Abstract
Objectives
The aim was to investigate the effect of fecal microbiota transplantation (FMT) on colonic enteroendocrine cells densities in patients with irritable bowel syndrome (IBS).
Materials and methods
This study is connected to the REFIT study, a double-blinded placebo-controlled trial to investigate using FMT for IBS treatment. Eighty-three subjects received either donor-FMT or placebo FMT (own feces) by colonoscope to cecum. Biopsies were obtained from sigmoid colon. Ten responders and ten non-responders consented to new biopsy one-year after FMT. Sixteen patients received donor-FMT and four received placebo FMT. Biopsies were immunostained for all of the colonic enteroendocrine cells and were quantified using computerized image analysis.
Allocation sequence was revealed after obtaining re-biopsies and cells quantification.
Results
Scores for IBS-SSS (mean ± SEM) of responders (eight of 10 patients who received donor FMT) and non-responders changed from baseline to one year after FMT (297 ± 11 and 81 ± 16, p < .0001, and 270 ± 17 and 291 ± 16, p = .15, respectively). Using paired t-test to compare enteroendocrine cells densities one-year after FMT to baseline showed significant increase only in somatostatin immunoreactive cells density in the total IBS responders group (p = .023) and who received donor-FMT (p = .038). The densities of peptide YY and enteroglucagon immunoreactive cells increased significantly (p = .04 and .035, respectively) in donor-FMT recipients. No significant changes were noted in placebo FMT or nonresponders subgroups.
Conclusion
This study shows that colonic enteroendocrine cells densities significantly change in responders group that received donor-FMT. The mechanisms for the cross talks between gut microbiota and colonic enteroendocrine cells remain to be investigated.
Introduction
There are at least 15 different types of enteroendocrine cells that are scattered among the epithelial cells lining the lumen of the gastrointestinal (GI) tract [Citation1,Citation2]. The enteroendocrine cells have microvilli that sense the intestinal luminal contents and stimulate the release of their neuroendocrine peptides/amines to regulate the functions of the GI tract [Citation3–6]. The colon contains five different types of enteroendocrine cells, namely serotonin-containing (enterochromaffin) cells, peptide YY (PYY), enteroglucagon (oxyntomodulin), pancreatic polypeptide (PP) and somatostatin cells [Citation7]. PYY and enteroglucagon (oxyntomodulin) are expressed by the same enteroendocrine cells (L-cells) [Citation8,Citation9].
Patients with irritable bowel syndrome (IBS) have abnormal densities of enteroendocrine cells in the colon [Citation10] that tend to normalize following dietary change with low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) [Citation7,Citation11]. Alterations in the gut microbiota, known as dysbiosis occur in IBS [Citation12–14] for example, the abundance of Lactobacillus and Bifidobacterium spp. decreased and that of Clostridium spp. increased [Citation15]. Gut microbiota interacts with the enteroendocrine cells [Citation16]. Therefore, changes in certain bacterial species can alter luminal host nutrient-sensing and gut peptide signaling [Citation6]. Prebiotics affect the differentiation of enteroendocrine cells [Citation17] such as glucagon-like peptide-1 and PYY [Citation18] cell types.
In a recent study by our group, fecal microbiota transplantation (FMT) through a gastroscope into the descending part of the duodenum in IBS patients improved their symptoms, quality of life and changed the gut microbiota [Citation19] as well as the enteroendocrine cells densities of the duodenum after the transplantation [Citation20,Citation21]. The aim of the present study was to investigate the effect of FMT on the enteroendocrine cells densities of the colon in IBS patients. The current data set was generated from Fecal Microbial Transplantation in Treatment of Irritable Bowel Syndrome; a Double Blinded Placebo Controlled Trial - the REFIT project, where the treatment of IBS with FMT, introduced via a colonoscope in the cecum of the colon, was investigated in a double-blinded, placebo-controlled trial [Citation22].
