Abstract
Background
Endoscopic full-thickness resection (EFTR) has been shown to be a feasible and safe technique in several studies since the introduction of the full-thickness resection device (FTRD®). This study aimed to describe our clinical experience and long-term follow up in in patients who underwent EFTR of benign and malignant colon lesions using FTRD.
Methods
All patients with difficult adenomas or early adenocarcinomas referred for an EFTR to two centres in Denmark were included in this prospective consecutive study. The primary outcome was technical success with R0 resection and relapse-free follow up. The secondary outcome was procedure-related adverse events.
Results
Twenty-six patients were enrolled in the study. Technical success was achieved in 81% patients and R0 resection rate was 86%. Full-thickness resection was achieved in 86% patients. In 13 patients with malignant lesions, we obtained follow-up in 10 cases (two patients underwent surgery and one was non-compliant). Findings of the three-month follow up showed no residual tumour in all 10 cases. At the 12-month follow up, one patient had a late relapse. There were no residual or recurrent adenomas in the benign subgroup. Overall, adverse events were observed in 11.5% (3/26) patients with a perforation rate of 7.7%.
Conclusion
EFTR with FTRD proves to be an additional technique for the treatment of difficult non-lifting colorectal lesions. For malignant lesions, EFTR is technically safe and feasible and can potentially treat small early low-risk tumours; however, some cases may require subsequent surgery according to the histological staging observed in the resected specimen.
Keywords:
Introduction
The standard treatment for benign colonic neoplasia is polypectomy, endoscopic mucosal resection (EMR), or endoscopic submucosal dissection (ESD) depending on the morphology and size of the lesion and available expertise. These are minimally-invasive techniques that are effective for the treatment of adenomas [Citation1,Citation2]. There is a risk of incomplete resection with these techniques and the recurrence rate is reported to be 15–30% in cases undergoing piecemeal EMR [Citation3,Citation4]. Although the recurrence rate is lower with ESD, it is accompanied by challenges such as higher complication rates and longer procedural time [Citation5,Citation6]. Furthermore, it has been estimated that 19% of the interval cancers stem from an incomplete adenoma resection [Citation7].
These standard techniques are also limited to resection of lesions located above the submucosal layer. Often recurrent lesions and early cancers are anchored below the submucosal layer and have features that may indicate deep invasion such as the “non-lifting” of the mucosa after submucosal injection of fluid. Although often small, these “non-lifting” lesions may be difficult to remove and have a higher rate of procedure-related complications [Citation8]
To overcome the challenges of removal of difficult adenomas, the one-step endoscopic full-thickness resection (EFTR) has been developed as an addition to the armamentarium of the endoscopist for the treatment of colonic lesions. In late 2014, a full-thickness resection device (FTRD®) was introduced to clinical practice and different studies with a short follow-up period have shown the feasibility and safety of the procedure [Citation9,Citation10].
The aim of the study was to observe and describe the clinical aspects of EFTR using FTRD with a long-term follow up in patients with difficult colonic adenomas and adenocarcinomas that would have been otherwise treated by surgical resection. The primary outcome was technical success with R0 resection and relapse-free follow up. The secondary outcome was procedure-related adverse events.
Materials and methods
Study design and patient selection
All patients referred to undergo EFTR at the two centres (Zealand University Hospital and Odense University Hospital) between December 2016 and December 2017 were included consecutively in this prospective study. We included patients with non-lifting colonic lesions, who were not eligible for standard endoscopic resection with polypectomy, EMR, or ESD. The eligible lesions were estimated to have a diameter below 25 mm and exhibit a non-lifting sign or characteristics of submucosal invasion. In cases with previous attempts of treatment, the non-lifting sign was confirmed by submucosal injection. The lesions were assessed with high-definition white-light and digital chromoendoscopy (narrow band imaging, NBI). Important characteristics of submucosal invasion were morphologically depressed surface (Paris III, IIc and IIa + IIc), fold convergence, and also irregular surface pattern and vessels in NBI. The included lesions were untreated, residual, or recurrent adenomas or early adenocarcinomas. All eligible cases were discussed at a multidisciplinary team (MDT) conference in order to ensure the necessary paraclinical examinations and achieve a consensus on the treatment strategy. Written informed consent was obtained from all patients. Patients under the age of 18, pregnant women, or those incapable of providing written consent were excluded.
