Abstract
Objectives
Peptic ulcers and erosions are the most common causes of upper gastrointestinal bleeding. The aim of this study was to investigate the management and outcomes of these patients.
Materials and methods
A total of 543 patients with endoscopically confirmed bleeding from peptic ulcers and erosions were included from March 2015 to December 2017. The patient characteristics, endoscopic findings, Forrest classification and endoscopic treatment were recorded. Moreover, the rebleeding rates, repeated endoscopies and transcatheter angiographic embolization and surgery incidences were registered. A follow-up endoscopy after discharge from the hospital was scheduled.
Results
Among the patients, high-risk stigmata ulcers were present in 36% (198/543) and low-risk stigmata ulcers and erosions in 60% (327/543) at first endoscopy. Endoscopic therapy was performed in 30% (165/543) of the patients, and hemostasis was achieved in 94% (155/165). The incidence of rebleeding was 9% (49/543) for the whole cohort and 14.8% (23/155) for those patients who had received successful endoscopic treatment. Moreover, rebleeding was significantly more frequent in duodenal ulcers than in gastric ulcers (11.9% vs 4.0%, p = .004). In a multivariable analysis, rebleeding was significantly related to comorbidity and Forrest classification. Transcatheter angiographic embolization and surgery were required in 6% (34/543) and 0.07% (4/543) of patients, respectively. Complete peptic ulcer healing was found at follow-up in 73.3% (270/368) of patients.
Conclusions
Endoscopic hemostasis was achieved in the majority of patients with high-risk ulceration, although the occurrence of rebleeding is a significant challenge, especially in patients with duodenal ulcers. Clinical trial registration: Bleeding Ulcer and Erosions Study (BLUE Study), ClinicalTrials.gov identifier: NCT03367897.
Introduction
The most common causes of acute upper gastrointestinal bleeding are peptic ulcers and erosions, which account for up to 60% of all cases [Citation1,Citation2]. The reported incidence rates vary from 20 to 60/100 000 adults per year [Citation3]. Despite the increased use of endoscopic therapy and the introduction of new modalities for endoscopic treatment in recent years, the mortality in this patient group has been rather stable and varies between 5% and 11% [Citation3–5].
Several guidelines and recommendations for the management and treatment of acute upper gastrointestinal bleeding have been published [Citation6–10], and it has been documented that adherence to guidelines is important and improves patient outcomes [Citation11]. Management practices have advanced, and contemporary endoscopic treatments, including injection, thermal and mechanical therapy, have been shown to be superior to conservative therapy in the management of high-risk patients with ulcers that have ongoing bleeding or major stigmata of recent hemorrhage at gastroscopy [Citation12,Citation13].
Previous studies have demonstrated that in patients with peptic ulcer bleeding, hemostasis is achievable in more than 90% of cases with endoscopic therapy [Citation14,Citation15]. However, recurrent bleeding still occurs in 10–25% of patients [Citation14,Citation15] and is associated with a two- to five-fold mortality increase depending on the presence of other risk factors [Citation16]. To further improve patient management and avoid rebleeding, the early use of proton pump inhibitors (PPIs) has been adopted as the standard approach [Citation17]. Although second-look endoscopy after endoscopic hemostasis has often been performed, it is not routinely recommended [Citation8–10].
The BLeeding Ulcer and Erosions (BLUE) study was conducted at two hospitals in southeastern Norway, and we previously reported that the incidence of bleeding from peptic ulcers and erosions is decreasing compared with that in previous Norwegian studies. In addition, we found that the mortality rate was unchanged due to older patients with more comorbidities [Citation4].
In this part of the study, we aimed to present more detailed information with regard to the endoscopic findings, endoscopic therapy, and other treatment options use, rebleeding rate, and patient cohort outcomes.