Material and methods
Study design
The participants’ selection, screening and transplantation process are detailed in a previous publication [Citation22]. Briefly, 83 patients with diarrhea-predominant and mixed type IBS according to Rome III criteria who were referred to the University Hospital of North Norway at Harstad, were included. They were allocated to receive donor-FMT (either fresh or frozen feces) or placebo FMT (own feces), in a ratio 2:1, by colonoscope to cecum in a double-blinded, randomized, placebo-controlled study after a serial of medical and physical tests to establish eligibility. To standardize the transplantation procedure, feces were collected from only two donors who fulfilled predetermined inclusion criteria. The randomization sequence was sealed in non-transparent envelopes and reveled to researchers when all participants completed a 12-month follow-up. Biopsy samples were obtained from sigmoid colon at baseline as part of the FMT procedure. Using the same cohort of IBS patients in the REFIT study [Citation22], 10 recipients among of the best responders (6 females and 4 males, age range 19–66, mean 44 years old), defined by >100 points improvement in IBS-symptom severity score (IBS-SSS) after FMT compared to baseline, and 10 non-responders (5 females and 5 males, age range 32–69, mean 53 years old) without any changes in IBS-SSS (<100 point change), consented to a new biopsy one year after FMT. They had diarrhea-predominant IBS (n = 10) and mixed-IBS (n = 10) subtypes. Out of these participants (n = 20), 16 received donor-FMT and four received placebo FMT.
The study was approved by the regional committee of medical ethics (REK-NORD: 2013/971) and registered in ClinicalTrials.gov Identifier NCT02154867) [Citation22]. All authors had access to the study data and had reviewed and approved the final manuscript.
Colonoscopy, biopsies and immunohistochemistry
The participants underwent a standard bowel preparation with sodium picosulfate and magnesium citrate (Picoprep, Ferring Pharmaceuticals AS, Switzerland) prior to colonoscopy. Tissue biopsy samples were obtained from the sigmoid colon at baseline before FMT and one year after FMT. The biopsy samples were fixed in 4% buffered paraformaldehyde, paraffin-embedded and cut into 5-μm-thick sections. The samples were then stained with hematoxylin–eosin and immunostained with an ultraView Universal DAB Detection Kit (cat. no. 760-500, Ventana Medical Systems, Basal, Switzerland) and the BenchMark Ultra IHC/ISH staining module (Ventana Medical Systems). The sections were then incubated for 32 min at 37 °C with the following primary antibodies, diluted according to the suppliers’ recommendations as described previously (7, 11): monoclonal mouse anti-N-terminal of purified chromogranin A (CgA) primary antibody (code no. M869; Dako, Glostrup, Denmark), monoclonal mouse anti-serotonin (code no. 5HT-209, Dako, Glostrup, Denmark), polyclonal anti-mouse peptide YY (PYY; code no. PYY 11 A, Alpha Diagnostic, San Antonio, TX, USA), polyclonal rabbit against synthetic human pancreatic polypeptide (code no. 114, Diagnostic Biosystems, Pleasanton, CA, USA), polyclonal rabbit anti-porcine oxyntomodulin (code no. BP508, Acris Antibodies, Herford, Germany) and polyclonal rabbit against synthetic human somatostatin (code no. A566, Dako). Labeled secondary antibodies were used to locate the specific antibody. The complex is then visualized with hydrogen peroxide substrate and 3, 3′-diaminobenzidine tetrahydrochloride (DAB) chromogen and counterstained with hematoxylin.
Computerized image analysis
The densities of the enteroendocrine cells were quantified under a light microscope with a ×40 objective using the Cell^B imaging program (Olympus, Tokyo, Japan). The number of the enteroendocrine cells was quantified in 10 randomly chosen fields, where each frame (field) of epithelial cells measured 0.09 mm2. This method was validated earlier [Citation23]. The density of each enteroendocrine cells type was expressed as the number of cells per square millimeter of epithelium. The identity of the slides was concealed during the quantification of the enteroendocrine cells that was performed by T.M.
Statistical analysis
The paired t-test is used to compare the immunoreactive cells densities of the patients at baseline to one year after receiving FMT. The data are presented as mean ± standard error of mean (SEM) values. We considered p < .05 to be statistically significant.