The patients enrolled for the EFTR procedure received standard oral bowel preparation. During the procedures, the patients could receive intravenous conscious sedation (benzodiazepines and opiates), nurse administered propofol sedation (NAPS), or general anaesthesia.
EFTR using FTRD
Endoscopists with expertise in advanced endoscopy performed the EFTR. Furthermore, all involved endoscopists had participated in a training course on EFTR in ex-vivo pig models. The resection was performed using the FTRD system from Ovesco (Tuebingen, Germany) with standard colonoscopes from the Olympus Exera system (Tokyo, Japan).
The EFTR procedure was initiated with the identification of the lesion and marking of the lateral margins of the lesion using a marking probe supplied with the system. The colonoscope was withdrawn to mount the FTRD and reintroduced. In some cases, a guidewire was placed in the colonic lumen during the first insertion of the scope to alleviate the advancement of the scope with the FTRD cap. Subsequently, the lesion was grasped with the forceps and pulled into the cap until the circumferential markings were visible inside. The clip was released and the integrated hot snare was closed to perform the resection. The endoscope with the resected specimen, still grasped and in the cap, was then extracted. Finally, the colonoscope was reintroduced to the resection site for a final inspection. The specimen was pinned down on a cork plate and preserved in formalin for pathological assessment.
All procedures were performed with CO2 insufflation. Prophylactic broad-spectrum antibiotics were given peri-interventionally and anticoagulation treatment was handled according to the national guidelines. All patients were hospitalized overnight and clinically assessed by the endoscopist before discharge. A follow-up colonoscopy was scheduled for 3 and 12 months after the date of procedure. The follow-up colonoscopy was with inspection and image documentation of the resection site. Biopsies were taken if there was any suspicion of relapse.
Study outcomes
A case report form was filled out to register the indication, histology before and after EFTR, location of the lesion, size, technical success, complications and follow-up findings. Total time duration of the procedure as well as time spent on sub-procedures such as reaching and marking the lesion, mounting the FTRD on the scope, advancing the scope with the FTRD and deploy the clip was also registered.
We defined technical success as lesion resected in one piece, en bloc, and macroscopically complete. Histologically complete R0 resection was defined as a specimen with microscopic tumour negative resection margins and and full thickness resection was defined histologically as including mucosal, submucosal and muscular layers. The primary outcome of the study was technical success with R0 resection and relapse-free follow up (1 year). The secondary outcome was the incidence of procedure-related adverse events based on the Clavien–Dindo classification [Citation11]. The data pertaining to primary and secondary outcomes has been presented as frequencies and percentages, mean, median, and ranges. The study was approved by the National Committee on Health Research Ethics (SJ-558) and the Danish Data Protection Agency (REG-130-2016).
Results
Patient and lesion characteristics
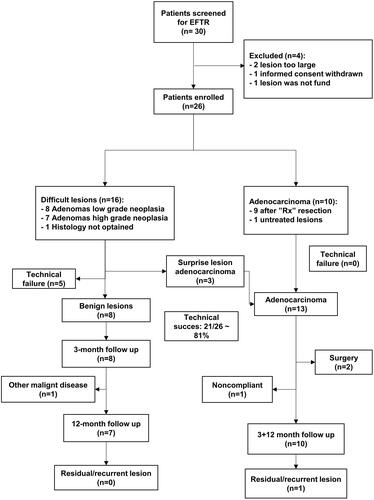
Between December 2016 and December 2017, 30 patients were referred to Zealand University Hospital and Odense University Hospital to undergo EFTR. We excluded two cases because of the lesions being too large. Furthermore, one case was excluded due to withdrawal of informed consent and one case was excluded since the lesion could not be found. Finally, 26 patients were included in the study. The patient selection has been shown in .
