Abstract
Background
This study aimed to compare the clinical outcomes between submucosal tunneling endoscopic resection (STER) and endoscopic submucosal dissection (ESD) for large subepithelial esophageal lesions (SELs) and analyze risk factors for perforation and piecemeal resection.
Methods
The clinicopathological features and outcomes of endoscopic treatment of 56 patients with SELs with diameters ≥30 mm, diagnosed between June 2017 and December 2020, were reviewed in this retrospective cohort study. Patients were divided into two groups (ESD group and STER group).
Results
The complete resection rates of the STER and ESD groups were 88.1% and 78.6%, respectively (p = .398). The operation time of STER was longer than ESD (p = .03), while the hospital stay of STER was shorter than ESD (p = .02). The rate of major adverse events associated with ESD was considerably higher than STER group (p = .035). The extraluminal growth pattern was a risk factor for piecemeal resection, and ESD was an independent risk factor for perforation. Regarding tumors with extraluminal growth patterns, the ESD group's perforation rate was significantly higher than the STER group (p = .009). There were no recurrence or metastases found during a mean follow-up of 24.4 months.
Conclusion
The STER technique has advantages of shorter hospital stays and fewer major adverse events than ESD. The extraluminal growth pattern seems to be a risk factor for piecemeal resection in both ESD and STER. STER appears to be a preferable choice for large SELs with extraluminal growth patterns.
Introduction
Subepithelial esophageal lesions (SELs) are a heterogeneous group of bulging lesions or masses covered with an intact mucosa in the esophagus. SELs are rare, accounting for less than 1% of all esophageal tumors [Citation1]. Most SELs do not show any typical symptoms, and they are usually found incidentally during endoscopic examinations. While SELs larger than 3 cm may cause symptoms, the most common symptoms include dysphagia, heartburn, and retrosternal pain [Citation2]. Most SELs are benign. Esophageal leiomyoma represents the most common benign neoplasm in the esophagus. However, a proportion of SELs arising from the muscularis propria are gastrointestinal stromal tumors (GISTs), which potentially have malignant behavior. An accurate diagnosis and assessment of the malignant potential determine the initial management of SELs. Bite-on-bite forceps biopsy sampling of SELs is frequently nondiagnostic, primarily due to its limited penetration depth. EUS-FNA yields high cytopathologic diagnostic accuracy for solid pancreatic tumors. However, EUS-FNA may fail to differentiate GISTs from other gastrointestinal mesenchymal tumors and assess the malignant potential of GISTs, which requires further immunohistochemical analysis and evaluation of mitotic index [Citation3]. Submucosal endoscopic tumor resection turns out to be the best technique to confirm the final diagnosis.
The American Society for Gastrointestinal Endoscopy (ASGE) recommended that all gastrointestinal submucosal tumors ≥3 cm in size be removed, while smaller gastrointestinal submucosal tumors (<3 cm) without any imaging signs of malignancy may be managed with active surveillance [Citation4]. Thoracic enucleation has been previously regarded as the first-line treatment for large SELs, while imaging surveillance is recommended for small SELs [Citation5]. With the significant advances in endoscopic technologies, therapeutic endoscopic techniques have become potential alternatives for removing upper gastrointestinal submucosal tumors. Therapeutic endoscopic resection has also been extensively studied for a definitive histologic diagnosis as a minimally invasive treatment. Endoscopic submucosal dissection (ESD) is a novel but effective strategy for tumors originating from the muscularis mucosa and submucosa [Citation6]. The ESD technique mainly consists of circumferential cutting of the surrounding mucosa of the lesion and direct dissection of the tumor. Nevertheless, it is risky and challenge to use ESD to completely resect tumors originating from the deep muscularis propria (MP) layer, particularly those tumors that are growing outside the esophageal wall. Submucosal tunneling endoscopic resection (STER), a combination of ESD with peroral endoscopic myotomy techniques, emerged as a novel technique for removing upper gastrointestinal submucosal tumors. Compared with conventional ESD, STER can resect SELs, originating from the muscularis propria, under direct vision, while maintaining the superficial mucosa integrity and reducing the risk of postoperative esophageal leakage and secondary infection. Reports regarding STER have been increasing and the technique has been widely applied for removing SELs. There are few studies comprehensively comparing the treatment of upper gastrointestinal submucosal tumors between STER and ESD [Citation7–9]. However, no study has focused on a comprehensive comparison of the treatment outcomes of large SELs between STER and ESD. Therefore, the primary purpose of this retrospective study was to evaluate the efficacy and safety of ER for large SELs in a real-world setting and to explore the risk factors for perforation and piecemeal resection.
