow-up (median 1.5 years), 52% of the 75 patients were on maintenance therapy and had a median trough level of 8.8 mg/L.
Conclusion
Higher trough levels in the first 3 months of adalimumab treatment are associated with lower rates of discontinuation due to loss of response during the first year.
Introduction
Monoclonal antibodies to tumor necrosis factor-alpha (TNFα) agents infliximab and adalimumab have become the mainstay of therapeutic treatment for moderate to severe pediatric inflammatory bowel disease (PIBD) [Citation1–4]. This is comparable with the management of adult IBD. In Europe, the patent for infliximab expired in 2015 and for adalimumab in 2018, and currently, there are several biosimilars to the original drugs Remicade (Janssen Biologics) and Humira (Abbvie) on the market [Citation5–7]. The induction phase of infliximab is six weeks, and the three first doses are administered intravenously usually at 5 mg/kg at weeks 0, 2, and 6. The maintenance phase and the fourth dose follow at 8 weeks [Citation1–5]. For adalimumab, the approved induction regimen in PIBD ranges from 40 mg at week 0 and 20 mg at week 2 to 160 mg at week 0 and 80 mg at week 2 based on the weight of the patient, and disease severity assessed by a physician. Maintenance therapy with adalimumab is administered biweekly but may be enhanced to weekly therapy and/or by doubling the maintenance dose [Citation8,Citation9].
Infliximab and its biosimilars are licensed for use in children who are at least six years of age and have moderate to severe Crohn’s disease or ulcerative colitis, and adalimumab only recently for such patients with ulcerative colitis in addition to Crohn’s disease. Several countries, including Finland, allow off-label use of anti-TNFα in younger children at the physicians’ discretion. This includes shortening of the interval and increasing the dose [Citation10,Citation11].
Most patients with PIBD initially respond to anti-TNFα therapy but will need dose escalation during the first year of therapy [Citation12–15]. To guide therapeutic decisions, the use of trough level measurements of infliximab has been established [Citation9,Citation16]. In PIBD, however, published reports on adalimumab levels are scarce. We have had access to adalimumab trough level measurements since 2012 and aim in this study to summarize our experience of the association between adalimumab levels and therapeutic outcomes in PIBD, i.e., treatment continuation rate.
Materials and methods
We traced altogether 75 pediatric patients with PIBD and ongoing adalimumab therapy with trough level measurements between February 2012 and December 2018 at Children’s Hospital, University of Helsinki, Helsinki, Finland and at the Department of Pediatrics, Tampere University Hospital, Tampere, Finland. Drug levels at one year and at the latest follow-up for maintenance therapy were related to the therapy outcome in the total patient cohort. Of all 75 patients, 52 had at least one drug level measurement in the first 3 months of therapy and these patients were included in a sub-analysis and assessment of the impact of induction drug levels on therapy outcome at one year. The background data of the included patients are shown in .
Table 1. Background data of the patients with pediatric-onset inflammatory bowel disease and treated with anti-TNF-alpha antagonist adalimumab between 2012 and 2018 at two tertiary care hospitals in Finland.
In Helsinki, the patients on adalimumab were identified from the prospective register of PIBD patients treated with biologics at Children’s Hospital, University of Helsinki, Finland. This register includes data on all patients introduced to biologics, dates of introduction, dates of discontinuation, and reasons for discontinuation. As the register is in routine use, it includes all patients treated at the hospital. In Tampere, the patients were traced from the files of the outpatient clinic of pediatric gastroenterology using a retrospective chart review and laboratory files of adalimumab measurements.
Decisions on management were made at the discretion of the treating physician and were based on the routine use of the clinical symptom index [Citation17], fecal calprotectin [Citation18–20], blood inflammatory marker and drug level measurements, and the presence of drug antibodies when appropriate [Citation7,Citation16]. Clinical disease activity was retrospective scored according to Physician Global Assessment (PGA) on a scale of 1–3 [Citation21]. Fecal calprotectin <100 mg/g is considered as remission and values >1000 mg/g as exceedingly high [Citation18]. The endoscopy or imaging results were also used when they were available. We reviewed data on patient age and gender, a subtype of PIBD, disease duration at adalimumab induction, immunosuppressive medication, adalimumab induction doses, drug levels, and the presence of drug antibodies. Our analysis also included the duration of the adalimumab therapy, the reasons for discontinuing the drug, and any adverse events. As most adalimumab injections are given at home, the day of blood sampling varies from 0 to 3 d prior to the injection but counts as trough level. There were in total 328 measurements of adalimumab levels in 75 patients, but as the sampling time points varied, we evaluated the levels and inflammatory markers at the following time points: 1) in the first 3 months (corresponding induction period), 2) at one year, and 3) at the latest follow-up visit.