The patients have been divided into the benign and malignant lesion groups according to the histological description of their lesions at referral; 16 patients had benign lesions and 10 had malignant lesions. The patient and lesion characteristics have been outlined in .
Table 1. Patient and lesion characteristics.
We achieved technical success in 21 of the 26 cases (81%). In three cases, we were unable to advance the FTRD to the lesion site. In the remaining two cases, technical failure was due to snare malfunction and defected release of the clip. The snare malfunction was handled by performing standard snare resection of the tissue above the clip. The defect release of the clips resulted in a perforation which could not be treated endoscopically and thus the patient underwent surgery for the closure of the perforation.
The total EFTR procedural time was median 69 min. The most time (median, 21 min) was spent advancing the endoscope with the FTRD to the lesion, deploying the clip, and retracting the endoscope. Further details on procedural data have been presented in .
Table 2. Procedural data.
The mean diameter of the resected specimens as assessed by the pathologist was 23 mm (range, 10–35 mm). We achieved full-thickness resection in 18/22 (85.7%) cases and R0 resection in 18/22 (85.7%) cases. The histologic characteristics of the EFTR specimens have been detailed in . Among those who underwent EFTR procedures with technical success, the length of hospital stay was 1 day for 20/21 (95.2%) patients and 2 days for one patient.
Table 3. Data on resected specimens.
Nine of the initially malignant lesions had been previously treated with endoscopic resection, with snare polypectomy and histology of these lesions showed an accidentally malign lesion with less than 1 mm to the resection margin. Due to the size of the lesions as well as the patients’ comorbidity, the lesions were considered most suitably treated with EFTR. All EFTR specimens in this subgroup were R0. One patient had a small, untreated malignant lesion and multiple comorbidities including hypertension, diabetes, and chronic obstructive pulmonary disease, with an ASA score III and performance status 1. After consideration at MDT conference and with the patient’s informed consent, we chose to perform local resection with EFTR. The EFTR went uneventful with a R0 resection, and the follow up assessment showed no relapse.
Of the 16 initially benign lesions, 8 were adenomas with low-grade neoplasia and 7 were adenomas with high-grade neoplasia. Although the initial histological diagnosis declared them benign, three of the EFTR specimens were observed to contain adenocarcinoma. Two of these patients underwent subsequent surgery because the EFTR specimen analysis showed R1 resection. However, the surgical resection specimens in both cases revealed neither residual tumour nor lymph node metastasis. The third patient underwent EFTR which was R0 and based on the histologic assessment and patient’s preference, we chose the watchful waiting approach. At the 3-year follow up, we observed no signs of relapse.
Adverse events
Overall, adverse events were observed in 3/26 (11.5%) patients out of which one had Clavien–Dindo grade 1 and two had grade 3 b events. One patient experienced late bleeding three weeks after the EFTR procedure and was admitted to the hospital. There was no need for an intervention or blood transfusion and the patient was discharged after 3 days. This patient was receiving direct oral anticoagulant (Dabigatran etexilate/Pradaxa).
Perforation occurred in two patients (7.7%) after the EFTR procedures. One perforation, as mentioned above, was recognized during the endoscopic resection, and was due to the lack of release of the clip from the cap. The patient underwent surgery, and the defect was sutured. After the 6-day hospitalization, the patient was discharged without sequelae and they attended the follow-up program subsequently.
The other case with perforation was a late perforation where the patient was hospitalized 5 days after an otherwise uncomplicated EFTR procedure. Surgery revealed a perforation located at the EFTR site. The perforation was sutured without further intervention. The patient was hospitalized for 4 days and discharged without sequelae and was later followed up.
