Abstract
Background and objectives
The association between abdominal obesity and reflux esophagitis (RE) has been extensively evaluated, but the current findings are mixed and more convincing epidemiological evidence urgently needs to be established. To thoroughly explore this relationship, we summarized the latest studies, performed an updated meta-analysis, and examined the dose–response relationship.
Methods
We performed a systematic search of PubMed, Web of Science, and Embase up to 28 March 2021, using prespecified terms to identify studies investigating the association between abdominal obesity and RE. Odds ratios (ORs) with 95% confidence intervals (CIs), mean differences (MDs) or standardized mean differences (SMDs) with 95% CIs were taken as effect-size estimates.
Results
Forty-two observational studies, including 11 cohort studies, were meta-analyzed. Overall, a statistically significant association was observed between abdominal obesity and RE, by both the pooled OR (adjusted OR = 1.51, 95% CI: 1.37–1.66, p < .001) and the pooled SMD (SMD = 0.36, 95% CI: 0.30–0.42, p < .001). Moreover, this significant relationship persisted with subgroup stratification. In subgroup analyses, we found that study design, abdominal obesity measurement, adjustment for covariates and sex were possible sources of between-study heterogeneity. For the dose–response analyses, the risk of RE increased with the degree of abdominal obesity, and the increasing trend accelerated when waist circumference (WC) reached 87.0 cm.
Conclusion
This meta-analysis indicated a significant association between abdominal obesity and RE, and the risk of RE increased with abdominal obesity especially when the WC was over 87.0 cm.
Background
Gastroesophageal reflux disease (GERD) is a highly prevalent gastrointestinal disease affecting 8–33% of the global population depending on the geographical region [Citation1,Citation2]. It causes economic losses of up to billions of dollars worldwide each year and consumes many medical resources [Citation3]. Reflux esophagitis (RE) is an essential branch of GERD. Recent studies suggest that changes in the trans-diaphragmatic pressure gradient, motility disturbance of the esophagus, and the existence of esophageal hiatal hernia may destroy the balance of protective mechanisms and aggressive chemical substances, further resulting in endoscopic esophageal mucosal erosion and ulceration [Citation4–6]. RE symptoms are associated with reduced health-related quality of life [Citation1]. More importantly, chronic RE may be closely related to the occurrence and development of Barrett's esophagus and even esophageal adenocarcinoma [Citation7,Citation8].
Previous studies have demonstrated that obesity is a high-risk lifestyle factor associated with RE [Citation9], and recent research indicates that the regional distribution of adipose tissue, especially abdominal obesity, is considered a more important pathogenic factor than total body obesity [Citation10]. Accumulated abdominal fat increases the occurrence of reflux by increasing intra-abdominal pressure [Citation11]. Over the past two decades, research has gradually intensified, although the results have been mixed [Citation12–16]. Interestingly, in 2013, Siddharth and colleagues pooled the results of 17 independent studies that did not restrict the analysis based on study design and observed a significant association between abdominal obesity and RE (OR: 1.87, 95% CI: 1.51–2.31) [Citation12]. However, the number of included studies was insufficient. In recent years, literature exploring the relationship between abdominal obesity and RE has flourished, including several high-quality cohort studies, which can further expand the sample size. Additionally, the dose–response analyses in the original research need to be improved, as they cannot adequately reflect the association between abdominal obesity and RE.
To provide more convincing epidemiological evidence for further studies, the present meta-analysis expanded the scope of the included studies to comprehensively assess the relationship between abdominal obesity and RE and further conducted a dose–response analysis between them. Meanwhile, we aimed to eliminate the influence of covariates on pooled ORs and explored possible sources of heterogeneity.
Method
Search strategy
The PubMed, Web of Science, and Embase databases were systematically searched for articles up to 28 March 2021. The combination of medical subject terms used included: (adiposity* OR abdominal obesity OR abdominal fat OR anthropomet* OR BMI OR body composition OR body fat distribution OR body fat patterning OR body mass index OR body size OR central obesity OR girth circumference OR intra-abdominal fat OR retroperitoneal fat OR visceral fat OR visceral obesity OR waist circumference OR waist–hip ratio OR visceral adiposity OR visceral adipose tissue) [All Fields] AND (Erosive esophagiti OR Esophag*, Reflux OR Esophag*, Peptic OR Gastroesophageal Reflux OR gastrooesophageal reflux OR Gastric Acid Reflux OR GERD OR Peptic Esophag*) [Title/Abstract]. Additionally, a manual search of the reference lists of major articles was performed to avoid potential omissions.
The search process was performed independently by two investigators (J. Z. and M. Y.), and the articles were not restricted by language or publication. If necessary, we contacted authors to try to obtain more relevant information. Reporting of the study conforms to the PRISMA statement [Citation17], which is listed in Supplementary Table 1. This meta-analysis was based on published studies, and no prior ethics committee approval or informed consent was required.