Patients and methods
Patients
The clinicopathological features and outcomes of endoscopic treatment of 56 consecutive patients at Shanghai Xuhui Center Hospital who received ESD or STER to treat SELs from June 2017 to December 2020 were reviewed in this retrospective cohort study. Patients who matched the following conditions were enrolled: (1) SELs with maximal diameter ≥30 mm; (2) the patient consented to undergo a STER or ESD procedure for gastrointestinal symptoms or mental pressure from potentially malignant tumors. The exclusion criteria were (1) intolerance to anesthesia with tracheal intubation; (2) multiple SELs; (3) blood coagulation disorders before procedure (international normalized ratio > 2.0, platelet count < 70,000/mm3); and (4) malignant manifestation. SELs originating from the submucosal layer and those originating from muscularis propria with a predominantly intraluminal growth pattern on EUS scanning were included in ESD technique, while SELs arising from muscularis propria with an extraluminal growth pattern were included in the STER. A flow diagram for the study participant screening and grouping is shown in . If the SELs tended to be high-risk or malignant, a surgical approach was required to guarantee a complete resection. All procedures were performed by the same chief physician, who has been committed to endoscopic surgery for over 10 years and successfully performed 200 cases of ESD and 60 cases of STER for SELs every year. The Shanghai Xuhui Central Hospital Ethics Committee approved the present study (Issue No. 112(2021). IRB), and written informed consent was obtained from the patients before endoscopic treatment.
Endoscopic submucosal dissection
ESD was carried out with a single channel endoscope (GIT-H260; Olympus, Japan). Before inserting the gastroscope, a short and transparent cap (ND-201-11802, Olympus, Japan) was attached to its tip to provide a constant endoscopic view and constant tension to the connective tissue for dissection. The other equipment and accessories employed for these procedures included a hybrid Knife system (ICC 200, Erbe, Germany), an argon plasma coagulation unit (APC 300; ERBE), an injection needle (INJ1-A1; Medwork, Germany), an insulated-tip (IT) knife (KD-611; Olympus), a hook knife (KD-620LR; Olympus), and hemostatic forceps (FD-410-LR; Olympus). All the ESD procedures were performed with patients under general anesthesia. The standard ESD steps are performed as follows (). The knife and hemostatic forceps were utilized for hemostasis whenever active bleeding was detected. Argon plasma coagulation was employed to coagulate any exposed vessels to decrease the risk of delayed bleeding.
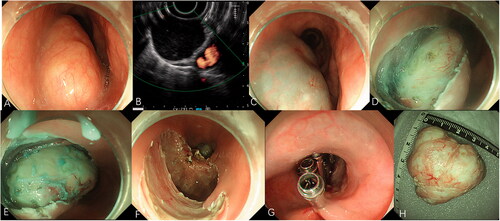
Figure 2. Endoscopic submucosal dissection for a large subepithelial esophageal lesion. (A) Endoscopic view of oval-like tumor. (B) Endoscopic ultrasonography view of the tumor. (C) Submucosal injection with fluid mixture. (D) Incision of the covering mucosa. (E) Peeling the lesions. (F) The wound after resection. (G) Closure of the mucosal incision by clips. (H) The resected specimen.
Submucosal tunneling endoscopic resection
The equipment and accessories necessary for the STER technique were analogous to those of ESD. The critical steps of STER are summarized as follows (): (1) a 2-cm longitudinal mucosal incision was made 5 cm away from the oral side of the lesion to establish submucosal tunnel; (2) a shallow a submucosal tunnel was created between the mucosal and MP layers until the lesion was exposed; (3) the lesion was removed directly; and (4) the mucosal lesion was closed with metal clips.