Follow up
Of the 21 patients with a successful EFTR, 3 patients were not followed up. As stated above, two patients had unexpected malignancy in the EFTR specimen and subsequently underwent uncomplicated surgery with oncological, segmental colon resection. Further follow up was therefore done according to national guidelines for surveillance after colorectal cancer. One patient was lost to follow up. Thus, 18 patients were available at the 3-month follow up. At 12-month follow up, one patient turned out to be terminally ill from a disseminated small bowel neuroendocrine tumour and it was decided not to perform any further procedures ().
Based on the observations from the 3-month follow up colonoscopy, the clip had disappeared in 15 out of the 18 (84%) cases and that from 12-month follow up, it was present in only one patient. At the 3-month follow up biopsies were taken from the resection site in 9 patients, of which 7 were from the adenocarcinoma subgroup. One patient who showed a R1-resection in the EFTR-specimen had a bioptically confirmed residual adenoma with low-grade neoplasia at 3-month follow up. The residual adenoma was 6 mm in diameter and was successfully re-treated endoscopically (snare resection), with no residual adenoma tissue on the subsequent follow up. Apart from this case, there were no residual or recurrent adenomas observed during the 12-month follow up in the group with benign lesions.
Among the adenocarcinoma subgroup of 13 patients, we obtained follow up in 10 cases (two patients underwent surgery and one was non-compliant). The 3-month follow up showed no residual tumour in all 10 cases. At the 12-month follow up, one patient had a late relapse despite no evidence of residue on endoscopic assessment and biopsy at the 3-month follow up. The colonoscopy at 12 months showed stenosis and the biopsy revealed adenoma with high-grade dysplasia. Subsequently, this patient underwent sigmoid colectomy and the specimen histology revealed a T2 adenocarcinoma and one lymph node metastasis out of 73 retrieved lymph nodes. Subsequently, the patient developed hepatic metastases and underwent liver resection with curative intent.
Discussion
This study show our experience with the EFTR on colon lesions using the FTRD. Our aim of the study was to report our clinical results on the efficacy and safety of EFTR as well as on the long-term follow up. Our study demonstrated a total technical success rate of 81% which is in accordance with other studies with technical success rates ranging from 75% to 100% [Citation10,Citation12–16]. In this study, half of the cases with technical failure were due to inaccessibility to the lesion after mounting the FTRD. The Wall Resect study reported that all target lesions were successfully reached with a technical success of 89.5% [Citation9]. In some studies, the patients were excluded if the lesion was not reached with prOVE cap (Ovesco Endoscopy), a dummy cap of the same size as that of FTRD [Citation16]. The prOVE cap was optional and we did not use it in our study in order to reduce the number of colonoscopies.
A histologically complete resection, R0, was achieved in 86% cases in our study, which is comparable with that reported in a recent meta-analysis (84.9%) [Citation17]. Furthermore, the rate of full-thickness resection was 85.7% which is in line with other studies. However, the confirmation of a full-thickness resection is not reported in half of the studies mentioned in the meta-analysis. This was speculated to be because the pathologist was unfamiliar with the nature of the resection [Citation14]. In the present study, we had a dedicated pathologist to assess the specimens if in doubt.
The maximal recommended size of lesions to be removed by the FTRD is 30 mm in diameter, but with scarring, it is decreased to 20–25 mm. Since most of the lesions in the present study were expected to exhibit some degree of scarring due to previous endoscopic attempts of removal, we decided to accept lesions with a diameter of up to 25 mm. In this study, the mean size of the resected specimen was 23 (10–35) mm which is in accordance with other published studies (12–35 mm) [Citation17].
