Abstract
Background/aims
Originator-adalimumab, an established treatment for patients with Crohn’s disease (CD), showed no difference in efficacy or adverse events versus adalimumab biosimilar SB5 (SB5-adalimumab) over 10 weeks (W) of treatment. To understand the long-term effectiveness of SB5-adalimumab in CD, patients switched from originator-adalimumab to SB5-adalimumab were compared with patients remaining on originator-adalimumab over 104 W.
Methods
Data on patients aged ≥18 years, diagnosed with CD and treated at ISCARE, were collected prospectively from July 2018 to January 2021. Primary outcome: clinical disease activity at W52, measured by Harvey-Bradshaw index (HBI). Secondary outcomes: C-reactive protein (CRP), faecal calprotectin (FC) and adalimumab concentrations at W10, 26, 52 and 104, and treatment persistence. To ensure comparable cohorts, patients were propensity score (PS)-matched for age, gender and disease activity.
Results
After matching, 54 patients remained per cohort. At W52, mean (SD) HBI score was 3.2 (2.5) for originator-adalimumab and 4.0 [3.6] for SB5-adalimumab (difference [95% CI] −0.78 [−2.8, 1.3]; n = 18/cohort); no clinically meaningful differences in CRP, FC or drug concentrations were noted. Kaplan–Meier’s estimates (95% CI) of remaining on treatment were originator-adalimumab: 0.870 (0.785–0.965) versus SB5-adalimumab: 0.648 (0.533–0.789) at W52 and significantly lower for SB5-adalimumab versus originator-adalimumab (p < .001) over 104 W. Local skin reaction events/pain was the main reason for treatment discontinuation in the SB5-adalimumab cohort (n = 20/54 [37%]).
Conclusions
These long-term results of CD patients receiving originator-adalimumab or following nonmedical switch to SB5-adalimumab show similar therapeutic effects on clinical disease activity, biological parameters and pharmacokinetic profile in both cohorts from 52 to 104 W. A separation in persistence was observed beyond W26, mainly due to differences in local reactions at the injection site.
Keywords:
Introduction
Adalimumab, a recombinant, fully human, immunoglobulin G1 monoclonal antibody specific for human tumour necrosis factor-α, was first licenced in 2002 for the treatment of rheumatoid arthritis and has subsequently been approved to treat several autoimmune conditions, including inflammatory bowel disease (IBD). Since the patent expiry of originator-adalimumab in the European Union, several biosimilar medications have become available. SB5-adalimumab (ImraldiTM; Biogen, Cambridge, MA/Samsung Bioepis, Incheon, South Korea) is one such biosimilar [Citation1,Citation2]. The abbreviated approval pathway for biosimilars as specified by the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) provides the opportunity for biosimilars such as SB5-adalimumab to be cost-effective alternatives to their reference products and to improve access to effective treatment for patients for whom they are indicated [Citation3].
SB5-adalimumab fulfilled all the requirements for biosimilarity with reference adalimumab, as per guidance from the EMA [Citation4] and FDA [Citation5] with respect to structural, biochemical, physicochemical and biological properties [Citation6,Citation7]; pharmacokinetics [Citation8,Citation9]; immunogenicity [Citation8,Citation9]; and clinical safety and effectiveness [Citation9] and is approved by the EMA and FDA for all eligible indications of reference adalimumab [Citation10,Citation11] (Humira®; AbbVie, Lake Bluff, IL).
The evidence for similar efficacy and safety for SB5-adalimumab versus originator-adalimumab comes from a phase 3 randomized controlled trial in patients with rheumatoid arthritis [Citation9]. As such, data of SB5-adalimumab in patients with IBD are limited. A retrospective analysis of SB5-adalimumab in patients with IBD in the Czech Registry of IBD Patients on Biological and Innovative Therapy (CREdIT registry) found that the switch from originator-adalimumab to SB5-adalimumab had no short-term impact on treatment efficacy and no influence on drug serum concentrations nor on the development of anti-drug antibodies [Citation12]. There was also no difference in adverse events between SB5-adalimumab and originator-adalimumab during the 10-week follow-up period [Citation12]. While the 10-week results provide valuable information from routine clinical practice on the effectiveness, safety and immunogenicity of SB5-adalimumab, long-term data on these parameters are required to provide clinicians with knowledge on the long-term utility of SB5-adalimumab.