Materials and methods
Data collection
The methods used for the inclusion of patients and collection of data in this observational study, including demographic variables, use of risk medication (NSAIDs, ASA, non-ASA antiplatelet agents, warfarin, direct-acting anticoagulants, low-molecular-weight heparin) and PPI, Helicobacter pylori testing, comorbidity and mortality in the BLUE study, have previously been described [Citation4].
Endoscopy
Time from referral to endoscopy and the first endoscopic examination was registered and then divided into four time periods: 0–24 h, 24–48 h, 48–72 h and >72 h.
Forrest classification was used to evaluate the ulcers, which were classified as spurting hemorrhage (Forrest 1A), oozing hemorrhage (Forrest 1B), nonbleeding visible vessel (Forrest 2A), adherent clot (Forrest 2B), hematin on ulcer base (Forrest 2C) and clean ulcer base (Forrest 3) [Citation18]. Forrest 1A–2B ulcers were considered high-risk stigmata for persistent bleeding or rebleeding, and Forrest 2C and 3 ulcers were considered low-risk stigmata. For cases with more than one ulcer, the ulcer with the most severe Forrest classification was used in the classification and analysis.
Repeated endoscopies were performed during the hospital stay in several patients, and the time between the examinations together with the endoscopic findings were recorded.
At discharge from the hospital, a follow-up control endoscopy was scheduled to assess ulcer healing, which was defined as complete macroscopic normalization of the mucosa.
Endoscopic therapy
In our study, the following strategies using different endoscopic modalities were applied based on the endoscopist’s judgment [Citation1]: monotherapy, which included injection therapy with diluted epinephrine solution, thermal therapy (heater probe, gold probe or argon plasma coagulation), mechanical therapy (endoclips), or topical therapy (hemospray); and [Citation2] combination therapy, which included two or more of the abovementioned methods. When endoscopic therapy was performed, the success of achieving hemostasis was assessed by the endoscopist at the end of the procedure.
Other treatment options
PPIs were administered intravenously either as 40 mg twice daily or 80 mg three times daily or by an 80 mg bolus followed by continuous infusion of 8 mg/h for 72 h.
The need for radiological transcatheter angiographic embolization (TAE) and surgery was recorded.
Rebleeding and persistent bleeding
Rebleeding was defined as endoscopically verified bleeding from the same lesion or a decrease in hemoglobin of at least 1.24 mmol/l (2 g/dl) after initial endoscopic hemostasis.
Persistent bleeding was defined as endoscopically verified active bleeding and with no endoscopic intervention, and still ongoing bleeding after the endoscopy was completed.
Statistical analysis
Student’s t-test for continuous variables and Pearson’s chi-squared test for categorical variables were used in the univariate analyses. A multivariable logistic regression model was used to predict rebleeding according to sex, comorbidities, smoking status, current H. pylori infection, Forrest classification and ulcer location. All analyses were performed using the statistical software IBM SPSS Statistics for Windows, version 25.0 (IBM Corp, Armonk, NY) and Microsoft Office Excel 2016 (Microsoft, Redmond, Washington, USA).
Ethics
The study was approved by the Norwegian Regional Ethics Committee (REK 2014/2068).
Results
In total, 543 patients were included in this study. The demographic parameters and different variables, including the history of previous ulcers, smoking status, H. pylori infection, use of risk medication and PPI, time to endoscopy, location of the ulcer, length of the hospital stay, rebleeding and mortality for the whole patient group and subgroups according to findings at the first endoscopy, are summarized in . In general, there were only minor differences in demographic characteristics between the Forrest classified ulcers.
Table 1. Characteristics of the patient groups according to the findings of the first endoscopy.