Ethics
This was a register-based study, and, according to Finnish legislation, neither ethics approval nor informed consent was needed since the patients were not contacted. The study was licensed at Helsinki University Hospital and Tampere University Hospital.
Statistical analysis
Data are presented as the median and interquartile range (IQR) unless otherwise stated. We used Fisher’s exact test to determine differences in binary variables. Non-parametric Mann–Whitney test was used to compare continuous variables between the groups as appropriate (Graph Pad Prism version 7.0, GraphPad Software, San Diego, CA, USA). When comparing the therapy outcomes related to disease subtype, patients with ulcerative colitis (UC) were combined with unclassified colitis (IBDU) due to the low number of patients. The level of significance was set at p < .05.
Results
Background data of the 75 patients with PIBD treated with adalimumab are shown in . One-fifth of the patients were anti-TNF-naïve at therapy induction, 44% had concomitant immunosuppression with azathioprine and/or methotrexate, and half were on glucocorticoids. Four patients (5%) had undergone colectomy and three (4%) ileocecal resection.
The induction dose of subcutaneous adalimumab was 160 mg in 3, 120 mg in one, 80 mg in 48, 60 mg in 2, 40 mg in 20, and 25 mg in one patient, whereafter all except one patient were introduced to maintenance therapy at 40 mg biweekly ().
Table 2. Therapy with anti-TNF-alpha antagonist adalimumab (ADA) of the patients with pediatric-onset inflammatory bowel disease.
At one year, 46/75 (61%) were on maintenance therapy and 31/46 patients (67%) were receiving adalimumab biweekly, 7 (15%) with a shorter interval of one week, and 7 (15%) with a longer interval of up to 3 weeks (for one patient, data were not available). In seven patients follow-up was less than one year and 22 patients had discontinued the therapy (see below).
During the first year, 22 patients (29%) discontinued adalimumab due to loss of response (20%, n = 15/75), anti-drug antibody formation (5.3%, n = 4), or adverse events (4.0%, n = 3). One patient developed drug antibodies already during the first 3 months of therapy. The dosing of adalimumab was enhanced during the follow-up in 38/75 patients (51%), and 31 patients underwent endoscopy during the therapy, mostly due to loss of response and switching to alternate therapy. During the entire follow-up, 48% of patients discontinued the therapy (). There were no routine follow-up endoscopies to assess mucosal healing during treatment.
Regarding trough levels during induction, 9/16 patients (56%) with trough levels <5 mg/L and 12/20 patients (60%) with trough levels <7.5 mg/L at 3 months discontinued the therapy during the first year. In contrast, only 8/32 patients (25%) with trough levels >7.5 mg/L discontinued treatment during the first year (p = .005). shows the probability of continuing maintenance therapy at one year stratified to the levels of adalimumab during the first 3 months of therapy. At the latest follow-up at a median of 1.5 years, 39 (52%) of the 75 patients were on maintenance therapy with adalimumab and had a median trough level of 8.8 mg/L (). Disease activity at induction and the latest follow-up of ongoing maintenance adalimumab therapy are shown in . The albumin values were within the normal range in the majority of patients, with a median value of 37 g/L at induction and 38 g/L at one year. More than two-thirds were steroid-free at the latest follow-up and combination therapy was in use in less than one-third (). At the one-year follow-up, 8 patients and at the latest follow-up 12 patients were on concomitant methotrexate therapy. Their median levels of adalimumab were 8.3 mg/L (n = 6) and 7.3 mg/L (n = 7), respectively, comparable to the rest. There were altogether 7 patients with concomitant azathioprine therapy with available adalimumab levels at last follow-up, with a median level of 6.6 mg/L.
Figure 1. Trough levels during the first 3 months of adalimumab therapy are related to the probability of ongoing therapy during the first year.
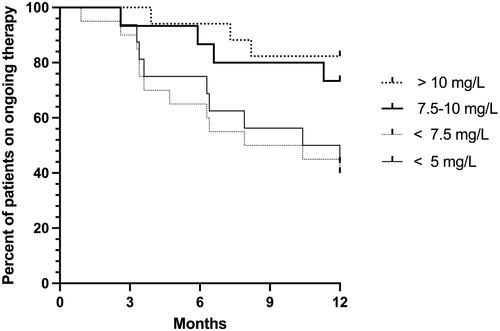
Table 3. Disease activity at induction and last follow-up of ongoing adalimumab therapy.