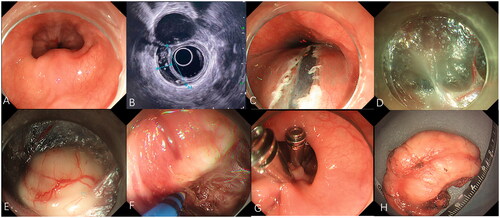
Figure 3. Submucosal tunneling endoscopic resection for a large submucosal esophageal lesion. (A) Endoscopic view of a ring-like tumor surrounding the esophagus. (B) Endoscopic ultrasonography view of the tumor. (C) A 2-cm longitudinal mucosal incision was made approximately 5 cm proximal to the SEL. (D) The submucosal tunnel is established. (E) The SEL is exposed using the submucosal tunnel technique. (F) Separating the tumor from the MP layer using the hybrid knife. (G) The mucosal entry incision is sealed with several clips. (H) Irregularly-shaped completely resected specimen (maximal diameter).
Definition
According to pathological reports, all tumors were measured in two dimensions: with the maximal longitudinal length and maximal transverse diameter. The operation speed was calculated as the ratio of the tumor area to the procedure time (tumor area = π×T/2 × L/2, T = maximum transverse diameter, L = maximum longitudinal length) [Citation10]. Complete resection was achieved when entire tumors and surrounding tissue were resected in a single piece with negative vertical and lateral margins. The time from inserting the gastroscope to the withdrawal of the resected tumor was defined as the operation time. Perforation was considered to have occurred if there was visualization of any extra gastrointestinal structure during the procedure. The length of postoperative hospital stay was calculated as the time interval from the date of surgery to discharge from the hospital.
The primary endpoint of the current study was the incidence of major ER-related adverse events, including perforation, pneumothorax or pleural effusion, massive intraoperative bleeding, delayed bleeding, and pulmonary embolism, all of which was treated invasive therapy. The minor technical adverse events included slight intraoperative hemorrhage, a mild degree of pleural effusion, and a minor amount of carbon dioxide that accumulated in the thorax, mediastinum, subcutis, or abdomen that could be rapidly absorbed by the body, and these were not regarded as ER-related adverse events. The mucosal incision for the tunnel entrance of STER was not recorded as a complication, while mucosal injury in other areas were defined as perforations during STER.
Follow-up
All patients agreed to receive follow-up. All SEL patients after ER treatment were required to undergo follow-up esophagogastroduodenoscopy and CT in the first, sixth, and 12th month postoperatively and annually to recheck wound healing and check for any residual tumor recurrence or metastasis. Recurrence was defined as certain radiologic findings on CT imaging or biopsy results of suspicious lesions. A periodic chest contrast-enhanced CT scan was performed postoperatively every 6 to 12 months for patients with malignant tumors during the first three years.
Statistical analysis
The clinicopathologic features and follow-up data were collected for further analysis. These data were then used to compare the STER and ESD groups and analyze the risk factors for piecemeal resection and perforation. The statistical analysis between the groups was carried out using Student’s test, the Mann-Whitney U test, Pearson’s chi-square test, or Fisher’s exact test. Multivariate analysis was performed with factors that had a univariate p value less than .05 in the univariate analysis. Statistical analysis was performed using commercial software (SPSS statistics, version18.0; SPSS Inc). p Values < .05 were considered statistically significant.
Results
Clinicopathologic characteristics
Fifty-six patients diagnosed with SELs were included in this retrospective study. The baseline characteristics of the patients and tumors are provided in . The average age of 56 patients was 47.2 years (median 45 years, range 18 to 79 years) with a male: female ratio of 1.6:1. Of the 56 patients, 14 (25%) had typical symptoms, such as dysphagia and retrosternal pain. Seven patients (12.5%) reported atypical symptoms, such as epigastric discomfort. In the STER group, the median longitudinal length and transverse diameters were 25 mm (15–65 mm) and 41 mm (20–110 mm), respectively, and in the ESD group, the median longitudinal length and transverse diameters were 47.5 mm (20–135 mm) and 30 mm (20–70 mm), respectively. Among the SELs, 40 had regular shapes (71.4%) and 16 had irregular shapes (28.6%). Our study included 47 leiomyomas (83.9%), two leiomyosarcomas (3.6%), one lymphoma (1.8%), one adenosarcoma (1.8%), one lipoma (1.8%), one bronchogenic cyst (1.8%), one schwannoma (1.8%), one granular cell (1.8%), and one Castleman (1.8%). Patients diagnosed with leiomyosarcomas and adenosarcomas refused further open surgery despite our strong recommendation. The patient diagnosed with lymphoma was required to undergo further chemotherapy. No statistically significant difference in age, sex, tumor size, tumor location, or tumor shape was observed between the two groups. The percentage of leiomyoma in the STER group was significantly higher than that in the ESD group (p = .03).