Endoscopy
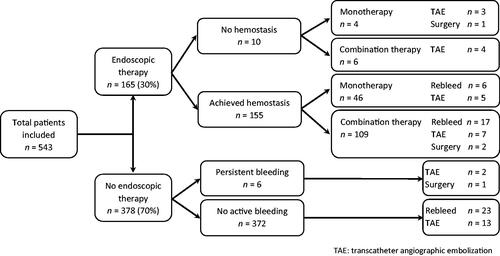
The first endoscopic examination was performed within 24 h in 414 (76%) patients and within 48 h in 471 (86.7%) patients. Peptic ulcers classified as Forrest 1A–2B were found in 198 (36.5%) patients, and those classified as Forrest 2C–3 or erosions were found in 327 (60.2%) patients (). Failure to identify a bleeding lesion due to limited visibility was the case in 18 (3.3%) patients, and the reasons were mainly excessive bleeding, with a large quantity of blood in the lumen or the presence of a large clot.
Endoscopic treatment
Endoscopic therapy was performed in 165 (30%) patients at the first endoscopy and included either monotherapy (50 patients) or combined therapy (115 patients) (). Detailed information regarding the application of different endoscopic modalities is given in . Endoscopic intervention based on the injection of diluted epinephrine solution combined with endoclips was most frequently applied. Hemostasis was achieved in 155 (94%) patients. Unachieved hemostasis was predominately observed in patients with ulcers classified as Forrest 1A and ulcers located in the duodenum. Seven of these patients underwent TAE (), one died as a direct cause of uncontrolled bleeding and the last two patients had a duodenal Forrest 1B ulcer without the need for any further intervention due to spontaneous hemostasis.
Table 2. Endoscopic therapy, hemostasis and rebleeding by ulcer stigmata according to the Forrest classification and location on first endoscopy.
In addition, endoscopic therapy was performed in 39 patients who underwent repeated endoscopies during the hospital stay, and hemostasis was achieved in 35 (90%) of these cases.
Rebleeding
Rebleeding occurred in 23 patients (14.8%) who had been successfully treated endoscopically and achieved hemostasis ( and ), and no significant difference was found between those patients who received monotherapy (13%) and combination endoscopic therapy (15.6%) (p = .896). Among those patients who did not receive any endoscopic treatment, the rate of rebleeding was 6.2% (23/372). In most patients who rebleed, rebleeding occurred within 1–3 days (65%) and was significantly more frequent in patients with duodenal ulcers (32/270, 11.9%) than in those with gastric ulcers (7/176, 4.0%) (p = .004) (). Moreover, rebleeding was more common in patients with high-risk stigmata ulcers (17.2%) than in those with low-risk stigmata ulcers (4.6%) (p < .001). No patients experienced rebleeding twice.
Table 3. Multivariable logistic regression model to predict rebleeding.
The multivariable analysis () revealed that comorbidity was a significant positive predictor for rebleeding with OR 3.193 (97.5% CI 1.22–10.21) while sex, age and H. pylori infection were not. An effect was observed between rebleeding and duodenal ulcers, although the largely associated uncertainty resulted in OR 2.638 (97.5% CI 0.73–8.92).
Persistent bleeding
No endoscopic therapy was applied in six patients who had ongoing bleeding at the first endoscopy () due to either failure to identify the lesion or the ulcer being located in a difficult position that made endoscopic therapy impossible. However, all patients received continuous high-dose PPI infusion. One of these patients underwent surgery, and one patient was referred directly for TAE. Another patient underwent TAE as well, but two attempts with endoscopic therapy failed to achieve hemostasis. The remaining three patients were hemodynamically stable, and no further intervention was needed. Two of them had a second endoscopy, and low-risk stigmata ulcers were found.
Other treatments
In total, 34 (6.3%) patients were referred for TAE because the initial endoscopic treatment either failed or was not applied based on the endoscopist’s judgment (). Severe complications related to the procedure were experienced in three patients. Perforation occurred in one patient, and pancreatitis developed in two patients, which resulted in pancreatic necrosis in one patient. In addition, one patient died within 10 days after TAE due to infection and myocardial infarction type 2. Rebleeding occurred in 2 patients after TAE and was managed conservatively with blood transfusion and intravenous PPI.
Surgery was performed in three patients due to perforated ulcers and, as previously stated, in one patient where TAE was complicated with a perforation.