Regarding disease subtypes, no difference was present in the first measured adalimumab level in the UC/IBDU group compared with the group with Crohn’s disease (median 7.5 and 7.8 mg/L, respectively), although their fecal calprotectin levels were higher at induction (median 1071 mg/g; IQR 264–1533; n = 18 and median 597 mg/g; IQR 761–2455; n = 53; p < .0114, respectively). In the first three months, adalimumab levels were <7.5 mg/L in 7/13 patients with UC/IBDU (with available levels) and 5 of these patients discontinued the therapy within the first year. At one year, adalimumab levels were higher in the group with Crohn’s disease than in the UC/IBDU group (median 12 vs. 7.5 mg/L; p < .011), but there were only 8 patients in the latter group at this time point. At one year, there was no difference in calprotectin levels between patients with Crohn’s disease (median 272 mg/g, IQR 51–528, n = 16) and those with UC/IBDU (median 411 mg/g, IQR 226–962, n = 5) or in the corresponding proportions of patients on maintenance therapy at one year (39% and 44%, respectively). No significant differences emerged in blood or fecal inflammatory markers, in PGA, or proportions of patients on combination therapy at the end of follow-up when the Crohn’s and UC/IBDU groups were compared (data not shown).
Adverse events observed during adalimumab therapy included severe infections (septicemia, n = 1; abscesses, n = 2), tubulointerstitial nephritis (n = 2), psoriasis (n = 1), leukocytoclastic vasculitis (n = 1), recurrent ear infections. (n = 1), tinnitus (n = 1), and an increase in the level of liver enzymes (n = 1). On six occasions, these resulted in the discontinuation of therapy.
Discussion
Although therapeutic drug monitoring for adalimumab is widely available and might allow for improved treatment outcomes, optimal serum trough levels for children according to clinical and biological remission have not yet been clearly defined. The objective of this study was to evaluate the relationship between serum concentration and efficacy of adalimumab in pediatric patients with IBD in a real-life setting. We observed that adalimumab levels above 7.5 mg/L during the first 3 months of therapy were associated with sustained maintenance therapy at one year. Most patients were exposed to infliximab prior to the start of adalimumab and almost every other patient discontinued adalimumab during the follow-up (median 1.5 years). This suggests that the proportion of anti-TNF non-responsive patients was somewhat higher than if all patients had been anti-TNF-naïve at adalimumab induction [Citation22].
A recent retrospective study reported outcomes of 32 pediatric patients with UC and treated with adalimumab after exposure to infliximab [Citation23]. At one year, 59% of the patients had maintained their therapy and 41% were in steroid-free remission in the UC group. This is comparable to our study results, although of our patients only 13% had UC. In a study on pediatric patients with Crohn’s disease, the one-year maintenance rate and clinical remission rates were higher (73% and 78% of those on therapy, respectively) [Citation24]. The proportion of patients receiving combination therapy in the above-mentioned studies was not reported, but intriguingly, an adult study showed no difference in the levels of adalimumab between patients with and without combination therapy [Citation25]. This is similar to our finding, although the number of patients receiving combination therapy was low. Moreover, pediatric gastroenterologists favor withdrawal of immunosuppressants in responders to adalimumab (or infliximab), as observed in a recent multinational survey [Citation26].
Fecal calprotectin <100 μg/g is an appropriate surrogate marker for transmural and histological remission [Citation18,Citation27]. This target was reached in less than 10% of patients during the first 3 months of therapy but in 24% at one year. It is remarkable that at one year most (89%) were steroid-free. A recent pediatric study reported histological healing in 11% of patients with Crohn’s disease and 5.3% of those with UC when on adalimumab. Mucosal healing was within the same range (12%) in both disease subtypes, although the time of assessment varied from 7.6 to 18 months [Citation28]. In adults, patients with Crohn’s disease and low levels of adalimumab (<3 μg/mL) had significantly higher bowel wall thickness when measured with ultrasound, indicating poor response to therapy [Citation29].
Most (80%) of our patients were exposed to infliximab prior to the introduction of adalimumab. Infliximab has been available longer and has been the drug of choice in severe diseases in our clinics. However, this partly reflects the reimbursement policy in our country, as patients receive drugs free of charge when administered at a hospital but need to pay annually 600 euros for prescribed drugs, including adalimumab, whereafter the costs are covered by the state. Thus, only 20% of the patients were introduced to adalimumab as first-line therapy and 28% had a disease duration of less than one year, both facts potentially having an impact on the outcomes. At the time of the study, there were no biosimilars to adalimumab available.