Our median procedure time was 69 min, which is comparable to other studies where it ranged from 50 min to 95 min [Citation9,Citation10]. The maximum time was expended in the advancement of the scope with FTRD during the procedure (median, 21 min). In some cases, the resection site and the colon were flushed after marking to achieve a more seamless colonoscopy with the FTRD which represented a large proportion of the procedure time. Unfortunatly, this time slot used to clean the colon was not registered separately but has been included in the total procedural time. Additionally, it is important to mention a major limitation of the FRTD which is the substantial reduction in the flexibility of the colonoscope and the visibility of colon when the device is mounted on the endoscope [Citation18].
The procedures were conducted with different types of medication; conscious sedation was used in 42%, NAPS in 12%, and general anaesthesia in 46% of our cases, which demonstrates the possibility of performing the procedure with conscious sedation. However, in all three cases involving the inability to reach the lesions with the FTRD, the patients were only conscious sedated which could advocate for deeper sedation using NAPS or general anaesthesia.
The overall adverse events were observed in 11.5% of the cohort, including one late bleeding and two perforations. The perforation rate was somewhat higher in our study compared with other studies. In the Wall Resect study, perforation occurred in 6 out of 181 patients (3.3%) where 4 cases were handled endoscopically in the same session [Citation9]. Although post polypectomy syndrome has been mentioned as a common complication [Citation12], we did not observe it in the present study. Moreover, we did not observe any complications with injury to adjacent organs or the bowel. Consequently, perforations continue to be concerning, especially delayed perforations can cause unfortunate complications on patients that have been already discharged.
Recently, two, Dutch and German, registry-based studies on the efficacy and safety of FTRD were published [Citation19,Citation20]. These studies involved 367 and 1178 procedures, respectively, spread over 20 and 65 hospitals, respectively, for over 3–4 years. Their observations with regards to technical success, R0, full-thickness resection, and overall adverse events are comparable to those in our study. However, their follow-up times are short (median, 14 and 16 weeks, for Dutch and German studies, respectively) compared with our one-year follow up. Furthermore, the number of procedures was 2–3 times higher in this study compared to the average procedures per centre annually in the two studies [Citation19,Citation20].
The indication for EFTR in our study was removal of adenomas or small adenocarcinomas most of which had been previously treated with EMR or polypectomy. These previously treated colon lesions with scarring and non-lifting areas have given the endoscopists limited options of treatment until now. ESD is an option but it is time-consuming, technically demanding and is accompanied with a high risk of perforation (reportedly up to 15%) [Citation2,Citation21]. In cases with adenocarcinoma, surgery can undoubtedly determine the invasiveness of the lesions as well as lymph node metastasis, but at the expense of a more invasive procedure with a higher risk of procedure-related surgical and medical complications [Citation22]. Therefore, EFTR has been suggested to be feasible and safe in order to obtain histological risk stratification and avoid surgery on small and low-risk lesions [Citation23]. In the subgroup with T1 adenocarcinomas that was followed for 12 months, one patient had a late relapse. Moreover, the biopsy and colonoscopy findings at 3 months had showed no recurrence; therefore, we recommend a follow up duration longer than 3 months in cases with adenocarcinomas. Only one other case series with EFTR performed in five patients with T1 early rectal cancer has a 12-month follow up; however, they revealed no relapse in all five cases [Citation24]. The selection of patients for a bowel preservation strategy is one of the greatest challenges within this field, and there is need for prediction models building on the general phenotype of the patient and the particular lesion removed.
In conclusion, the EFTR with the FRTD is an additional minimally-invasive technique in the treatment of difficult non-lifting colorectal lesions. For the treatment of adenomas, FTRD appears effective and safe taking into account the limitations in terms of lesion size and access. For malignant lesions, EFTR is technically safe and feasible and has the potential to treat small, early low-risk tumours, though some cases may require subsequent surgery based on the histological staging of the resected specimen. Further prospective studies are warranted to investigate the role of EFTR in treatment of malignant lesions and its long-term outcomes.
Ethics approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. The study was approved by the National Committee on Health Research Ethics (SJ-558) and the Danish Data Protection Agency (REG-130-2016).