PPI
Prehospital use of PPIs was registered in 46 (8.5%) patients. Overall, prior to the first endoscopy, 424 (78%) patients had received at least one dose of PPI, but after the first endoscopy, all 543 patients were treated with PPI.
Repeat endoscopy
During hospitalization, two or more endoscopies were performed in 154 (28%) patients (). Nine patients underwent a second endoscopy on the same day as the initial endoscopy. Moreover, 54, 33 and 58 patients underwent a second endoscopy after 24–48 h, 48–72 h and more than 72 h, respectively.
Table 4. Changes in endoscopic findings according to the Forrest classification comparing the first and second endoscopies.
A second endoscopy was performed due to rebleeding in 39 patients and due to failed endoscopic hemostasis or persistent bleeding at the first endoscopy in five and six patients, respectively. Eighteen patients underwent a second endoscopy to identify the source of bleeding ().
Patients who had a high-risk stigmata ulcer at the first endoscopic examination were at the greatest risk for a second endoscopy (). In 37 (24%) patients, the endoscopic finding was unchanged. Worsened and improved findings according to the Forrest classification were seen in 15 (11%) and 87 (63%) of the cases, respectively ().
Hospitalization
The mean duration of hospitalization was 6.7 days for all patients, although already in-hospital patients had a significantly longer mean hospital stay than acutely admitted patients at 16.1 days versus 4.9 days (p < .001), respectively. There were no differences in the mean length of hospital stay between the different Forrest groups and erosion. However, 55 patients, including 48 patients with low-risk stigmata ulcers and erosions, had a prolonged hospital stay after further examination via colonoscopy as inpatients.
Mortality
Mortality was registered in 41 patients in this study. Only three patients died as a direct consequence of a bleeding ulcer, and the remaining died due to complications of comorbidities, including cardiovascular events or acute infection. The mortality rates for patients treated with endoscopic intervention or not were 7.9% and 7.7% (p = .848), respectively.
Follow-up
Of the 543 patients, 368 (67.7%) underwent a follow-up upper endoscopy within a mean time of 73 days after discharge from the hospital. Overall, ulcer healing was observed in 270 (73.3%). The remaining patients were lost to follow-up, with 45 (8.3%) patients dying before the scheduled control, 33 (6.1%) not attending the control, 38 (7%) not scheduled for the control and 59 (10.9%) refusing a follow-up endoscopy.
Discussion
This is the first Norwegian real-world observation study to describe endoscopic therapy, other treatment options and outcomes in consecutively recruited patients with upper gastrointestinal bleeding due to peptic ulcers or erosions. Approximately one-third of the patients received endoscopic therapy with a high success rate of achieving hemostasis. However, rebleeding occurred in almost 15% of these patients. Some patients required TAE and very few needed surgery. The mortality rate was as expected and almost exclusively related to comorbidities.
Current international guidelines [Citation6–10] recommend early endoscopy (<24 h) for all patients with bleeding symptoms. However, Laursen et al. suggest that this timeframe may not be optimal for all patients, and usage of time to optimize resuscitation and manage comorbidities before endoscopy may improve outcome [Citation19]. Altogether, 76% of the patients in our study cohort underwent the first endoscopy within 24 h, which is acceptable when compared with other studies [Citation11,Citation20,Citation21] but nevertheless implies opportunities for improvement. In a French prospective multicenter study that included 3298 patients admitted for upper gastrointestinal bleeding, 79% had their first endoscopy within 24 h [Citation20,Citation21]. In contrast, a UK audit performed in 2007 [Citation22] revealed that only 50% of the patients presenting with acute upper gastrointestinal bleeding underwent endoscopy within 24 h, and in a Finnish prospective observational study, the percentage of patients examined with early endoscopy was even lower at only 45% [Citation23].
A large meta-analysis of 75 studies demonstrated that while the use of thermal therapy and mechanical clips are successful methods in achieving hemostasis, no single therapy has been proven to be superior to other therapies, regardless of the site of the lesion [Citation6,Citation12]. Furthermore, injection therapy as a single modality is no longer advocated [Citation6,Citation7,Citation9].