Literature search
The present meta-analysis included studies that met criteria [Citation1,Citation2], and [Citation3] with either [Citation4] or [Citation5] of the following criteria: (1) observational studies, including cross-sectional, case–control, and cohort studies, assessing of the association between abdominal obesity and RE; (2) abdominal obesity defined using visceral fat measured by abdominal computed tomography (CT), the waist–hip ratio (WHR), or waist circumference (WC); (3) RE diagnosed using an upper gastrointestinal endoscope; (4) the use of odds ratio (OR) or hazard ratio (HR) with a 95% confidence interval (95% CI) to measure the magnitude of the association between abdominal obesity and RE; and (5) continuous data sufficient to calculate the MD or SMD with the 95% CI.
Studies were excluded if they were published in the form of case reports or case series, editorials, and narrative reviews.
Data abstraction and quality assessment
Data extraction was performed using predefined forms, and the details were as follows: (1) characteristic information: first author, country, baseline age, gender, study publication time, study design, population type, and sample size. (2) Exposure assessment: measurement of abdominal obesity (CT, WHR, or WC) and the reference standard for abdominal obesity. (3) Information for calculation: adjusted OR or HR with the 95% CI or the sample size, mean, and standard deviation of continuous data. In addition, we recorded adjustments for confounding factors in each study in detail, such as body mass index (BMI), age, sex, smoking and drinking, and the presence of an esophageal hiatus hernia, for the next subgroup analysis.
Data were independently abstracted from each qualified article by two investigators (J. Z. and M. Y.). Disagreements in data abstraction were resolved by consensus and, if necessary, by a third author (Y. Z.).
The quality of cohort studies and case–control studies was assessed using the Newcastle–Ottawa Scale (NOS) tool [Citation18]. The NOS mainly evaluates a study based on the following aspects: the selection of the study groups, the comparability of the study groups, and the assessment of exposure and outcome. The quality of cross-sectional studies was assessed using the criteria recommended by the Agency for Healthcare Research and Quality (AHRQ) [Citation19]. The criteria consisted of 11 items, and the answers to each item were ‘yes’, ‘no’, or ‘unknown’.
Exposure and outcome assessment
When multiple indicators for abdominal obesity measurement were reported in the same study, we prioritized CT-measured visceral fat (visceral fat area (VFA cm2), visceral adipose tissue (VAT cm3), or visceral fat area to subcutaneous fat area ratio (V/S)), followed by WHR and lastly WC. Since the international community does not have a unified standard for the definition of abdominal obesity, the definition provided by the study or the lowest reference category of categorical variables was regarded as the benchmark when extracting data. We included more than one estimate from the studies (e.g., if a study reported an OR for persons with a WC 90–99.9 cm and an OR for persons with a WC ≥ 100 cm, both ORs were included in the summary estimate as WC ≥ 90 cm). The outcome measurement was defined as RE diagnosed according to endoscopy.
Statistical analysis
Data management and analysis were conducted using STATA 14.1 (StataCorp, College Station, TX). Effect-size estimates were expressed as either the OR or HR (for classified variables) or the MD or SMD (for continuous variables), with 95% CI for the RE risk. We converted HRs into RRs [Citation20,Citation21] to provide approximate OR values, which were combined with the reported ORs. Summary OR estimates were calculated based on the assumption of random effects; even in the absence of significant heterogeneity, all differences between studies were considered.
The generalized least squares regression proposed by Greenland and Longnecker was used to examine the dose–response relationship between abdominal obesity and RE [Citation22]. The restricted cubic splines of exposure distributions with three knots (25th, 50th, and 75th percentiles) were used to test the nonlinear relationship.
The inconsistency index (I2) was used to quantify the heterogeneity between various studies. AnI2 > 50% indicates significant heterogeneity, and the degree of heterogeneity increases with an increasing percentage. To explore potential sources of heterogeneity among the included studies, a considerable number of prespecified subgroup analyses were conducted based on population characteristics, research characteristics and whether covariates were adjusted. Sensitivity analysis was used to evaluate the impact of any single article on the stability of the overall results. In addition, publication bias was evaluated by Begg's funnel plots and Egger's regression asymmetry test, while the number of theoretically missing studies was estimated by the trim-and-fill method.
