Abstract
Purpose
To examine whether positive associations between alcohol and liver enzymes were modified by coffee consumption, smoking, or weight status in a female population.
Methods
Regular consumption of beer, wine, and spirits was assessed in a representative cohort of 1462 Swedish women aged 38–60 in 1968, and re-assessed in 1974. In 1980, gamma-glutamyltransferase (GGT) and aspartase transaminase (AST) were measured in 1130 women. Exposures were averaged over values obtained in 1968 and 1974. Multivariable linear regression linked total ethanol intake to log-transformed enzyme values, including interactions by coffee, smoking, and overweight in mutually adjusted models.
Results
Coffee consumption significantly modified the association between ethanol intake and liver enzymes. One g/day higher ethanol intake was associated with 5.5 (3.5, 7.5)% higher values of GGT, and 1.2 (0.4, 2.1)% higher values of AST in women consuming 0–1 cups of coffee per day, while smaller or no effects were observed in women consuming ≥2 cups/day. Synergistic interactions were observed for ethanol and smoking, and for ethanol and overweight. Average alcohol-related effects on GGT in smokers and non-smokers were given by 3.8 (2.7, 4.9)% and 2.1 (0.9, 3.2)% per g ethanol/day, and by 0.9 (0.4, 1.4)% and 0.2 (−0.3, 0.7)% for AST. Similarly, in overweight women, 1 g/day higher ethanol intake was associated with 4.3 (3.0, 5.6)% higher GGT compared to 1.6 (0.7, 2.5)% in non-overweight women.
Conclusions
The results suggest that coffee consumption reduces the enzyme-raising effect of ethanol in the presence of synergistic interactions with smoking and overweight, specifically in women.
Introduction
Alcohol consumption in any amount is known to be associated with increased risk of disease in the digestive system [Citation1,Citation2]. Previous studies showed that regular coffee consumption may reduce the alcohol-related risk of liver cirrhosis [Citation3–6]. Effect modification by coffee consumption has also been observed for several liver enzymes, all showing weaker interaction effects in women than in men [Citation7–10]. Thus, the previously reported reduction in alcohol-related risk of liver cirrhosis might be driven by higher alcohol consumption in men. In contrast to alcohol and coffee consumption, synergistic interactions have been reported for alcohol consumption and smoking [Citation10–12], again with larger effect sizes or only investigated in men. Because the use of coffee and cigarettes are correlated behaviors [Citation13], non-accounting for one exposure may lead to inconsistent results for the other exposure in different populations. Weight status is another health indicator associated with smoking, alcohol, and coffee consumption [Citation14]. Recent results suggest that overweight and obesity enhance alcohol-related effects on indicators of liver health [Citation15–20], but interactions between alcohol and smoking or coffee consumption were not considered simultaneously.
The present study aims to fill in two important gaps: first, we investigate the modulating effects of coffee, smoking, and weight status on the association between ethanol intake and indicators of liver function in one model, accounting for potentially opposite effects of correlated behaviors. Second, to add evidence for effect modification of alcohol-related liver damage in women we based the analyses on data from the Prospective Population Study of Women in Gothenburg, Sweden, a representative sample of women aged 38–60 in 1968, and followed over up to 50 years. Lifestyle behaviors and weight status were assessed in 1968 and 1974, and average exposures were defined. Gamma-glutamyltransferase (GGT) as well as aspartate transaminase (AST) are markers for liver function and were both measured 12 years after baseline. We aimed to characterize the correlations between exposure variables, and the modulating effect of coffee, smoking, and weight status on alcohol-related rise of liver enzymes in a female population.