Results
The scores of IBS-SSS (mean ± SEM) for responders before and one year after receiving FMT were (297 ± 11 and 81 ± 16, p < .0001) and for nonresponders were (270 ± 17 and 291 ± 16, p = .15), . In the total group of IBS patients, the densities of almost all of the enteroendocrine cells in the sigmoid colon significantly increased after FMT compared to before FMT except for densities of serotonin immunoreactive cells (p = .11), and CgA and somatostatin immunoreactive cells that did not reach the cut-off level of significance (p = .06 and .051, respectively), . When comparing the densities of the enteroendocrine cells in the total group of responders after FMT to before FMT, both the densities of somatostatin (Supplementary figure 1) and enteroglucagon (oxyntomodulin) immunoreactive cells increased (p = .023 and .056, respectively) though enteroglucagon (oxyntomodulin) immunoreactive cells densities didn’t reach the cut-off level of significance, . No significant changes were shown in the densities of the enteroendocrine cells in the total group of non-responders, . Moreover, the total group of IBS patients who received donor-FMT (n = 16) showed significant increases after FMT compared to before FMT only in the immunoreactive cells densities (mean ± SEM) of PYY (77.1 ± 7, 54.8 ± 5.9, p = .04, respectively) and enteroglucagon (53.7 ± 6.1, 35.3 ± 4.5, p = .035, respectively). No statistically significant change was found in the densities of the enteroendocrine cells in the total group of IBS patients who received placebo FMT. Furthermore, the subgroup of IBS responders who received donor-FMT showed a significant increase only in the densities of somatostatin immunoreactive cells after FMT compared to before FMT (37 ± 7, 21 ± 4, p = .038, respectively).
Figure 1. IBS-SSS scores for (A) responders and (B) nonresponders IBS patients after receiving fecal microbiota transplantation.
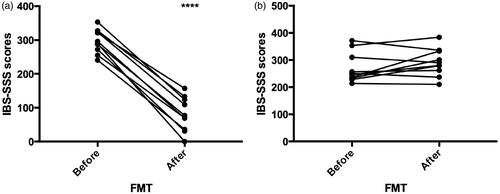
Table 1. The densities of the enteroendocrine cells in the sigmoid colon of the total group of IBS patients before and after transplantation.
Table 2. The densities of the enteroendocrine cells in the sigmoid colon of the total groups of IBS responder and non-responder patients before and after transplantation.
Discussion
This study is connected to the REFIT study, where the treatment of IBS with FMT is investigated in a double-blinded placebo-controlled trial and 10 responders and 10 nonresponders consented to a new biopsy one year after FMT. The densities of the different enteroendocrine cells have shown changes in the total IBS group and specifically in those who have received donor-FMT. Neither the group of patients that have received placebo FMT nor nonresponders group has shown any statistically significant changes.
Patients with IBS have altered densities of enteroendocrine cells throughout the GI tract [Citation10,Citation24–27] that tend to change following manipulations of diet [Citation7,Citation11,Citation28–32] and of the gut microbiota [Citation20,Citation21]. Previous publication shows that dietary manipulation also affects the gut microbiota [Citation33]. The dynamic changes that occur to the enteroendocrine cells following their interactions with the surrounding stimuli stimulate their release of the gut hormones to regulate the different functions of the GI tract [Citation5,Citation34]. Chromogranin A is a common marker for the enteroendocrine cells [Citation35–38]. Serotonin modulates the GI visceral sensitivity [Citation24,Citation39–42] stimulates large intestinal motility, and accelerates intestinal transit time [Citation39–47]. Somatostatin inhibits intestinal contraction [Citation34,Citation48], and stimulates the absorption of water and electrolytes [Citation34]. PYY stimulates the absorption of water and electrolytes and is a major regulator of the ‘ileal brake’ [Citation24,Citation49]. Enteroglucagon (oxyntomodulin) inhibits gastric and pancreatic secretions and reduces gastric motility [Citation48]. Pancreatic polypeptide inhibits pancreatic secretion; relaxes gall bladder; and stimulates motility of stomach and small intestine [Citation48].
Previous publications have shown that the densities of several types of enteroendocrine cells in the upper part of the GI tract; namely duodenum, have changed after receiving donor-FMT [Citation20,Citation21]. In the current study, the densities of almost all of the enteroendocrine cells types in the sigmoid colon of IBS patients have changed one year after receiving donor-FMT, namely somatostatin, PYY, enteroglucagon (oxyntomodulin), PP and PYY. Taking into consideration the different roles of these gut hormones; being especially involved in the motility of the different parts of the GI tract, one may speculate that these significant changes may be related to the improvement in the symptoms of IBS in the majority of the responders group. The densities of CgA and somatostatin cells have also changed in IBS patients who received donor-FMT, but these changes have not reached the cut-off level of significance. This may be a type-II error due to the small sample size of the present study. The changes in the densities of the enteroendocrine cells in the placebo FMT group may also be a type-II error. As stated earlier, intestinal luminal stimuli may affect the enteroendocrine cells such as changes occurring to the transplants’ microbiota during the preparation process and dietary changes. In the previous study [Citation22], dietary changes were monitored for up to three months after FMT, though changes were insignificant at that time point, one cannot exclude any dietary changes occurring by the time the second colonoscopy was performed one year after FMT. Nevertheless, it is interesting to see that mainly the group of IBS patients that received donor FMT has shown significant changes in the densities of several types of enteroendocrine cells that persisted for one year after FMT.