The majority (72%) of our patients with actively bleeding ulcers (Forrest 1A and 1B) were treated according to the European guidelines [Citation9] with a combination of epinephrine injection and another hemostasis modality. However, nonadherence to guidelines occurred in 17 patients with a peptic ulcer classified as Forrest 1B and 2A.
The treatment of peptic ulcers with adherent clots (Forrest 2B) is still controversial [Citation6–8], and we were not able to demonstrate any difference between endoscopic therapy and IV PPI infusion with regard to the occurrence of rebleeding. Selection bias is possible as the decision for intervention was up to the endoscopist. However, the mean hospital stay was 2 days shorter in patients who received endoscopic therapy.
Our success rate of achieving hemostasis with endoscopic therapy was high and did not differ from that of other studies [Citation15]. Nevertheless, we believe there is an opportunity for improvement with regard to the management of this patient group. The available treatment tools must be further improved, and ideal clinical scenarios for their implementation must be determined. In addition, the endoscopist’s skills are an important factor for success.
Rebleeding after initial successful endoscopic therapy is still a major challenge and one of the most significant predictive factors for mortality [Citation16,Citation24]. In our study, rebleeding occurred more often among patients who had peptic ulcers with high-risk stigmata than among those with low-risk stigmata ulcers. In addition, the rate of rebleeding was significantly higher when the ulcer was located in the duodenum. These findings are consistent with previous studies that reported a rebleeding rate in the range of 10–20% [Citation11,Citation16,Citation21,Citation25].
The ulcer location or massive acute bleeding may limit the possibility of performing endoscopic therapy to achieve hemostasis. In our study, such limitations were observed in six patients, and additional treatment was required in further management, including a second endoscopy, TAE, surgery and high-dose IV PPI, as recommended by the guidelines [Citation6,Citation8,Citation9].
If endoscopic treatment fails or is not technically feasible, TAE should be considered [Citation6,Citation8,Citation9]. Thirty-four of our patients underwent TAE, especially those with duodenal ulcers and ulcers classified as Forrest 1A. Only five of these patients had complications related to the procedure; therefore, we concluded that TAE is a safe treatment option to achieve hemostasis in difficult cases. In a meta-analysis by Tarasconi et al. [Citation26] and a recent study by Nykanen et al. [Citation27], the safety and efficacy of TAE and surgery in the treatment of bleeding peptic ulcers were compared and TAE was indicated as the preferred hemostatic method when endoscopy fails. The overall rate of surgery in our study was very low.
The European Society of Gastrointestinal Endoscopy (ESGE) recommends initiating a high-dose PPI and intravenous bolus followed by continuous infusion (80 mg then 8 mg/h) in patients awaiting upper endoscopy and presenting with acute upper gastrointestinal bleeding; however, PPI infusion should not delay the performance of early endoscopy [Citation9]. According to the Cochrane meta-analysis, the use of PPIs before endoscopy reduces the stigmata of bleeding and the need for endoscopic hemostasis but does not affect mortality, subsequent surgery, or rebleeding [Citation28]. At least one intravenous PPI dose was given to 67% of the patients in our study before endoscopy, although detailed data on post endoscopy administration of PPIs were not recorded.
Current guidelines recommend against routine second-look endoscopy in patients who have received adequate endoscopic therapy; however, repeated endoscopy for patients with evidence of recurrent bleeding is indicated [Citation6,Citation8–10]. Routine second-look endoscopy is defined as scheduled repeat endoscopic assessment of previously diagnosed bleeding lesions that are usually performed within 24 h following the first endoscopy [Citation9], and the main purpose is to lower the risk of rebleeding and the need for surgery [Citation29,Citation30]. In our study, repeat endoscopy was defined by any endoscopy performed during hospitalization (also scheduled endoscopy). In 69 out of 154 patients (45%), the reason for repeat endoscopy was either rebleeding, persistent bleeding, failed endoscopic therapy, or no lesion identified on the first endoscopy. The remaining 85 patients should not have undergone repeat endoscopy according to the guidelines [Citation6,Citation8–10]. Interestingly, 15 patients showed worsening with regard to Forrest classification at the second endoscopy.