Participants and methods
Study population
In 1968, a representative sample of 1622 women aged 38, 46, 50, 54, or 60 years and living in Gothenburg, Sweden, was invited to the Prospective Population Study of Women in Gothenburg [Citation21]. A total of 1462 women (90%) accepted the invitation and attended physical examinations with questionnaires about education, lifestyle, and medical history. Follow-up examinations were carried out 1974, 1980, 1992 and 2000, with participation rates among those still alive of 89% in 1974 and 71% in 2000 [Citation22]. Participants provided informed oral consent in 1968, 1974, and 1980, and written consent in 1992 and later. Since 1980, all examinations have been approved by the Regional Ethics Review Board in Gothenburg in accordance with the Declaration of Helsinki.
Exposures (1968–1974)
The frequency of consumption of beer, wine, and liquor was assessed in terms of seven categories ranging from never to daily consumption. For each type of alcoholic beverage, ethanol intake in g/day was calculated using standard portion size and ethanol content [Citation23]. Total ethanol intake in g/day was the main exposure in this study, and beverage-specific intakes were studied in sensitivity analyses. In addition, total ethanol intake was dichotomized at 1 unit (12 g) per day, which is the recommended upper limit for low-risk alcohol consumption in Sweden [Citation24]. The question about habitual coffee consumption included seven categories given by 0, 1, 2–3, 4–6, 7–10, 11–20, and >20 cups/day, irrespective of type of coffee or preparation method. Smoking status was assessed as current versus former or never smoking, and in terms of cigarettes/day. Anthropometric measures included body weight, height, and waist and hip circumferences using identical procedures in 1968 and 1974 [Citation25]. Waist-to-hip ratio (WHR) as well as body mass index (BMI) were calculated. Overweight was defined as BMI ≥25 kg/m2, and central adiposity as WHR ≥0.8.
Liver enzymes (1980)
GGT and AST were measured in fasting blood samples of 1145 women participating at the examination in 1980 using standard spectrophotometric methods [Citation26].
Potential confounders (1968–1974)
Higher education was defined as more than compulsory education (5–6 years, depending on birth cohort). Self-rated leisure time physical activity (LTPA) distinguished low (sedentary lifestyle), moderate, and high LTPA. At baseline, participants reported on past and present diseases and symptoms. Prevalent heart disease or nervous heart complaints as well as stomach ulcer or bleeding might affect coffee consumption and were included as potential confounders for associations with liver enzymes. Self-reported health problems also included jaundice, for which only few prevalent cases were reported in this population. Because jaundice is not specific to liver disease it was included as potential confounder. Finally, we adjusted for use of sleeping pills, which are associated with induction of GGT [Citation27] as well as with consumption and metabolism of coffee.
Study design and definition of analytic sample
Because the question about regular coffee consumption was not included after 1974, we restricted ourselves to the investigation of associations with exposures measured in 1968 and 1974. As behaviors may have changed over time the average of exposures at the first two examinations was considered the most reliable measure [Citation28]. Mean values were calculated for continuous exposures, and classification of ethanol intake (>12 g/day versus less), and overweight was based on respective mean values. Average coffee consumption was combined into four categories of 0-1, 2-3, 4-5, and ≥ 6 cups/day. Additionally, we defined a binary variable for high (≥2 cups/day) versus low coffee consumption (0–1 cup/day) that was used to further visualize the interaction between alcohol and coffee consumption with respect to liver enzymes. Current smoking, use of sleeping pills, heart problems, stomach problems, and jaundice were defined as prevalent condition in 1968 or 1974. LTPA describes the maximal activity in 1968 or 1974. Average values of exposures and potential confounders were then related to liver enzyme values measured about 9 years later at the second follow-up examination (1980).
All women who participated at baseline (1968) as well as at first and second follow-up (1974 and 1980) were eligible for the analyses of liver enzymes (n = 1138). Of these, 1130 women had values for relevant covariates and at least one liver enzyme. The final number of observations differed slightly by outcome: 1125 for GGT and 1116 for AST.