Consent to participate
All patients provided written informed consent before participation in the study.
Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection were performed by MB, NB, SK and LB. Analysis were performed by MB and IG. The first draft of the manuscript was written by MB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declare that they have no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, MB, upon reasonable request.
Additional information
Funding
References
- Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2017;49(3):270–297.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2015;47(9):829–854.
- Belderbos TDG, Leenders M, Moons LMG, et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46(05):388–400.
- Knabe M, Pohl J, Gerges C, et al. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109(2):183–189.
- Arezzo A, Passera R, Marchese N, et al. Systematic review and meta-analysis of endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal lesions. United European Gastroenterol J. 2016;4(1):18–29.
- De Ceglie A, Hassan C, Mangiavillano B, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: a systematic review. Crit Rev Oncol Hematol. 2016;104:138–155.
- Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63(6):949–956.
- Hong SN, Byeon JS, Lee BI, et al. Prediction model and risk score for perforation in patients undergoing colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2016;84(1):98–108.
- Schmidt A, Beyna T, Schumacher B, et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut. 2018;67(7):1280–1289.
- Albrecht H, Raithel M, Braun A, et al. Endoscopic full-thickness resection (EFTR) in the lower gastrointestinal tract. Tech Coloproctol. 2019;23(10):957–963.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Vitali F, Naegel A, Siebler J, et al. Endoscopic full-thickness resection with an over-the-scope clip device (FTRD) in the colorectum: results from a university tertiary referral center. Endosc Int Open. 2018;6(1):E98–E103.
- Aepli P, Criblez D, Baumeler S, et al. Endoscopic full thickness resection (EFTR) of colorectal neoplasms with the full thickness resection device (FTRD): clinical experience from two tertiary referral centers in Switzerland. United European Gastroenterol J. 2018;6(3):463–470.
- Ichkhanian Y, Vosoughi K, Diehl DL, et al. A large multicenter cohort on the use of full-thickness resection device for difficult colonic lesions. Surg Endosc. 2021;35(3):1296–1306.
- Richter-Schrag HJ, Walker C, Thimme R, et al. [Full thickness resection device (FTRD). Experience and outcome for benign neoplasms of the rectum and colon]. Chirurg. 2016;87(4):316–325.
- Andrisani G, Soriani P, Manno M, et al. Colo-rectal endoscopic full-thickness resection (EFTR) with the over-the-scope device (FTRD®): a multicenter Italian experience. Dig Liver Dis. 2019;51(3):375–381.
- Li P, Ma B, Gong S, et al. Efficacy and safety of endoscopic full-thickness resection in the Colon and rectum using an over-the-scope device: a meta-analysis. Surg Endosc. 2021;35(1):249–259.
- Schmidt A, Bauerfeind P, Gubler C, et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47(8):719–725.
- Zwager LW, Bastiaansen BAJ, Bronzwaer MES, et al. Endoscopic full-thickness resection (eFTR) of colorectal lesions: results from the Dutch colorectal eFTR registry. Endoscopy. 2020;52(11):1014–1023.
- Meier B, Stritzke B, Kuellmer A, et al. Efficacy and safety of endoscopic full-thickness resection in the colorectum: results from the German Colonic FTRD registry. Am J Gastroenterol. 2020;115(12):1998–2006.
- Kuroki Y, Hoteya S, Mitani T, et al. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol. 2010;25(11):1747–1753.
- Ahlenstiel G, Hourigan LF, Brown G, et al. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the Colon. Gastrointest Endosc. 2014;80(4):668–676.
- Kuellmer A, Mueller J, Caca K, et al. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc. 2019;89(6):1180–1189.e1.
- Soriani P, Tontini G, Neumann H, et al. Endoscopic full-thickness resection for T1 early rectal cancer: a case series and video report. Endosc Int Open. 2017;5(11):E1081–E1086.