Result
Study selection
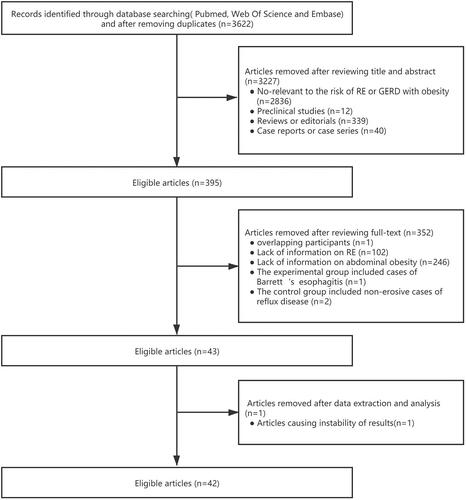
Among the initially identified 3622 unique publications searched by prespecified search strategies, 42 relevant studies (37 independent populations with more than 400,000 participants) met the inclusion criteria of the present meta-analysis. For studies with overlapping participants, different analysis methods were used to combine effect sizes to reduce the impact of autocorrelation. The detailed search strategy and inclusion and exclusion process are shown in .
Study characteristics and quality assessment
Among the 42 eligible studies, 22 were combined to calculate OR as effect-size estimates [Citation14,Citation16,Citation23–42], and 20 were combined to calculate SMD [Citation15,Citation43–61]. The baseline characteristics of the eligible studies included in each outcome are recorded in and . Most of the included studies were carried out in Asia (11 in China [Citation26,Citation28,Citation30,Citation31,Citation35,Citation38,Citation51,Citation54,Citation55,Citation57,Citation60], 6 in Japan [Citation16,Citation33,Citation34,Citation43,Citation53,Citation58], and 21 in Korea [Citation14,Citation24,Citation25,Citation27,Citation28,Citation32,Citation36,Citation37,Citation39–42,Citation44–46,Citation48–50,Citation52,Citation59,Citation61]), 2 studies were performed in Europe [Citation15,Citation56], 1 study was conducted in South America [Citation23], and 1 study was carried out in Africa [Citation47]. Twelve articles reported results upon stratification by sex [Citation14,Citation16,Citation24,Citation29,Citation33,Citation35,Citation36,Citation44,Citation49,Citation51,Citation53,Citation58], and 6 articles provided results for patients [Citation16,Citation23,Citation27,Citation33,Citation43,Citation60].
Table 1(A). The baseline characteristics of studies presenting adjusted effect size estimates with 95% CI.
Table 1(B). The baseline characteristics of studies presenting adjusted effect size estimates with 95% CI.
Table 2. The baseline characteristics of studies presenting continuous data.
In terms of research characteristics, 11 articles adopted cohort studies as the experimental design [Citation14,Citation23–25,Citation33,Citation37,Citation43,Citation45,Citation46,Citation48,Citation50], 14 adopted case–control studies [Citation15,Citation29,Citation31,Citation35,Citation38–41,Citation47,Citation53,Citation55,Citation56,Citation59,Citation61], and the remaining 17 adopted cross-sectional studies [Citation16,Citation26–28,Citation30,Citation32,Citation34,Citation36,Citation42,Citation44,Citation49,Citation51,Citation52,Citation54,Citation57,Citation58,Citation60]. Ten studies used CT-measured visceral fat to assess abdominal obesity [Citation28,Citation36,Citation41,Citation43,Citation46,Citation50,Citation53,Citation54,Citation58,Citation61], 9 studies used WHR [Citation15,Citation35,Citation36,Citation40,Citation47,Citation55,Citation56,Citation60,Citation61], and 36 studies used WC [Citation14,Citation16,Citation23–27,Citation29–39,Citation41,Citation42,Citation44–55,Citation57–60].
Supplementary Table 2 shows the quality assessment of the cohort studies using the NOS tool. The average total score was 7.36 (range: 6–8), with a standard deviation of 1.25. Supplementary Table 3 shows the assessment of case-control studies, which had an average score of 6.86 (range: 5–9), with a standard deviation of 1.36. In addition, the quality assessment of the cross-sectional studies using AHRQ criteria is shown in Supplementary Table 4.
Meta-analyses
According to the pooled OR (adjusted OR= 1.51, 95% CI: 1.37–1.66, p < .001), abdominal obesity is significantly associated with an increased risk of RE. Meanwhile, in the continuous data, the pooled SMD also revealed a similar conclusion (SMD= 0.36, 95% CI: 0.30–0.42, p < .001).
To explore possible sources of potential heterogeneity in the overall results and to test the robustness of the results in this meta‐analysis, some prespecified subgroup analyses were conducted. Subgroup analyses of the two conclusions were discussed separately and presented only if more than one study was included.