The overall mortality rate was low, as previously reported [Citation4], and initially related to coexisting morbidity and not to the actual bleeding episode. No difference in the mortality rate was demonstrated between patients with high-risk and low-risk stigmata ulcerations (7.6% vs 7.9%) or between patients who received endoscopic treatment or not (7.9% vs 7.7%). These findings are consistent with that of other studies [Citation23,Citation25,Citation31].
An important strength is that our study is population-based. Moreover, 90% of the patients were included prospectively. Real-life clinical practice was observed at two hospitals with a catchment area of 800,000 inhabitants. A large number of endoscopically verified cases of peptic ulcer bleeding provide detailed information concerning treatment and outcome, which may be applicable to gastroenterological wards in other hospitals in Norway. There are some limitations to this study. First, we did not record data on the prescription of low-dose ASA or NSAIDs or the use of PPIs after discharge in terms of whether such therapy could have contributed to a delay in ulcer healing.
In addition, a more detailed description of the size and ulcer location could have provided valuable information regarding how the different methods used for endoscopic hemostasis may have influenced the rebleeding rate.
Finally, the study did not compare the comorbidities of the different Forrest classifications. Because comorbidities are risk factors for rebleeding, further studies are needed to analyze the rebleeding rates and risk factors corresponding to the Forrest classification.
In summary, we demonstrated in the current study that 36.5% of the patients presented with high-risk stigmata ulcers, with 80% of these patients receiving endoscopic therapy and successful achievement of hemostasis observed in 94%. The management of ulcers in real-world clinical practice is not always performed according to current clinical guidelines. Rebleeding occurred significantly more often in patients who had ulcers with high-risk stigmata and in those with duodenal ulcers than in those with gastric ulcers. In addition, rebleeding was associated with comorbidities. TAE is a safe treatment option to achieve hemostasis in difficult cases. The overall rate of surgery in our study was very low.
Acknowledgments
Thanks to all participants in the BLUE study group who assisted with the data collection and to Professor Truls Micheal Leegaard M.D., Astri Lervik Larsen M.D. and Heidi Johanne Espvik M.D. at the Department of Microbiology, Akershus University Hospital for analyzing the H. pylori tests.
Disclosure statement
The authors report no conflict of interest. The funders had no role in the study design, data collection and analysis, or interpretation of data.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Hearnshaw SA, Logan RF, Lowe D, et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60(10):1327–1335.
- van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22(2):209–224.
- Lau JY, Sung J, Hill C, et al. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84(2):102–113.
- Romstad KK, Detlie TE, Søberg T, et al. Gastrointestinal bleeding due to peptic ulcers and erosions - a prospective observational study (BLUE study). Scand J Gastroenterol. 2020;55(10):1139–1145.
- Lanas A, Aabakken L, Fonseca J, et al. Clinical predictors of poor outcomes among patients with nonvariceal upper gastrointestinal bleeding in Europe. Aliment Pharmacol Ther. 2011;33(11):1225–1233.
- Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107(3):345–360.
- Hwang JH, Fisher DA, Ben-Menachem T, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012;75(6):1132–1138.
- Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the international consensus group. Ann Intern Med. 2019;171(11):805–822.
- Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2015;47(10):1–46.
- Sung JJ, Chiu PW, Chan FKL, et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67(10):1757–1768.
- Barkun A, Sabbah S, Enns R, et al. The canadian registry on nonvariceal upper gastrointestinal bleeding and endoscopy (RUGBE): endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol. 2004;99(7):1238–1246.
- Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on Meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009;7(1):33–47.