Table 1. Characteristics of 56 patients and subepithelial esophageal lesions after resection.
Treatment outcomes and complications
In this comparison study, the complete resection rate were 88.1% (37 of 42) and 78.6% (11 of 14) by STER and ESD, respectively. No statistically significant difference in complete resection rate was observed between the STER and ESD groups (p = .398). The median duration of STER was 99 min (45–200 min), and the median duration of ESD was 62.5 min (22–165 min). Overall, the median duration of STER was significantly longer than that of ESD (p = .03). In addition, the resection speed of ESD was significantly faster than that of STER (p < .001). The median follow-up time of ESD (22 months, range 11–44 months) was shorter than that of STER (24, range 11–48 months) with a non-significant difference (p = .496). No recurrence or metastasis was observed during the follow-up examinations.
The incidence of major adverse events in the STER group (9.5%) was lower than that in the ESD group (35.7%) (p = .035) (). One patient (2.4%) suffered from pneumothorax or pleural effusion after STER and was treated with a small-caliber thoracic tube. Three patients (21.4%) developed pneumothorax or pleural effusion after ESD, which required therapeutic intervention. Massive bleeding occurred in one patient (2.4%) after the STER operation, which needed a second gastroscopy check and was successfully treated with endoscopic hemostasis. Both of the patients, after STER and ESD, were diagnosed with low-risk pulmonary thromboembolism through computed tomography pulmonary angiography (CTPA). Both of these patients were conservatively managed with anticoagulant and mask oxygen inhalation. One out the 14 patients in the ESD group suffered from a pulmonary infection after perforation. All of the complications were successfully managed with conservative treatment and no patients died during perioperative period. The median duration of hospitalization for the STER group and ESD group was six days (range 4–12 days) and nine days (range 4 to 28 days), respectively; The duration of hospitalization for the STER group was significantly shorter than that of the ESD group (p < .01). Pulmonary thromboembolism was the most severe adverse event that caused the most prolonged hospital stay after ESD operation, and it was up to 28 days.
Risk factors associated with piecemeal resection and perforation
To investigate the risk factors for piecemeal resection, we divided the patients into the complete resection group and piecemeal group. After univariate logistic regression analysis of the correlation between the baseline clinicopathologic characteristics and piecemeal resection, a transverse diameter ≥35 mm, an irregular shape, and an extraluminal growth pattern were significant risk factors for piecemeal resection (). However, the extraluminal growth pattern remained significant based on the multivariate logistic regression analysis (OR: 14.222; 95% CI 1.339, 151.008; p = .028). The other clinicopathologic factors, such as age, sex, the longitudinal length, location, histology and endoscopic treatment, were insignificant.
Table 2. Outcomes after resection in patients in submucosal tunneling endoscopic resection and endoscopic submucosal dissection.
Table 3. Comparison between the complete resection group and piecemeal resection group.
All of the risk factors for perforation are provided in . A transverse diameter ≥35 mm, an extraluminal growth pattern, and a procedure time ≥100 min were significant risk factors for perforation. In addition, ESD was also a contributor to perforation. The incidence of perforation in the ESD group was significantly higher than that in the STER group (p = .020). The multivariate logistic regression analysis revealed that ESD was a significant independent predictor of perforation (OR: 0.053; 95% CI 0.003, 0.864; p = .017). For all of the tumors with extraluminal growth patterns, the perforation rate of ESD (100%) was significantly higher than that of STER (25%) (p = .009).
Table 4. Comparison between nonperforation group and perforation group.