The present study has several strengths and limitations. The strength points of the study are placebo-controlled, having repeated measures before and after FMT, IBS-SSS scores change parallel to changes in the densities of the enteroendocrine cells and the blinding of the investigator to the identity of the slides while performing the quantification of the enteroendocrine cells using a validated method [Citation23] that can detect differences even in small sample size. The limitations of the study were the small sample size, the explorative nature of this study, and probably a low statistical power. Although the sample size was small a significant change in the densities of the colonic enteroendocrine cells could be detected.
Conclusion
This study shows that the densities of enteroendocrine cells in the colon change following donor FMT. This observation emphasizes the interaction between gut microbiota and enterendocrine cells following manipulation of the gut microbiota. The mechanisms behind these interactions remain to be investigated. Further studies with larger cohorts are recommended to confirm our present results.
Supplemental Material
Download MS Word (1.5 MB)Acknowledgement
The authors thank Dr. Peter Holger Johnsen, Dr. Rasmus Goll and Dr. Per Christian Valle for their support to the project but recruiting the patients and performing the colonoscopy procedures with biopsy sampling from the sigmoid colon.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Moran GW, Leslie FC, Levison SE, et al. Enteroendocrine cells: neglected players in gastrointestinal disorders? Therap Adv Gastroenterol. 2008;1(1):51–60.
- Moran-Ramos S, Tovar AR, Torres N. Diet: friend or foe of enteroendocrine cells-how it interacts with enteroendocrine cells. Adv Nutr. 2012;3(1):8–20.
- Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of 'taste' in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15(1):73–78.
- El-Salhy M, Mazzawi T, Hausken T, et al. Interaction between diet and gastrointestinal endocrine cells. Biomed Rep. 2016;4(6):651–656.
- Mazzawi T, El-Salhy M. Effect of diet and individual dietary guidance on gastrointestinal endocrine cells in patients with irritable bowel syndrome (review). Int J Mol Med. 2017;40(4):943–952.
- Bauer PV, Hamr SC, Duca FA. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell Mol Life Sci. 2016;73(4):737–755.
- Mazzawi T, Hausken T, Gundersen D, et al. Dietary guidance normalizes large intestinal endocrine cell densities in patients with irritable bowel syndrome. Eur J Clin Nutr. 2016;70(2):175–181.
- El-Salhy M, Grimelius L, Wilander E, et al. Immunocytochemical identification of polypeptide YY (PYY) cells in the human gastrointestinal tract. Histochemistry. 1983;77(1):15–23.
- Spangeus A, Forsgren S, el-Salhy M. Does diabetic state affect co-localization of peptide YY and enteroglucagon in colonic endocrine cells? Histol Histopathol. 2000;15(1):37–41.
- El-Salhy M, Gundersen D, Ostgaard H, et al. Low densities of serotonin and peptide YY cells in the Colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57(4):873–878.
- Mazzawi T, Gundersen D, Hausken T, et al. Increased chromogranin a cell density in the large intestine of patients with irritable bowel syndrome after receiving dietary guidance. Gastroenterol Res Pract. 2015;2015:823897.
- Hong SN, Rhee PL. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J Gastroenterol. 2014;20(10):2470–2481.
- Carroccio A, Brusca I, Mansueto P, et al. A cytologic assay for diagnosis of food hypersensitivity in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2010;8(3):254–260.
- Ohman L, Simren M. Intestinal microbiota and its role in irritable bowel syndrome (IBS). Curr Gastroenterol Rep. 2013;15(5):323.
- Kassinen A, Krogius-Kurikka L, Makivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133(1):24–33.
- Greiner TU, Backhed F. Microbial regulation of GLP-1 and L-cell biology. Mol Metab. 2016;5(9):753–758.
- Everard A, Lazarevic V, Derrien M, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–2786.
- Neyrinck AM, Van Hee VF, Piront N, et al. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes. 2012;2:e28.
- Mazzawi T, Lied GA, Sangnes DA, et al. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS One. 2018;13(11):e0194904.
- Mazzawi T, Eikrem Ø, Lied GA, et al. Abnormal uroguanylin immunoreactive cells density in the duodenum of patients with Diarrhea-Predominant irritable bowel syndrome changes following fecal microbiota transplantation. Gastroenterol Res Pract. 2020;2020:3520686.