In adults with IBD, higher trough levels (>7.3 mg/L), but not concomitant thiopurine therapy, were associated with clinical remission, and patients with drug antibodies against adalimumab experienced treatment failure [Citation25]. In our pediatric study, adalimumab levels above 7.5 mg/L during the first 3 months of therapy were associated with improved outcomes thereafter. It is widely recognized that loss of response is frequent during maintenance therapy with TNFα-blockers and is not always overcome with therapy escalation [Citation9]. Therapeutic drug monitoring can be used for monitoring and guiding the dosing of different types of medications. With anti-TNF medication, drug monitoring can be used either reactively, where trough level measurements are made when loss of response is suspected, or prospectively, where optimal trough levels are pursued to prevent loss of response. Here, the therapeutic decisions and therapy adjustments were made at the discretion of the treating physician. Interestingly, a recent study identified genomic biomarkers associated with non-response in adalimumab- and infliximab-treated patients with PIBD [Citation30]. Three genes, FCGR1A, FCGR1B, and GBP1, were found to be over-expressed in non-responders. FCRG1A and FCGR1B are expressed on the surface of neutrophils, and GBP1 is an interferon-stimulated, guanylate-binding protein involved in defense against pathogens and inflammation. This study is an example of many to come aiming at identifying non-responders at an early phase of therapy. Previously, it has also been reported that the microbiome is related to therapeutic response [Citation31–33]. Based on these studies, it is likely that in the future drug level monitoring will be combined with testing of the microbiome and/or genetic variants when the therapeutic response is suboptimal.
The strength of the study is that it combines comprehensive real-life data from two major tertiary care centers of our country. The data on patients with PIBD treated with adalimumab and their drug levels were traced from the hospital files and a patient registry covering all patients treated with biologics. Therefore, the number of eligible, missing patients is considered very low. As a limitation, the timing of the drug level measurements was decided by the treating physicians and not all patients had values during the first three months of therapy. Also, decisions on continuing or discontinuing the therapy were made at the discretion of the treating pediatric gastroenterologists. As in most pediatric studies, endoscopy data as routine follow-up were not available.
In conclusion, higher trough levels of adalimumab during the first three months of treatment are associated with improved outcomes and lower rates of discontinuation due to loss of response during the first year. Therefore, therapeutic drug monitoring is an essential part of the management of PIBD patients treated with adalimumab.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Turner D, Levine A, Escher JC, et al. Management of pediatric ulcerative colitis: joint ECCO and ESPGHAN evidence-based consensus guidelines. J Pediatr Gastroenterol Nutr. 2012;55(3):340–361.
- Ruemmele F, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8(10):1179–1207.
- Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care – an evidence-based guideline from European Crohn’s and Colitis Organization and uropean Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67(2):257–291.
- van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2020:jjaa161.
- de Ridder L, Waterman M, Turner D, ESPGHAN Paediatric IBD Porto Group, et al. Use of biosimilars in paediatric inflammatory bowel disease: a position statement of the ESPGHAN paediatric IBD Porto group. J Pediatr Gastroenterol Nutr. 2015;61(4):503–508.
- de Ridder L, Assa A, Bronsky J, et al. Use of biosimilars in pediatric inflammatory bowel disease: an updated position statement of the pediatric IBD porto group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2019;68(1):144–153.
- Nikkonen A, Kolho KL. Infliximab and its biosimilar produced similar first-year therapy outcomes in patients with inflammatory bowel disease. Acta Paediatr. 2020;109(4):836–841.
- Aardoom MA, Veereman G, de Ridder L. A review on the use of anti-TNF in children and adolescents with inflammatory bowel disease. Int J Mol Sci. 2019;20(10):2529.
- Winter DA, Joosse ME, de Wildt SN, et al. Pharmacokinetics, pharmacodynamics, and immunogenicity of infliximab in pediatric inflammatory bowel disease: a systematic review and revised dosing considerations. J Pediatr Gastroenterol Nutr. 2020;70(6):763–776.
- Frymoyer A, Piester TL, Park KT. Infliximab dosing strategies and predicted trough exposure in children with crohn disease. J Pediatr Gastroenterol Nutr. 2016;62(5):723–727.
- Merras-Salmio L, Kolho KL. Clinical use of infliximab trough levels and antibodies to infliximab in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;64(2):272–278.