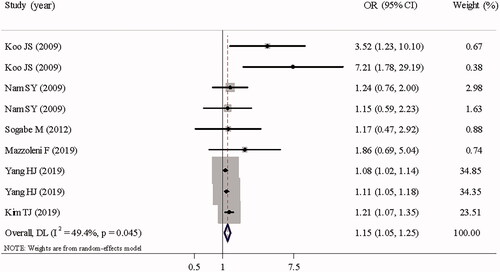
shows the overall and subgroup analyses of studies with adjusted ORs. Stratified by study design, significant associations between abdominal obesity and RE were noted across the three schemes, especially in case–control studies (adjusted OR = 1.92, 95% CI: 1.52–2.43, p < .001) and cross-sectional studies (adjusted OR =1.52, 95% CI: 1.29–1.80, p < .001). In addition, the association was still significant in cohort studies (adjusted OR = 1.15, 95% CI: 1.05–1.25, p = .002) (). Stratified by abdominal obesity measurement, statistically significant associations between abdominal obesity and RE were still identified when abdominal obesity was defined by WC (adjusted OR = 1.40, 95% CI: 1.28–1.54, p < .001), WHR (adjusted OR = 2.47, 95% CI: 1.85–3.30, p < .001), and CT-measured visceral fat (adjusted OR= 1.74 95% CI: 1.47–2.06, p < .001). Moreover, the results were stable across geographic region and sex subgroups. Notably, the existence of historical diseases, such as MS (adjusted OR = 1.23, 95% CI: 0.65–2.32, p = .530), may have an impact on this correlation. Interestingly, the effect of abdominal obesity on increased risk of RE persisted regardless of whether the covariates were adjusted. Sensitivity analysis was conducted by omitting each article sequentially, which revealed that no single study had a significant impact on the pooled OR.
Table 3. Overall and subgroup analyses of studies presenting adjusted effect size estimates with 95% CI.
shows overall and subgroup analyses of studies providing continuous data. When the comparison was stratified by abdominal obesity measurement, the MD was calculated as the index of effect scale. The measurements of WC (MD = 2.87, 95% CI: 2.46–3.28, p < .001), VFA (MD = 0.41, 95% CI: 0.20–0.62, p < .001), VAT (MD = 0.50, 95% CI: 0.35–0.64, p < .001) all suggested higher risks of RE in people with abdominal obesity, while WHR (MD = 0.03, 95% CI: 0.00–0.06, p < .028) failed to show a significant association. The results were stable with stratification by geographic region, sex and study design, which is consistent with the results in . Sensitivity analysis indicated that pooled SMD (SMD = 1.04, 95% CI: 0.81–1.28, p < .001) was affected when an independent article was included [Citation62]. The possible reasons are inaccurate measurement of WC and participant selection bias. Accordingly, we omitted the article in the present meta-analyses.
Table 4. Overall and subgroup analyses of studies presenting continuous data.
Strong evidence of between-study heterogeneity was revealed by the overall analyses. Through subgroup analyses, including analyses stratified by a cohort study design, the use of visceral fat measured by CT to define abdominal obesity, and adjustments for covariates, the heterogeneity of both the pooled OR and the pooled SMD were improved in some, but not all.
Dose–response analyses
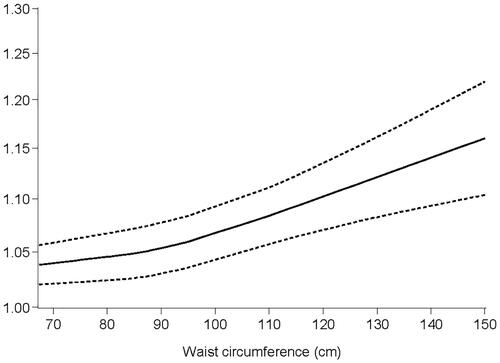
In dose–response analyses, the association was consistently significant with different methods of measurement. The risk of RE was positively related to abdominal obesity (). The dose–response relationship for the association of WC with RE is illustrated in , which shows a J-distribution. When the waist circumference reached 87 cm, the increasing trend of the RE risk was accelerated.
Table 5. Dose–response relationship of abdominal obesity with risk of RE.
Publication bias
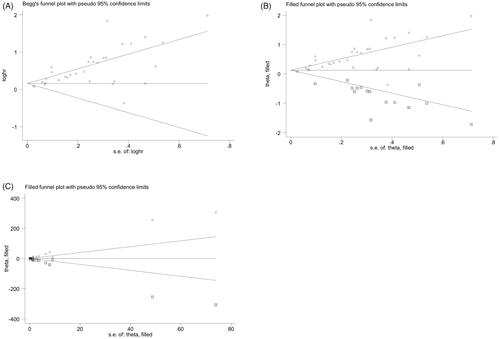
Begg's and filled funnel plots of the association between abdominal obesity and RE were asymmetric (), indicating that positive results may have been prioritized. Egger's tests further confirmed the publication bias (p < .01), which suggested that the meta-analysis may have been affected.