Discussion
Leiomyoma accounts for 70%–80% of SELs and represents the most common SELs, followed by gastrointestinal stromal tumors (GISTs). However, some SELs might be leiomyosarcomas or adenosarcomas, despite their rarity. The National Comprehensive Cancer Network Guidelines recommended surgical treatment for submucosal tumors larger than 2 cm, particularly those with malignant findings or symptoms [Citation11]. However, distinguishing between benign and malignant tumors based on endoscopic imaging and EUS is challenging. Moreover, EUS-FNA usually fails to obtain an adequate sample for an immunohistochemical diagnosis [Citation12]. An immunohistochemical assessment is essential to confirm the final diagnosis of SELs. As a minimally invasive approach, endoscopic resection, whether ESD or STER, can remove the tumors entirely and can provide a specimen for a pathological examination. The novel STER technique involves endoscopic resection in the submucosal space while keeping the overlying mucosa intact. Previous studies have demonstrated that STER is safe and effective in resecting SELs smaller than 3 in diameter [Citation9,Citation13]. Compared with ESD, STER has the advantage of using the 5-cm long submucosal tunnel, which theoretically reduces the risk of post-operative leak, digestive tract fistulae, and pleural infection. However, the smaller operating space in submucosal tunneling during STER might influence efficacy and safety. Despite the popularity of STER, it does not demonstrate significant advantages over ESD, either with complete resection or in the incidence of adverse events [Citation13]. However, few studies have focused on applying and comparing STER and ESD to treat large SELs [Citation7–9]. Additionally, no study has analyzed the risk factors for piecemeal resection and perforation after endoscopic resection of large SELs.
As demonstrated by our data, the complete resection rate in the STER group (88.1%) was higher than that in the ESD group, but the difference was not significant (p = .398). This finding was consistent with previous studies involving endoscopic resection of upper gastrointestinal submucosal tumors, which showed that STER did not show an advantage of complete resection over ESD [Citation8,Citation14]. Nevertheless, the complete resection rates of ESD and STER were lower than 100%, which were also reported by Xu et al. [Citation15]. The reason may be that we targeted SELs ≥35 mm, while the majority of SELs in the latter study were smaller than 35 mm in diameter. Since the inner diameter of the esophageal lumina is 35 mm, resecting tumors with transverse diameters ≥35 mm is challenging and time consuming when using the endoscopic techniques. Therefore, tumors with transverse diameters ≥35 mm were considered not appropriate for STER [Citation16]. After significant advances in the STER techniques, recent studies have showed that STER was safe and effective for large SELs, which is associated with a high risk of piecemeal resection [Citation8,Citation17,Citation18]. After using a minimally invasive approach, we found that STER reduced the postoperative hospital stay (six days vs. nine days; p = .020), which was in agreement with Chen et al. [Citation19]. The shortened hospitalization might be attributed to the mucosal integrity, minimal mucosal incision, and low major complication rates. The integrity of the overlying mucosa forms a barrier to prevent gas and liquid leakage, consequently lowering the perforation and secondary infection rate. The minimal mucosal incision contributed to patients being able to have a fluid diet sooner. In addition, the patients with adverse events stayed longer in the hospital than the patients without adverse events. Low rates of major complications reduced the hospital stay through the management of complications. Chen et al. comparatively evaluated ESD and STER and found no differences in the complication rate between the two groups [Citation20]. However, the rate of major adverse events in the STER group was significantly lower than that in the ESD group (9.5% vs. 35.7%, p = .035). The main contributing factors may be the long procedure time and the high perforation rate in the ESD group. A long surgical time was a risk factor for ER-related adverse events [Citation21], which is consistent with our result. Two patients suffered from low-risk pulmonary embolism after longer than 3-h surgery despite the different ER techniques. Our results demonstrated significant advantages in ESD for esophageal SELs in terms of the operation speed and operation time, and these results correspond to a previous study [Citation22]. Two reasons could include the fast operation speed and shorter procedure time. On the one hand, STER requires the additional creation of a submucosal tunnel that provides space for endoscopic dissection, which is technically challenging. On the other hand, some studies suggested that the large tumors ≥35 mm may be associated with endoscopic vision loss when using STER technique in the limited submucosal tunnel space [Citation23–26]. Moreover, the calcification of subepithelial esophageal tumors may exacerbate the loss of endoscopic vision. The loss of endoscopic vision was an essential factor that delayed the operation.