Statistical methods
Sample characteristics were presented by category of ethanol intake (>12 g/day versus less) using frequencies (%) and mean values with standard deviations (SD) for categorical and continuous variables, respectively. Because liver enzyme distributions were positively skewed, their values were log-transformed for linear regression. Results are given as relative change in liver enzyme value for a change in the value of the predictor by 1 unit, i.e., (exp(b) − 1) × 100%, based on the beta-coefficient b and its 95% confidence interval (CI). Interaction analyses were performed by including product terms for ethanol intake by categories of coffee consumption, smoking, and overweight into the regression model. Interaction results are presented as strata-specific associations. For interaction between ethanol intake and binary exposures (smoking, overweight, high versus low coffee consumption) p-values for individual interaction terms were given. For interaction results involving the 4-category variable for coffee consumption, we reported the p-value of an overall F-test comparing regression models with and without any interaction term. All regression models were adjusted for age, education, LTPA, heart problems, stomach problems, jaundice, and use of sleeping pills. Because of previously shown associations between WHR and liver cirrhosis [Citation29], interaction analyses were repeated replacing overweight by WHR dichotomized at 0.8, and shown in the Supplement. Analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC) and MATLAB (R2016b; The MathWorks, Inc., Natick, MA). Statistical significance was set at p-value < 0.05 (2-sided tests).
Results
Sample characteristics and correlations between exposure variables assessed 1968–1974
Women consumed on average 5.0 g ethanol/day, mostly from wine (43%) and beer (40%), and less from spirits (17%). shows the sample characteristics by category of ethanol intake (>12 g/day versus less). Higher than recommended alcohol intake was associated with lower values of BMI and TG. Higher than basic education, high LTPA, smoking, and use of sleeping pills were more common among women with high ethanol intake. Current smoking was associated with higher coffee consumption, and with less overweight (not shown). There was a weak positive association between coffee and overweight, which was strengthened when adjusted for age, education, smoking status, LTPA, and ethanol intake (odds ratio for overweight = 1.60 (1.01, 2.55), 1.56 (0.97, 2.51), 2.25 (1.35, 3.77) for 2–3 cups/day, 4–5 cups/day, ≥6 cups/day, compared to 0–1 cups/day).
Table 1. Cohort characteristics by regular ethanol intake above 12 g/day versus less.a
Associations with liver enzymes measured in 1980
Ethanol intake in g/day was positively associated with GGT and AST with effect sizes of 1.9 (1.2, 2.6)% and 0.5 (0.2, 0.8)%, respectively (adjusted for coffee consumption, smoking, overweight, age, education, LTPA, heart problems, stomach problems, jaundice, and use of sleeping pills). Coffee consumption showed a U-shaped association with respect to GGT, with effect sizes of −14.4 (−24.9, 2.3)%, −21.6 (−31.4, −10.4)%, −9.0 (−21.6, 5.6)% for 2–3 cups/day, 4–5 cups/day, ≥6 cups/day, compared to 0–1 cups/day. A similar but non-significant pattern was observed for AST (not shown). Overweight and smoking were positively associated with GGT as were age and use of sleeping pills. Current smoking showed a negative association with AST, while higher age was the strongest predictor of AST. None of the comorbidities were associated with liver enzyme values (not shown).
shows the results for effect modification of ethanol intake by coffee consumption, smoking, and overweight with respect to both liver enzymes. Because of the observed correlations between coffee consumption, smoking, and overweight interactions between ethanol intake and potential moderators were mutually adjusted for each other. There was an effect modification by coffee consumption such that the largest positive associations between ethanol intake and liver enzymes were observed in women with low coffee consumption, while no associations were observed for those with 4–5 cups of coffee per day, but differences were not statistically significant for AST. Consistent synergistic effects of ethanol intake and smoking were observed for both liver enzymes. A synergistic interaction between ethanol intake and overweight was demonstrated for GGT, but not AST.
Table 2. Effect modification of associations between ethanol intake and liver enzymes by coffee consumption, current smoking, and overweight in mutually adjusted interaction models (percent change in liver enzyme values per 1 g/day higher ethanol intake).