Discussion
To the best of our knowledge, this meta-analysis is the most comprehensive assessment exploring the relationship between abdominal obesity and RE. A total of 42 eligible studies with more than 400,000 participants were included in the present systematic review and meta-analysis. Our key finding suggests a 1.51-fold increased risk of RE among participants with abdominal obesity compared with individuals without abdominal obesity. Moreover, our subgroup analyses revealed that study design, abdominal obesity measurement, adjustment for covariates and sex were possible sources of between-study heterogeneity.
In previous literature, several prospective cohort studies have concluded that abdominal obesity was a significant risk factor for RE [Citation37,Citation43,Citation45,Citation50]. However, certain observational studies have yielded controversial findings. A cohort study including 8571 subjects in South Korea found that abdominal obesity was not independently associated with the risk of RE when adjusted for BMI (adjusted OR = 1.15, 95% CI: 0.59–2.23). However, after adjusting for WC, RE was the main consequence of increased BMI (adjusted OR = 4.15, 95% CI: 1.66–10.33) [Citation14]. Similarly, a cross-sectional study conducted in Japan showed no significant correlation between abdominal obesity and RE (adjusted OR = 0.687, 95% CI: 0.324–1.455) [Citation16]. After pooling the results of 17 independent studies, a meta-analysis by Siddharth and colleagues provided novel high-level evidence supporting abdominal obesity as a significant risk factor for the development of RE [Citation12]. Nevertheless, the number of included studies was insufficient, and the dose–response analyses also required improvement.
To fill the evidence-gap, several improvements were applied. First, we added to the number of studies to include 42 studies with 11 cohort studies to lower selective biases. Second, ORs were all adjusted by multiple factors, which may have better reduced the influence of covariates on the results. Third, subgroup analyses were designed to be more detailed. Furthermore, dose–response analyses were conducted according to WC, WHR and CT-measured visceral fat separately.
With extensive research on body fat distribution, abdominal obesity is considered to be as important as general obesity in the pathogenesis of GERD [Citation10]. Increased intra-abdominal pressure compromises the trans-diaphragm pressure balance, which is identified as the primary mechanism by which abdominal obesity increases the RE risk [Citation4,Citation63,Citation64]. First, increased intra-abdominal pressure may exceed the retention pressure of the esophagogastric barrier, disrupt the valvular mechanism and lead to reflux [Citation4,Citation5]. Then, whenever the lower esophageal sphincter (LES) is opened, this pressure increases the speed of gastric contents returning to the esophagus [Citation65,Citation66]. An increased frequency of transient LES relaxation and delayed recovery of the LES also contribute to more acid exposure [Citation4,Citation67]. Accordingly, esophageal clearance was delayed, and esophageal injury occurred. Additionally, disorders of gastric emptying and gastrointestinal motility driven by excessive leptin levels [Citation68], esophageal motility defects induced by fat accumulation at the gastroesophageal junction, and a high incidence of esophageal hiatal hernia are possible explanations for the higher prevalence of RE in central obesity [Citation8,Citation67].
In dose–response analyses, we observed increased abdominal obesity followed by a higher RE risk, which is consistent with the intra-abdominal pressure mechanism. The J-shaped curve shows the increasing trend of the RE risk. When gender and ethnic differences were considered, the significant peak in the trend close to 87 cm was consistent with the definition of abdominal obesity. However, the specific risk index needs to be verified by more literature in the future.
A healthy lifestyle is recommended as an alternative therapeutic method throughout the disease course of RE. Additionally, body fat distribution management is important [Citation69–71]. The present meta-analysis summarized the results of previous studies and highlighted the importance of improving abdominal obesity. Considering clinical practice, this article may provide more credible epidemiological evidence for lifestyle intervention in the treatment of RE.
Several limitations in the current context warrant further discussion. First, the subgroup analysis showed that the included studies were mostly concentrated in Asian populations, which may be related to the higher incidence of abdominal obesity in Asian populations [Citation72,Citation73], indicating that the universality and difference of this correlation cannot be reasonably explained in multiple ethnic backgrounds, and more studies in different regions are needed to confirm our conclusions. Second, although the adjusted effect size estimates were incorporated in our study, some important confounding factors were not considered, such as diet, physical activity, and proton pump inhibitor usage. Third, although a considerable number of subgroup analyses were used to explore the source of heterogeneity, significance persisted in several subgroups, which limited the interpretation of pooled effect-size estimates. Fourth, obvious publication bias existed in the included studies, but since the relevant biological mechanisms were clearly explained and a positive dose–response relationship was provided in the present meta-analysis, the clinical significance of such publication bias may be low.