Similar to our findings, Tang et al. [Citation27] showed that an irregular tumor shape was a risk factor for piecemeal resection in both ESE and STER. Since the esophageal measures up to 30 mm in diameter, they suggested that STER might be appropriate for SELs < 30 mm in diameter [Citation28]. However, studies reported that STER was successfully performed in tumors with a maximal diameter of 45 mm and 52 mm [Citation24,Citation29]. Chen et al. conducted a comparative study of 91 patients with SELs and found that a transverse diameter ≥35 mm was a significant factor for piecemeal resection, which consistent with our study [Citation30]. Therefore, closer attention should be given to such lesions to lower the rate of piecemeal resection during STER. Our experience shows that a complete resection of a large SEL could still be achieved unless the transverse diameter is small. However, Tang et al. [Citation27] did not assess the tumor growth patterns as a confounding variable. This should not be ignored because a higher rate of piecemeal resection could be connected with the extraluminal tumor shape. In the present study, the extraluminal growth pattern was found to be an independent risk factor for piecemeal resection based on a multivariate analysis, which is consistent with Ye et al. [Citation22]. Ye et al. also demonstrated that the extraluminal growth patterns could significantly increase the risk of perforation. Considering the risk of injuring the thoracic viscera and main blood vessels, a piecemeal resection of tumors with extraluminal growth patterns seems to be a safer alternative. In addition, it also carries the risk of severe bleeding. Notably, it is essential to note that a piecemeal resection resulted in the breakdown of tumor capsules, which violates the free-tumor principle. Piecemeal resection may influence the pathological analysis, particularly the resection margin. Previous findings have demonstrated that a piecemeal resection of leiomyoma does not affect the long-term outcomes [Citation31]. However, the long-term outcome of malignant tumors after a piecemeal resection remains unknown. Four (7.1%) out of the 56 SELs were diagnosed as malignant tumors. Although no residual tumor or recurrence was noted during the follow-up, the application of a piecemeal resection for large SELs should be carefully taken into consideration to ensure the best oncologic results. The combined evidence presented in the current study demonstrated that care should be taken when ER is carried out in large SELs with extraluminal growth patterns.
Although no previous studies have focused on the risk factors for ER-related perforation of large SELs, a large tumor size was predictive of perforation during the endoscopic treatment of upper gastrointestinal submucosal tumors [Citation22]. Our study showed that a transverse diameter ≥35 mm was a significant risk factor for perforation, while the longitudinal length did not increase the risk of perforation. Three lesions with small transverse diameters were resected without perforation despite a long longitudinal length of ≥70 mm. Perforation was reported to be more likely to occur in tumors arising from the deep MP layer and growing out of the esophageal lumen. Ye et al. demonstrated that the extraluminal growth pattern was an independent risk factor for perforation of gastrointestinal submucosal tumors [Citation22]. The perforation rate of tumors with extraluminal growth patterns in the ESD group was significantly higher than that in the STER group (p = .009). The difference in the rate of perforation of large SELs with extraluminal growth patterns is related to the selection of the endoscopic technique. In our opinion, STER may be preferable for large SELs with extraluminal growth patterns, where the mucosal integrity could reduce the influence of muscle perforation. A large cohort study is required to support this hypothesis. The procedure time, a confounding factor, has never been evaluated in any of previous studies, which cannot be ignored. A long procedure time can cause surgeon fatigue and may cause a high rate of perforation, particularly an injury to the mucosa during STER. The perforation rate in the procedure time ≥100 min group was significantly higher than that in the procedure time <100 min group. Previous studies [Citation21,Citation27] have showed that STER could significantly reduce the rate of perioperative perforation for upper gastrointestinal submucosal tumors, while ESD has a perforation rate ranging from 6.1% to 15% [Citation27]. We found that ESD was an independent risk factor for perforation based on the multivariate analysis. STER was preferred for large SELs, and this is consistent with previous studies [Citation31,Citation32]. The greatest concern about ER is how to manage the occurrence of perioperative perforation. Despite a relatively high frequency of perforation (19.6%), none of the patients received surgical intervention. Similar to the previous studies, surgical management was rarely necessary in patients who developed perforations [Citation33,Citation34]. The appropriate use of fully covered and retrievable metal stents or metal clips may facilitate the recovery of this benign course after medical accidents [Citation22,Citation30,Citation33,Citation35]. Nevertheless, surgical management should be taken into consideration who have a rapid worsening of clinical signs.