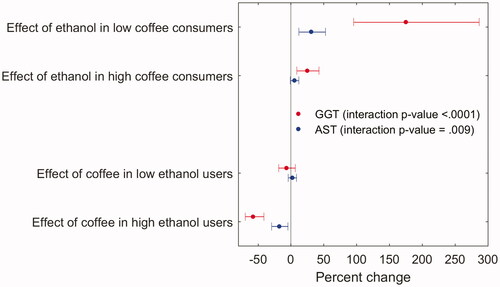
Effect modification by coffee consumption was also seen for ethanol intake dichotomized at 12 g/day, with high ethanol intake showing a larger association with liver enzymes in women with low coffee consumption compared to women drinking ≥2 cups/day (). Conversely, we saw a protective association with high versus low coffee consumption in women with high ethanol intake only.
Figure 1. Stratified presentation of interactions between ethanol intake (>12 g/day versus less) and coffee consumption (≥2 cups/day versus less) with respect to GGT or AST measured 1980. The upper two estimates show associations with ethanol intake in strata defined by coffee consumption, and the lower two estimates show associations with coffee consumption in strata defined by ethanol intake. Linear regression of log liver enzymes on ethanol intake (>12 g/day versus less) as well as coffee consumption (≥2 cups/day versus less), current smoking, and overweight, and their interactions with ethanol intake, adjusted for age, education, LTPA, heart problems, ulcer, jaundice, and use of sleeping pills (1 model per liver enzyme). High ethanol use (>12 g/day), low ethanol use (≤12 g/day), high coffee consumption (≥2 cups/day), low coffee consumption (<2 cups/day).
Sensitivity analyses
Separate analyses with one interaction term per regression model showed similar results as the combined model (), with slightly larger interaction effects for smoking and overweight in the combined interaction model for GGT (Tables S1a–S1c). A similar pattern of interaction results was observed when overweight was replaced by central adiposity (WHR ≥ 0.8 versus less, Table S2). Further adjusting for number of cigarettes per day did not change the interaction results presented in (not shown). Beverage-specific analyses showed qualitatively similar results as total ethanol intake, with larger effect sizes for ethanol from wine and liquor compared to beer (not shown). About 87% of women participating 1968–1974 were also participants in 1980, and this percentage did not differ by alcohol consumption (>12 g/day versus less, ) indicating that interaction results reported in were not affected by loss to follow-up between 1974 and 1980.
Discussion
Data from a female population were used to investigate to which extent the associations between ethanol intake and levels of GGT and AST were modified by regular coffee consumption, smoking and overweight status. In models including all 2-way interactions with ethanol intake, we show that the alcohol-related rise of liver enzyme values was stronger in women reporting low coffee consumption compared to women consuming two or more cups of coffee per day. Synergistic interactions between ethanol intake and smoking were demonstrated for both GGT and AST, while the synergy between alcohol and overweight was restricted to GGT. Higher than recommended compared to lower ethanol intake more than doubled average GGT values in women with low coffee consumption, while mean GGT was only 25% higher for high versus low ethanol intake among women who regularly drank two cups of coffee per day or more. Conversely, we saw a protective effect of higher coffee consumption (≥2 cups/day versus less) on GGT only among women, who consumed more than the recommended upper limit of 12 g ethanol/day.
Consistent interactions regarding both liver enzymes were observed for alcohol and smoking, and these are reported here for the first time for an entirely female population. Previous reports of effect modifications by smoking were either restricted to men [Citation10,Citation11,Citation30], or to combined samples, which might have been driven by associations in the male subsamples [Citation3,Citation31]. One reason for the synergy between alcohol and smoking observed here is likely to be the high prevalence of current smoking (43%) in this cohort from 1968 to 1974, a prevalence that is considerably lower in modern cohorts (e.g., 15% in 2000–02 [Citation10] or 8% in 2003–2010 [Citation31]). The fact that current smokers reported higher ethanol intake than women not currently smoking may contribute to the larger effect size of alcohol on liver enzymes observed in smokers.