- de Ridder L, Rings EH, Damen GM, et al. Infliximab dependency in pediatric Crohn’s disease: long-term follow-up of an unselected cohort. Inflamm Bowel Dis. 2008;14(3):353–358.
- Hyams JS, Lerer T, Griffiths A, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn’s disease. Inflamm Bowel Dis. 2009;15:816–822.
- De Bie CI, Hummel TZ, Kindermann A, et al. The duration of effect of infliximab maintenance treatment in paediatric Crohn’s disease is limited. Aliment Pharmacol Ther. 2011;33(2):243–250.
- Kolho KL, Sipponen T. The long-term outcome of anti-TNF-alpha therapy related to fecal calprotectin values during induction therapy in pediatric inflammatory bowel disease. Scand J Gastroenterol. 2014;49(4):434–441.
- Kolho KL. Therapeutic drug monitoring and outcome of infliximab therapy in pediatric onset inflammatory bowel disease. Front Pediatr. 2020;8:623689.
- Puolanne AM, Kolho KL, Alfthan H, et al. Rapid fecal calprotectin test and symptom index in monitoring the disease activity in colonic inflammatory bowel disease. Dig Dis Sci. 2017;62(11):3123–3130.
- Kolho KL, Raivio T, Lindahl H, et al. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;41(6):720–725.
- Hämäläinen A, Sipponen T, Kolho KL. Infliximab in pediatric inflammatory bowel disease decreases fecal calprotectin levels. WJG. 2011;17(47):5166–5171.
- Sipponen T, Kolho KL. Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. Scand J Gastroenterol. 2015;50(1):74–80.
- Haapamäki J, Roine RP, Sintonen H, et al. Health-related quality of life in paediatric patients with inflammatory bowel disease related to disease activity. J Paediatr Child Health. 2011;47(11):832–837.
- Macaluso FS, Fries W, Privitera AC, et al. A propensity score-matched comparison of infliximab and adalimumab in tumour necrosis factor-α inhibitor-naïve and non-naïve patients with Crohn’s disease: real-life data from the sicilian network for inflammatory bowel disease. J Crohns Colitis. 2019;13(2):209–217.
- Aloi M, Bramuzzo M, Arrigo S, et al. Efficacy and safety of adalimumab in pediatric ulcerative colitis: a real-life experience from the SIGENP-IBD registry. J Pediatr Gastroenterol Nutr. 2018;66(6):920–925.
- Alvisi P, Arrigo S, Cucchiara S, et al. Efficacy of adalimumab as second-line therapy in a pediatric cohort of Crohn’s disease patients who failed infliximab therapy: the Italian Society of Pediatric Gastroenterology, Hepatology, and Nutrition experience. Biologics. 2019;13:13–21.
- Holmstrøm RB, Mogensen DV, Brynskov J, et al. Interactions between thiopurine metabolites, adalimumab, and antibodies against adalimumab in previously infliximab-treated patients with inflammatory bowel disease. Dig Dis Sci. 2018;63(6):1583–1591.
- Meredith J, Henderson P, Wilson DC, et al. Withdrawal of combination immunotherapy in paediatric inflammatory bowel Disease – an international survey of practice. J Pediatr Gastroenterol Nutr. 2021. Epub ahead of print.
- Weinstein-Nakar I, Focht G, Church P, et al. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn’s disease. Clin Gastroenterol Hepatol. 2018;16(7):1089–1097.e4.
- Scarallo L, Alvisi P, Bolasco G, et al. Mucosal and histologic healing in children with inflammatory bowel disease treated with antitumor necrosis factor-alpha. J Pediatr Gastroenterol Nutr. 2021;72(5):728–735.
- Ungar B, Ben-Shatach Z, Selinger L, et al. Lower adalimumab trough levels are associated with higher bowel wall thickness in Crohn’s disease. United European Gastroenterol J. 2020;8(2):167–174.
- Salvador-Martín S, Kaczmarczyk B, Álvarez R, et al. Whole transcription profile of responders to anti-TNF drugs in pediatric inflammatory bowel disease. Pharmaceutics. 2021;13(1):77.
- Kolho KL, Korpela K, Jaakkola T, et al. Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol. 2015;110(6):921–930.
- Magnusson MK, Strid H, Sapnara M, et al. Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J Crohns Colitis. 2016;10(8):943–952.
- Estevinho MM, Rocha C, Correia L, et al. Features of fecal and colon microbiomes associate with responses to biologic therapies for inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2020;18(5):1054–1069.
- Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17(6):1314–1321.