- Barkun AN, Martel M, Toubouti Y, et al. Endoscopic hemostasis in peptic ulcer bleeding for patients with high-risk lesions: a series of meta-analyses. Gastrointest Endosc. 2009;69(4):786–799.
- Rosenstock SJ, Moller MH, Larsson H, et al. Improving quality of care in peptic ulcer bleeding: nationwide cohort study of 13,498 consecutive patients in the danish clinical register of emergency surgery. Am J Gastroenterol. 2013;108(9):1449–1457.
- Garcia-Iglesias P, Villoria A, Suarez D, et al. Meta-analysis: predictors of rebleeding after endoscopic treatment for bleeding peptic ulcer. Aliment Pharmacol Ther. 2011;34(8):888–900.
- Rockall TA, Logan RF, Devlin HB, et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316–321.
- Sung JJ, Barkun A, Kuipers EJ, et al. Intravenous esomeprazole for prevention of recurrent peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2009;150(7):455–464.
- Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;304(7877):394–397.
- Laursen SB, Leontiadis GI, Stanley AJ, et al. Relationship between timing of endoscopy and mortality in patients with peptic ulcer bleeding: a nationwide cohort study. Gastrointest Endosc. 2017;85(5):936–944.
- Nahon S, Hagege H, Latrive JP, et al. Epidemiological and prognostic factors involved in upper gastrointestinal bleeding: results of a French prospective multicenter study. Endoscopy. 2012;44(11):998–1008.
- Zeitoun JD, Rosa-Hezode I, Chryssostalis A, et al. Epidemiology and adherence to guidelines on the management of bleeding peptic ulcer: a prospective multicenter observational study in 1140 patients. Clin Res Hepatol Gastroenterol. 2012;36(3):227–234.
- Hearnshaw SA, Logan RF, Lowe D, et al. Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: results of a nationwide audit. Gut. 2010;59(8):1022–1029.
- Malmi H, Kautiainen H, Virta LJ, et al. Outcomes of patients hospitalized with peptic ulcer disease diagnosed in acute upper endoscopy. Eur J Gastroenterol Hepatol. 2017;29(11):1251–1257.
- Chiu PW, Ng EK, Cheung FK, et al. Predicting mortality in patients with bleeding peptic ulcers after therapeutic endoscopy. Clin Gastroenterol Hepatol. 2009;7(3):311–316.
- Bai Y, Du YQ, Wang D, et al. Peptic ulcer bleeding in China: a multicenter endoscopic survey of 1006 patients. J Dig Dis. 2014;15(1):5–11.
- Tarasconi A, Baiocchi GL, Pattonieri V, et al. Transcatheter arterial embolization versus surgery for refractory non-variceal upper gastrointestinal bleeding: a meta-analysis. World J Emerg Surg. 2019;14:3.
- Nykanen T, Peltola E, Kylanpaa L, et al. Bleeding gastric and duodenal ulcers: case-control study comparing angioembolization and surgery. Scand J Gastroenterol. 2017;52(5):523–530.
- Sreedharan A, Martin J, Leontiadis GI, et al. Proton pump inhibitor treatment initiated prior to endoscopic diagnosis in upper gastrointestinal bleeding. The Cochr Datab Syst Rev. 2010;(7):CD005415.
- Marmo R, Rotondano G, Bianco MA, et al. Outcome of endoscopic treatment for peptic ulcer bleeding: is a second look necessary? A meta-analysis. Gastrointest Endosc. 2003;57(1):62–67.
- El Ouali S, Barkun AN, Wyse J, et al. Is routine second-look endoscopy effective after endoscopic hemostasis in acute peptic ulcer bleeding? A meta-analysis. Gastrointest Endosc. 2012;76(2):283–292.
- Lanas A, Carrera-Lasfuentes P, Garcia-Rodriguez LA, et al. Outcomes of peptic ulcer bleeding following treatment with proton pump inhibitors in routine clinical practice: 935 patients with high- or low-risk stigmata. Scand J Gastroenterol. 2014;49(10):1181–1190.