Regarding effect modification by coffee we observed that alcohol-related effects on liver enzymes were largest in women consuming 0–1 cups/day, and smallest in women with 4–5 cups/day. The larger effect size in low coffee consumption was not explained by higher ethanol intake, because differences in ethanol intake by category of coffee consumption were small. Larger alcohol-related effect sizes were also observed for ≥6 cups of coffee per day compared to 2–5 cups/day, which was not explained by higher ethanol intake or more cigarettes/day. Mechanistically, the interaction results for coffee and ethanol suggest that the main protective effect of coffee on liver indicators consists of neutralizing the adverse effect of alcohol, for instance through antioxidation [Citation9], with lesser effect on other predictors of liver markers. A protective effect of coffee might therefore not be observable in populations with low alcohol consumption. Conversely coffee consumption may have beneficial effects in individuals with high alcohol consumption, which has been concluded before [Citation3–8], and is here shown explicitly ().
Synergistic effects between alcohol and excess body weight on GGT have been reported for mixed male and female populations [Citation16,Citation32], and are here demonstrated for an exclusively female population. No interaction between alcohol and overweight was observed for AST, which is largely consistent with the literature [Citation32], and may be partially due to the lack of an independent association between body weight and AST. Interestingly, the prevalence of overweight changed from 27% to 41% for categories of increasing coffee consumption. This observation is consistent with a recent meta-analysis that demonstrated correlations between coffee consumption and overweight status in women but not in men. The authors suggest that different drinking habits could explain the gender difference, for instance, that women preferred coffee with added sugar and cream while men tended to drink black coffee [Citation33]. It is possible that opposite main effects of coffee and overweight on GGT reduce alcohol-related associations if the former are correlated, explaining weaker effect modifications by coffee observed in women compared to men.
Taken together we show that lifestyle modification of alcohol-related harm on liver health is important for women, who are less often included in studies of liver health than men, probably due to their lower alcohol consumption. Though the old age of the data may be considered a disadvantage it allowed us to investigate effect modifications by smoking when the prevalence of smoking was still high. Recent trends of increasing overweight and alcohol consumption make our results on synergetic effects between alcohol and overweight even more relevant for modern female populations. Corresponding analyses are planned using data from new participants of the Prospective Population Study of Women in Gothenburg recruited in 2004–2005 and 2016–2017.
The simultaneous investigation of alcohol-related effects and their modification by correlated health and lifestyle variables is a strength of the study, as is the use of two important markers of liver function measured nine years after the exposure. Continuous liver function markers were used when the analysis of liver cirrhosis was impossible because of too few cases [Citation29]. Alcohol-related effects were stronger for GGT than for AST, consistent with the use of GGT as biomarker for regular alcohol consumption [Citation34]. Insufficient sample size to investigate disease endpoints is a limitation of our study. Lack of baseline values for liver enzymes is another limitation that did not allow us to exclude subjects with high values at baseline, or to investigate change in liver enzyme values due to alcohol exposure. However, the time-separation between exposure and outcome also adds validity to our results as it reduces the risk that the measured exposure is a consequence rather than a predictor of poor liver health. The reliance on self-reported alcohol consumption is a limitation, but the correlation with the ethanol marker GGT supports its validity. Because of lipid-raising effects of coffee [Citation35,Citation36] the association between self-reported coffee consumption and total cholesterol and triglycerides, mainly for ≥6 cups/day versus less, was affirmative but the evidence was weak. With these reservations, the study strengthens previous evidence that smoking and overweight reinforce alcohol-related liver damage, while coffee consumption attenuates adverse effects of alcohol consumption, specifically in women.