Conclusions
Our findings suggest that abdominal obesity and an unhealthy body fat distribution may independently increase the risk of RE. In addition, the degree of risk increases with the severity of abdominal obesity. However, to establish the causal relationship, large-scale and well-designed cohort studies are needed for future research.
| Abbreviations | ||
| AHRQ | = | Agency for Healthcare Research and Quality |
| BMI | = | Body Mass Index |
| CI | = | confidence interval |
| CT | = | computed tomography |
| GERD | = | gastroesophageal reflux disease |
| HR | = | hazard ratio |
| LES | = | lower esophageal sphincter |
| MD | = | mean difference |
| NOS | = | Newcastle–Ottawa Scale |
| OR | = | odds ratio |
| RE | = | reflux esophagitis |
| SMD | = | standardized mean difference |
| VFA | = | visceral fat area |
| VAT | = | visceral adipose tissue |
| V/S | = | visceral fat area to subcutaneous fat area ratio |
| WC | = | waist circumference |
| WHR | = | waist–hip ratio |
Supplemental Material
Download MS Word (17.8 KB)Supplemental Material
Download MS Word (18 KB)Supplemental Material
Download MS Word (14.5 KB)Supplemental Material
Download MS Word (28.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the data generated or analyzed in this study are included in this article and its supplementary information files.
Additional information
Funding
References
- Richter JE, Rubenstein JH. Presentation and epidemiology of gastroesophageal reflux disease. Gastroenterology. 2018;154(2):267–276.
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63(6):871–880.
- Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254–272.e11.
- Herbella FAM, Schlottmann F, Patti MG. Pathophysiology of gastroesophageal reflux disease: how an antireflux procedure works (or does not work). Updates Surg. 2018;70(3):343–347.
- Menezes MA, Herbella FAM. Pathophysiology of gastroesophageal reflux disease. World J Surg. 2017;41(7):1666–1671.
- Usai Satta P, Oppia F, Cabras F. Overview of pathophysiological features of GERD. Minerva Gastroenterol Dietol. 2017;63(3):184–197.
- Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371(9):836–845.
- Schlottmann F, Dreifuss NH, Patti MG. Obesity and esophageal cancer: GERD, Barrett’s esophagus, and molecular carcinogenic pathways. Expert Rev Gastroenterol Hepatol. 2020;14(6):425–433.
- Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67(3):430–440.
- Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404.
- Nadaleto BF, Herbella FA, Patti MG. Gastroesophageal reflux disease in the obese: pathophysiology and treatment. Surgery. 2016;159(2):475–486.
- Singh S, Sharma AN, Murad MH, et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clinical gastroenterology and hepatology: the official clinical practice journal of the. Clin Gastroenterol Hepatol. 2013;11(11):1399–1412.e7.
- Corley DA, Kubo A, Zhao W. Abdominal obesity, ethnicity and gastro-oesophageal reflux symptoms. Gut. 2007;56(6):756–762.
- Nam SY, Choi IJ, Nam BH, et al. Obesity and weight gain as risk factors for erosive oesophagitis in men. Aliment Pharmacol Ther. 2009;29(9):1042–1052.
- Mulholland HG, Cantwell MM, Anderson LA, et al. Glycemic index, carbohydrate and fiber intakes and risk of reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Cancer Causes Control. 2009;20(3):279–288.
- Sogabe M, Okahisa T, Yamanoi A, et al. Subtypes of metabolic syndrome and of other risk factors in Japanese women with erosive esophagitis. Medicine. 2014;93(28):e276.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin Res Ed). 2009;339(Jul21 1):b2535–b2535.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford; 2000.
- Rostom A, Dubé C, Cranney A, et al. Appendix D. Quality assessment forms. In: Celiac disease. Agency for healthcare research and quality (US). Rockville, MD: Agency for Healthcare Research and Quality; 2004.
- Shor E, Roelfs D, Vang ZM. The “Hispanic mortality paradox” revisited: meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants' mortality. Soc Sci Med. 2017;186:20–33.
- VanderWeele TJ. On a square-root transformation of the odds ratio for a common outcome. Epidemiology. 2017;28(6):e58–e60.
- Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309.
- Mazzoleni F, Mazzoleni LE, de Magalhães Francesconi CF, et al. Potential roles of Helicobacter pylori treatment, body mass index and waist circumference in the causation of erosive esophagitis: a randomized clinical trial (HEROES-GERD). Int J Obes (Lond). 2020;44(1):147–158.
- Yang HJ, Chang Y, Park SK, et al. Sex differences in the relation between waist circumference within the normal range and development of reflux esophagitis. JCM. 2019;8(1):67.
- Kim TJ, Lee H, Baek SY, et al. Metabolically healthy obesity and the risk of erosive esophagitis: a cohort study. Clin Transl Gastroenterol. 2019;10(9):e00077.