There is another concern about the intact retrieval of tumor specimens during STER. Since most of the large SELs demonstrate a longitudinal growth style, they are usually retrieved through a mucosal entry. However, Ng first reported a novel second distal mucosal incision technique to completely retrieve a 40-mm esophageal leiomyoma during STER [Citation36]. Chen et al. suggested that in the case of the retrieval of SELs through the submucosal tunnel, closer attention should be given to SELs with diameters ≥35 mm and irregular shapes [Citation30]. He also introduced another way to retrieve large SELs directly through externally incising the mucosa of the submucosal tunnel. Compared to externally incising the mucosa, widening the submucosal tunnel seems to be more time-consuming, subsequently increasing the risk of adverse events. In our study, complex retrieval of specimens occurred in four patients (9.5%) with a median transverse diameter of 30 mm. Among the four patients, two of the large SELs had irregular shapes. An externally incising mucosa of the submucosal tunnel was performed to reduce procedure time for all patients to retrieve specimens. Finally, the mucosa incision was closed with metal clips, and all patients were discharged with uneventful recoveries.
The present study has substantial limitations. First, selection bias was inevitable because of the retrospective nature of our study. Second, the inherent weakness of the current study is the limited sample size and insufficient experience of using the STER technique. Finally, the two-year follow-up period in this retrospective study is relatively short to determine the long-term oncologic outcomes. More cases would need to be analyzed with a longer follow-up time. Thus, regular endoscopic examinations should be continued and performed at regular intervals.
Conclusion
In conclusion, submucosal tunneling endoscopic resection is a promising treatment for large SELs. The technique has advantages of shorter hospital stays and fewer major adverse events than ESD. The extraluminal growth pattern seems to be a risk factor for piecemeal resection in both the ESD and STER groups. STER appears to be a preferable choice for large SELs with extraluminal growth patterns.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Seremetis MG, Lyons WS, deGuzman VC, et al. Leiomyomata of the esophagus. An analysis of 838 cases. Cancer. 1976;38(5):2166–2177.
- Lee LS, Singhal S, Brinster CJ, et al. Current management of esophageal leiomyoma. J Am Coll Surg. 2004;198(1):136–146.
- Watson RR, Binmoeller KF, Hamerski CM, et al. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci. 2011;56(6):1757–1762.
- American gastroenterological association I. American gastroenterological association institute medical position statement on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2215–2216.
- Shin S, Choi YS, Shim YM, et al. Enucleation of esophageal submucosal tumors: a single institution's experience. Ann Thorac Surg. 2014;97(2):454–459.
- Hwang JC, Kim JH, Kim JH, et al. Endoscopic resection for the treatment of gastric subepithelial tumors originated from the muscularis propria layer. Hepatogastroenterology. 2009;56:1281–1286.
- Lu J, Jiao T, Zheng M, et al. Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc. 2014;28(12):3401–3407.
- Du C, Chai N, Linghu E, et al. Treatment of cardial submucosal tumors originating from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation. Surg Endosc. 2018;32(11):4543–4551.
- Xu HW, Zhao Q, Yu SX, et al. Comparison of different endoscopic resection techniques for submucosal tumors originating from muscularis propria at the esophagogastric junction. BMC Gastroenterol. 2019;19(1):174.
- Schubert P, Kirchner M. Ellipse area calculations and their applicability in posturography. Gait Posture. 2014;39(1):518–522.
- von Mehren M, Kane JM, Bui MM, et al. NCCN guidelines insights: soft tissue sarcoma, version 1.2021: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(12):1604–1612.
- El Chafic AH, Loren D, Siddiqui A, et al. Comparison of FNA and fine-needle biopsy for EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2017;86(3):510–515.
- Ye LP, Zhang Y, Mao XL, et al. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28(2):524–530.