Statement of ethics
Participants provided informed oral consent in 1968, 1974, and 1980, and written consent in 1992 and later. Since 1980, all examinations have been approved by the Regional Ethics Review Board in Gothenburg in accordance with the Declaration of Helsinki.
| Abbreviations | ||
| AST | = | aspartate transaminase |
| BMI | = | body mass index |
| CI | = | confidence interval |
| GGT | = | Gamma-glutamyltransferase |
| HR | = | hazard ratio |
| LTPA | = | leisure time physical activity |
| TC | = | total cholesterol |
| TG | = | triglycerides |
Supplemental Material
Download MS Word (24.3 KB)Disclosure statement
DT has acted as a consultant for the Norwegian Coffee Information (Norsk Kaffeinformasjon, European Coffee Brewing centre). The other authors have no conflict of interest to declare.
Additional information
Funding
References
- Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol. 2000;95(12):3374–3382.
- Taylor B, Rehm J. Moderate alcohol consumption and diseases of the gastrointestinal system: a review of pathophysiological processes. Dig Dis. 2005;23(3–4):177–180.
- Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol. 1992;136(10):1248–1257.
- Corrao G, Lepore AR, Torchio P, et al. The effect of drinking coffee and smoking cigarettes on the risk of cirrhosis associated with alcohol consumption. A case–control study. Provincial group for the study of chronic liver disease. Eur J Epidemiol. 1994;10(6):657–664.
- Tverdal A, Skurtveit S, Selmer R, et al. Coffee and wine consumption is associated with reduced mortality from alcoholic liver disease: follow-up of 219,279 Norwegian men and women aged 30–67 years. Ann Epidemiol. 2018;28(11):753–758.
- Chung H-K Nj, Lee M-Y, Kim Y-B, et al. The increased amount of coffee consumption lowers the incidence of fatty liver disease in Korean men. Nutr Metab Cardiovasc Dis. 2020;30(10):1653–1661.
- Tanaka K, Tokunaga S, Kono S, et al. Coffee consumption and decreased serum gamma-glutamyltransferase and aminotransferase activities among male alcohol drinkers. Int J Epidemiol. 1998;27(3):438–443.
- Ikeda M, Maki T, Yin G, et al. Relation of coffee consumption and serum liver enzymes in Japanese men and women with reference to effect modification of alcohol use and body mass index. Scand J Clin Lab Invest. 2010;70(3):171–179.
- Danielsson J, Kangastupa P, Laatikainen T, et al. Dose- and gender-dependent interactions between coffee consumption and serum GGT activity in alcohol consumers. Alcohol Alcohol. 2013;48(3):303–307.
- Breitling LP, Raum E, Muller H, et al. Synergism between smoking and alcohol consumption with respect to serum gamma-glutamyltransferase. Hepatology. 2009;49(3):802–808.
- Wannamethee SG, Shaper AG. Cigarette smoking and serum liver enzymes: the role of alcohol and inflammation. Ann Clin Biochem. 2010;47(4):321–326.
- Whitehead TP, Robinson D, Allaway SL. The effects of cigarette smoking and alcohol consumption on serum liver enzyme activities: a dose-related study in men. Ann Clin Biochem. 1996;33(6):530–535.
- Treloar HR, Piasecki TM, McCarthy DE, et al. Relations among caffeine consumption, smoking, smoking urge, and subjective smoking reinforcement in daily life. J Caffeine Res. 2014;4(3):93–99.
- Jang ES, Jeong SH, Hwang SH, et al. Effects of coffee, smoking, and alcohol on liver function tests: a comprehensive cross-sectional study. BMC Gastroenterol. 2012;12:145.
- Puukka K, Hietala J, Koivisto H, et al. Additive effects of moderate drinking and obesity on serum gamma-glutamyl transferase activity. Am J Clin Nutr. 2006;83(6):1351–1354. quiz 448–9.
- Carter AR, Borges MC, Benn M, et al. Combined association of body mass index and alcohol consumption with biomarkers for liver injury and incidence of liver disease: a Mendelian randomization study. JAMA Netw Open. 2019;2(3):e190305.