- Hsieh YH, Wu MF, Yang PY, et al. What is the impact of metabolic syndrome and its components on reflux esophagitis? A cross-sectional study. BMC Gastroenterol. 2019;19(1):33.
- Baeg MK, Ko SH, Ko SY, et al. Obesity increases the risk of erosive esophagitis but metabolic unhealthiness alone does not: a large-scale cross-sectional study. BMC Gastroenterol. 2018;18(1):82.
- Ze EY, Kim BJ, Kang H, et al. Abdominal visceral to subcutaneous adipose tissue ratio is associated with increased risk of erosive esophagitis. Dig Dis Sci. 2017;62(5):1265–1271.
- Lee SW, Lien HC, Lee TY, et al. Impact of obesity on a Chinese population with erosive esophagitis and Barrett’s esophagus. Gut Liver. 2017;11(3):377–382.
- Hung WC, Wu JS, Yang YC, et al. Nonalcoholic fatty liver disease vs. obesity on the risk of erosive oesophagitis. Eur J Clin Invest. 2014;44(12):1143–1149.
- Loke SS, Yang KD, Chen KD, et al. Erosive esophagitis associated with metabolic syndrome, impaired liver function, and dyslipidemia. WJG. 2013;19(35):5883–5888.
- Jung JG, Kang HW, Hahn SJ, et al. Vegetarianism as a protective factor for reflux esophagitis: a retrospective, cross-sectional study between Buddhist priests and general population. Dig Dis Sci. 2013;58(8):2244–2252.
- Sogabe M, Okahisa T, Kimura Y, et al. Visceral fat predominance is associated with erosive esophagitis in Japanese men with metabolic syndrome. Eur J Gastroenterol Hepatol. 2012;24(8):910–916.
- Chiba H, Gunji T, Sato H, et al. A cross-sectional study on the risk factors for erosive esophagitis in young adults. Intern Med. 2012;51(11):1293–1299.
- Wu P, Ma L, Dai GX, et al. The association of metabolic syndrome with reflux esophagitis: a case-control study. Neurogastroenterology and motility: the official journal of the. Eur Gastrointest Motil Soc. 2011;23(11):989–994.
- Nam SY, Choi IJ, Ryu KH, et al. Abdominal visceral adipose tissue volume is associated with increased risk of erosive esophagitis in men and women. Gastroenterology. 2010;139(6):1902–1911.e2.
- Koo JS, Lee SW, Park SM, et al. Abdominal obesity as a risk factor for the development of erosive esophagitis in subjects with a normal esophago-gastric junction. Gut Liver. 2009;3(4):276–284.
- Chua CS, Lin YM, Yu FC, et al. Metabolic risk factors associated with erosive esophagitis. J Gastroenterol Hepatol. 2009;24(8):1375–1379.
- Park JH, Park DI, Kim HJ, et al. Metabolic syndrome is associated with erosive esophagitis. WJG. 2008;14(35):5442–5447.
- Lee HL, Eun CS, Lee OY, et al. Association between GERD-related erosive esophagitis and obesity. J Clin Gastroenterol. 2008;42(6):672–675.
- Chung SJ, Kim D, Park MJ, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case–control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57(10):1360–1365.
- Kang MS, Park DI, Oh SY, et al. Abdominal obesity is an independent risk factor for erosive esophagitis in a Korean population. J Gastroenterol Hepatol. 2007;22(10):1656–1661.
- Takahashi K, Seki Y, Kasama K, et al. Prevalence of reflux esophagitis in obese Japanese undergoing bariatric surgery. JGH Open. 2020;4(3):519–524.
- Tae CH, Jung HK, Kim SE, et al. Potential involvement of adiponectin in obesity-associated erosive esophagitis. J Clin Biochem Nutr. 2020;67(2):206–213.
- Chung TH, Lee J, Jeong ID, et al. Effect of weight changes on the development of erosive esophagitis. Korean J Fam Med. 2020;41(1):14–19.
- Nam SY, Kim YW, Park BJ, et al. Effect of abdominal visceral fat change on the regression of erosive esophagitis: a prospective cohort study. Gut Liver. 2019;13(1):25–31.
- Rafat MN, Younus HA, El-Shorpagy MS, et al. Adiponectin level changes among Egyptians with gastroesophageal reflux disease. JGH Open: Open Access J Gastroenterol Hepatol. 2018;2(1):21–27.
- Bang KB, Park JH. Weight loss as a nonpharmacologic strategy for erosive esophagitis: a 5-year follow-up study. Gut Liver. 2018;12(6):633–640.
- Park JK, Lim Y, Lee H, et al. Comparison of anthropometric measurements associated with the risk of endoscopic erosive esophagitis: a cross-sectional study. Obes Res Clin Pract. 2017;11(6):694–702.