- Ponte Neto FL, de Moura DTH, Sagae VMT, et al. Endoscopic resection of esophageal and gastric submucosal tumors from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation: a systematic review and meta-analysis. Surg Endosc. 2021;35(12):6413–6426.
- Xu MD, Cai MY, Zhou PH, et al. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc. 2012;75(1):195–199.
- Chai N, Du C, Gao Y, et al. Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic enucleation for esophageal submucosal tumors originating from the muscularis propria layer: a randomized controlled trial. Surg Endosc. 2018;32(7):3364–3372.
- Li Z, Gao Y, Chai N, et al. Effect of submucosal tunneling endoscopic resection for submucosal tumors at esophagogastric junction and risk factors for failure of en bloc resection. Surg Endosc. 2018;32(3):1326–1335.
- Zhang M, Wu S, Xu H. Comparison between submucosal tunneling endoscopic resection (STER) and other resection modules for esophageal muscularis propria tumors: a retrospective study. Med Sci Monit. 2019;25:4560–4568.
- Chen Y, Wang M, Zhao L, et al. The retrospective comparison between submucosal tunneling endoscopic resection and endoscopic submucosal excavation for managing esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc. 2020;34(1):417–428.
- Chen T, Zhou PH, Chu Y, et al. Long-term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg. 2017;265(2):363–369.
- Xiu H, Zhao CY, Liu FG, et al. Comparing about three types of endoscopic therapy methods for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Scand J Gastroenterol. 2019;54(12):1481–1486.
- Ye L-P, Zhang Y, Luo D-H, et al. Safety of endoscopic resection for upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: an analysis of 733 tumors. Am J Gastroenterol. 2016;111(6):788–796.
- Liu B-R, Song J-T, Kong L-J, et al. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc. 2013;27(11):4354–4359.
- Wang L, Ren W, Zhang Z, et al. Retrospective study of endoscopic submucosal tunnel dissection (ESTD) for surgical resection of esophageal leiomyoma. Surg Endosc. 2013;27(11):4259–4266.
- Zhang Y, Ye L-P, Mao X-L. Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J Gastroenterol. 2015;21(32):9503–9511.
- Li Q-y, Meng Y, Xu Y-y, et al. Comparison of endoscopic submucosal tunneling dissection and thoracoscopic enucleation for the treatment of esophageal submucosal tumors. Gastrointestinal Endosc. 2017;86(3):485–491.
- Tang X, Ren Y, Huang S, et al. Endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer: a single-center study. Gut Liver. 2017;11(5):620–627.
- Inoue H, Ikeda H, Hosoya T, et al. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44(3):225–230.
- Tan Y, Liu D. En bloc submucosal tunneling endoscopic resection for a giant esophageal leiomyoma. Gastrointest Endosc. 2015;82(2):399.
- Chen T, Lin ZW, Zhang YQ, et al. Submucosal tunneling endoscopic resection vs thoracoscopic enucleation for large submucosal tumors in the esophagus and the esophagogastric junction. J Am Coll Surg. 2017;225(6):806–816.
- Du C, Ma L, Chai N, et al. Factors affecting the effectiveness and safety of submucosal tunneling endoscopic resection for esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc. 2018;32(3):1255–1264.
- Tan Y, Lv L, Duan T, et al. Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic surgery for large esophageal leiomyoma originating from the muscularis propria layer. Surg Endosc. 2016;30(7):3121–3127.
- Liu BR, Song JT, Qu B, et al. Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg Endosc. 2012;26(11):3141–3148.
- Chen T, Zhang C, Yao LQ, et al. Management of the complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Endoscopy. 2016;48(2):149–155.
- Eloubeidi MA, Talreja JP, Lopes TL, et al. Success and complications associated with placement of fully covered removable self-expandable metal stents for benign esophageal diseases (with videos). Gastrointest Endosc. 2011;73(4):673–681.
- Ng JJ, Chiu PW, Shabbir A, et al. Removal of a large, 40-mm, submucosal leiomyoma using submucosal tunneling endoscopic resection and extraction of specimen using a distal mucosal incision. Endoscopy. 2015;47(Suppl 1)UCTN:E232–3.