- Aberg F, Farkkila M. Drinking and obesity: alcoholic liver disease/nonalcoholic fatty liver disease interactions. Semin Liver Dis. 2020;40(2):154–162.
- Shen Z, Li Y, Yu C, et al. A cohort study of the effect of alcohol consumption and obesity on serum liver enzyme levels. Eur J Gastroenterol Hepatol. 2010;22(7):820–825.
- Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3(12):1260–1268.
- Glyn-Owen K, Bohning D, Parkes J, et al. The combined effect of alcohol and body mass index on risk of chronic liver disease: a systematic review and meta-analysis of cohort studies. Liver Int. 2021;41(6):1216–1226.
- Bengtsson C, Blohme G, Hallberg L, et al. The study of women in Gothenburg 1968–1969–a population study. General design, purpose and sampling results. Acta Med Scand. 2009;193(1–6):311–318.
- Bjorkelund C, Bondyr-Carlsson D, Lapidus L, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care. 2005;28(11):2739–2744.
- Lapidus L, Bengtsson C, Bergfors E, et al. Alcohol intake among women and its relationship to diabetes incidence and all-cause mortality: the 32-year follow-up of a population study of women in Gothenburg, Sweden. Diabetes Care. 2005;28(9):2230–2235.
- Nordic Nutrition Recommendations 2012. Nordic council of ministers 2014. Available from: https://doi.org/http://dx.doi.org/10.6027/Nord2014-002
- Bjorkelund C, Andersson-Hange D, Andersson K, et al. Secular trends in cardiovascular risk factors with a 36-year perspective: observations from 38- and 50-year-olds in the population study of women in Gothenburg. Scand J Prim Health Care. 2008;26(3):140–146.
- Shaw LM, London JW, Fetterolf D, et al. Gamma-glutamyltransferase: kinetic properties and assay conditions when gamma-glutamyl-4-nitroanilide and its 3-carboxy derivative are used as donor substrates. Clin Chem. 1977;23(1):79–85.
- Braide SA, Davies TJ. Factors that affect the induction of gamma glutamyltransferase in epileptic patients receiving anti-convulsant drugs. Ann Clin Biochem. 1987;24(4):391–399.
- Chen YH, Ferguson KK, Meeker JD, et al. Statistical methods for modeling repeated measures of maternal environmental exposure biomarkers during pregnancy in association with preterm birth. Environ Health. 2015;14:9.
- Schult A, Mehlig K, Bjorkelund C, et al. Waist-to-hip ratio but not body mass index predicts liver cirrhosis in women. Scand J Gastroenterol. 2018;53(2):212–217.
- Breitling LP, Arndt V, Drath C, et al. Liver enzymes: interaction analysis of smoking with alcohol consumption or BMI, comparing AST and ALT to gamma-GT. PLoS One. 2011;6(11):e27951.
- Park EY, Lim MK, Oh JK, et al. Independent and supra-additive effects of alcohol consumption, cigarette smoking, and metabolic syndrome on the elevation of serum liver enzyme levels. PLoS One. 2013;8(5):e63439.
- Alatalo PI, Koivisto HM, Hietala JP, et al. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am J Clin Nutr. 2008;88(4):1097–1103.
- Lee A, Lim W, Kim S, et al. Coffee intake and obesity: a meta-analysis. Nutrients. 2019;11(6):1274.
- Agarwal S, Fulgoni VL 3rd, Lieberman HR. Assessing alcohol intake & its dose-dependent effects on liver enzymes by 24-h recall and questionnaire using NHANES 2001–2010 data. Nutr J. 2016;15(1):62.
- Strandhagen E, Thelle DS. Filtered coffee raises serum cholesterol: results from a controlled study. Eur J Clin Nutr. 2003;57(9):1164–1168.
- Cai L, Ma D, Zhang Y, et al. The effect of coffee consumption on serum lipids: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2012;66(8):872–877.