- Mazzawi T, El-Salhy M, Lied GA, et al. The effects of fecal microbiota transplantation on the symptoms and the duodenal neurogenin 3, musashi 1, and enteroendocrine cells in patients with Diarrhea-Predominant irritable bowel syndrome. Front Cell Infect Microbiol. 2021;11:524851.
- Johnsen PH, Hilpusch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-Centre trial. Lancet Gastroenterol Hepatol. 2018;3(1):17–24.
- el-Salhy M, Sandstrom O, Nasstrom E, et al. Application of computer image analysis in endocrine cell quantification. Histochem J. 1997;29(3):249–256.
- El-Salhy M, Gilja OH, Gundersen D, et al. Endocrine cells in the ileum of patients with irritable bowel syndrome. WJG. 2014;20(9):2383–2391.
- El-Salhy M, Gilja OH, Gundersen D, et al. Endocrine cells in the oxyntic mucosa of the stomach in patients with irritable bowel syndrome. WJGE. 2014;6(5):176–185.
- El-Salhy M, Gundersen D, Hatlebakk JG, et al. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. 2014;188:60–65.
- El-Salhy M, Hatlebakk JG, Hausken T. Reduction in duodenal endocrine cells in irritable bowel syndrome is associated with stem cell abnormalities. World J Gastroenterol. 2015;21(32):9577–9587.
- Mazzawi T, El-Salhy M. Dietary guidance and ileal enteroendocrine cells in patients with irritable bowel syndrome. Exp Ther Med. 2016;12(3):1398–1404.
- Mazzawi T, El-Salhy M. Changes in small intestinal chromogranin A-immunoreactive cell densities in patients with irritable bowel syndrome after receiving dietary guidance. Int J Mol Med. 2016;37(5):1247–1253.
- Mazzawi T, El-Salhy M. Changes in duodenal enteroendocrine cells in patients with irritable bowel syndrome following dietary guidance. Exp Biol Med (Maywood)). 2017;242(13):1355–1362.
- Mazzawi T, Gundersen D, Hausken T, et al. Increased gastric chromogranin a cell density following changes to diets of patients with irritable bowel syndrome. Mol Med Rep. 2014;10(5):2322–2336.
- Mazzawi T, Hausken T, Gundersen D, et al. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur J Clin Nutr. 2015;69(4):519–524.
- Hustoft TN, Hausken T, Ystad SO, et al. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol Motility. 2017;29(4). DOI:https://doi.org/10.1111/nmo.12969.
- El-Salhy M, Gilja OH, Gundersen D, et al. Interaction between ingested nutrients and gut endocrine cells in patients with irritable bowel syndrome (review). Int J Mol Med. 2014;34(2):363–371.
- Wiedenmann B, Huttner WB. Synaptophysin and chromogranins/secretogranins-widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(2):95–121.
- Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348(12):1134–1149.
- El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin a as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45(12):1435–1439.
- Deftos LJ. Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev. 1991;12(2):181–187.
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132(1):397–414.
- Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol. 2003;3(6):600–607.
- Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20(1):14–21.
- Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc. 2012;123:268–280. discussion 80.
- Tack JF, Janssens J, Vantrappen G, et al. Actions of 5-hydroxytryptamine on myenteric neurons in guinea pig gastric antrum. Am J Physiol. 1992;263(6 Pt 1):G838–46.
- Michel K, Sann H, Schaaf C, et al. Subpopulations of gastric myenteric neurons are differentially activated via distinct serotonin receptors: projection, neurochemical coding, and functional implications. J Neurosci. 1997;17(20):8009–8017.
- Tack J, Coulie B, Wilmer A, et al. Influence of sumatriptan on gastric fundus tone and on the perception of gastric distension in man. Gut. 2000;46(4):468–473.
- Gershon MD. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13(Suppl 2):15–30.
- Gershon MD, Wade PR, Kirchgessner AL, et al. 5-HT receptor subtypes outside the central nervous system. Roles in the physiology of the gut. Neuropsychopharmacology. 1990;3(5-6):385–395.
- El-Salhy M, Gundersen D, Gilja OH, et al. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20(2):384–400.
- El-Salhy M, Mazzawi T, Gundersen D, et al. The role of peptide YY in gastrointestinal diseases and disorders (review). Int J Mol Med. 2013;31(2):275–282.