- Nam SY, Kim YW, Park BJ, et al. Effect of abdominal visceral fat on the development of new erosive oesophagitis: a prospective cohort study. Eur J Gastroenterol Hepatol. 2017;29(4):388–395.
- Hung WC, Wu JS, Sun ZJ, et al. Gender differences in the association of non-alcoholic fatty liver disease and metabolic syndrome with erosive oesophagitis: a cross-sectional study in a Taiwanese population. BMJ Open. 2016;6(11):e013106.
- Chung H, Chon YE, Kim SU, et al. Noninvasive prediction of erosive esophagitis using a controlled attenuation parameter (CAP)-based risk estimation model. Dig Dis Sci. 2016;61(2):507–516.
- Matsuzaki J, Suzuki H, Kobayakawa M, et al. Association of visceral fat area, smoking, and alcohol consumption with reflux esophagitis and Barrett’s esophagus in Japan. PLoS One. 2015;10(7):e0133865.
- Wu YW, Tseng PH, Lee YC, et al. Association of esophageal inflammation, obesity and gastroesophageal reflux disease: from FDG PET/CT perspective. PLoS One. 2014;9(3):e92001.
- Wu P, Zhao XH, Ai ZS, et al. Dietary intake and risk for reflux esophagitis: a case–control study. Gastroenterol Res Pract. 2013;2013:691026.
- Mokrowiecka A, Daniel P, Jasinska A, et al. Serum adiponectin, resistin, leptin concentration and central adiposity parameters in Barrett’s esophagus patients with and without intestinal metaplasia in comparison to healthy controls and patients with GERD. Hepato-gastroenterology. 2012;59(120):2395–2399.
- Hsu CS, Wang PC, Chen JH, et al. Increasing insulin resistance is associated with increased severity and prevalence of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34(8):994–1004.
- Gunji T, Sato H, Iijima K, et al. Risk factors for erosive esophagitis: a cross-sectional study of a large number of Japanese males. J Gastroenterol. 2011;46(4):448–455.
- Chung SJ, Lim SH, Choi J, et al. Helicobacter pylori serology inversely correlated with the risk and severity of reflux esophagitis in Helicobacter pylori endemic area: a matched case–control study of 5,616 health check-up Koreans. J Neurogastroenterol Motil. 2011;17(3):267–273.
- Tai CM, Lee YC, Tu HP, et al. The relationship between visceral adiposity and the risk of erosive esophagitis in severely obese Chinese patients. Obesity (Silver Spring). 2010;18(11):2165–2169.
- Lee HL, Eun CS, Lee OY, et al. Association between erosive esophagitis and visceral fat accumulation quantified by abdominal CT scan. J Clin Gastroenterol. 2009;43(3):240–243.
- Kato M, Watabe K, Hamasaki T, et al. Association of low serum adiponectin levels with erosive esophagitis in men: an analysis of 2405 subjects undergoing physical check-ups. J Gastroenterol. 2011;46(12):1361–1367.
- Camilleri M, Malhi H, Acosta A. Gastrointestinal complications of obesity. Gastroenterology. 2017;152(7):1656–1670.
- Chang P, Friedenberg F. Obesity and GERD. Gastroenterol Clin North Am. 2014;43(1):161–173.
- McColl KEL. What is causing the rising incidence of esophageal adenocarcinoma in the West and will it also happen in the East? J Gastroenterol. 2019;54(8):669–673.
- Mitchell DR, Derakhshan MH, Wirz AA, et al. Abdominal compression by waist belt aggravates gastroesophageal reflux, primarily by impairing esophageal clearance. Gastroenterology. 2017;152(8):1881–1888.
- Lee YY, McColl KE. Disruption of the gastroesophageal junction by central obesity and waist belt: role of raised intra-abdominal pressure. Dis Esophagus. 2015;28(4):318–325.
- Yarandi SS, Hebbar G, Sauer CG, et al. Diverse roles of leptin in the gastrointestinal tract: modulation of motility, absorption, growth, and inflammation. Nutrition. 2011;27(3):269–275.
- Gyawali CP, Fass R. Management of gastroesophageal reflux disease. Gastroenterology. 2018;154(2):302–318.
- Fass R. Alternative therapeutic approaches to chronic proton pump inhibitor treatment. Clin Gastroenterol Hepatol. 2012;10(4):338–345. Quiz e39–40.
- Alamuddin N, Bakizada Z, Wadden TA. Management of obesity. J Clin Oncol. 2016;34(35):4295–4305.
- Lear SA, Kohli S, Bondy GP, et al. Ethnic variation in fat and lean body mass and the association with insulin resistance. J Clin Endocrinol Metab. 2009;94(12):4696–4702.
- Lear SA, Humphries KH, Kohli S, et al. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr. 2007;86(2):353–359.