The primary objective of the current study was to assess the effectiveness of SB5-adalimumab in patients with Crohn’s disease (CD) after switch from originator-adalimumab over 52 weeks. Clinical and laboratory data on the cohort of patients who were switched to SB5-adalimumab were compared with data from a control cohort of patients who remained on originator-adalimumab. Additional objectives included clinical disease activity and laboratory markers of inflammation up to 104 weeks after the switch, treatment persistence and occurrence of adverse events leading to discontinuation of therapy.
Materials and methods
ISCARE
The Clinical and Research Centre for IBD, ISCARE a.s. (Prague, Czech Republic), specializes in the diagnosis and treatment of patients with CD and ulcerative colitis. For patients with IBD treated with biologic therapies during routine medical practice, patient data including demographics; disease characteristics and activity; selected laboratory parameters; and data on treatment, dose and adverse events are prospectively entered into the CREdIT registry at each outpatient visit. The CREdIT project, initiated in 2016, is a multicentre registry of patients with IBD treated with biologic or innovative therapies in the Czech Republic. The aim of this registry is to monitor the number of IBD patients treated with these therapies in order to assess efficacy, safety and therapeutic regimens and monitor the duration of biologic and innovative therapy.
Study population
This study included data for all adult patients in the CREdIT registry who were diagnosed with CD and treated with originator-adalimumab. Patients were required to have been on maintenance treatment with originator-adalimumab for ≥3 months prior to week 0 (baseline), at which time they either were switched from originator-adalimumab to SB5-adalimumab for nonmedical reasons or continued with the originator drug. The nonmedical switch was not based on any prespecified criteria, and the decision to switch was made by the treating physician. Baseline dates spanned 18 July 2018 to 12 February 2019, with most switches occurring between November 2018 and December 2018. The study observation period ended in January 2021. The cohort of patients treated with originator-adalimumab over the assessment period served as an internal comparator against the switched cohort. In both cohorts, patients received standard maintenance treatment with 40 mg subcutaneous adalimumab every other week. Patients who expressed a wish to switch back to originator-adalimumab, in most cases were permitted to do so.
Study outcome assessments
Primary effectiveness measure of clinical disease activity at week 52 in both cohorts was assessed using the Harvey-Bradshaw index (HBI). Secondary outcome measures included HBI, C-reactive protein (CRP), faecal calprotectin (FC) and adalimumab concentration at weeks 10, 26, 52, 78 and 104; adverse events leading to therapy withdrawal; and treatment persistence. Reasons for treatment discontinuation and subsequent biological therapies during the 104-week follow-up were also assessed. Actual time of data collection may vary within a 4–6-week window around the predefined timepoints (i.e., weeks 26, 52, 78 and 104).
HBI scores were calculated by specialized IBD nurses during patient visits at the outpatient department. CRP serum levels were detected by immunophelometry and FC concentrations were detected by fluoroimmunoanalysis. Adalimumab serum concentrations were measured using the ImmunoGuide® assay based on tumour necrosis factor-α as a target antigen (AybayTech Biotechnology, Ankara, Turkey).
Matching and statistical analysis
No formal sample size was calculated; all available data were already captured on the CREdIT registry and were utilized for study analyses [Citation13]. Patients from the SB5-adalimumab switched cohort were pairwise matched to patients from the originator-adalimumab cohort. Propensity score (PS) matching using a greedy nearest matching algorithm with a specified calliper of 0.2 (of the standard deviation of the logit of PS) was used to match each pair 1:1 for confounders of age, gender and disease activity (Supplementary Table 1) [Citation14]. If one member of the pair withdrew from study/treatment, the other of the pair was removed from analysis.
Propensity scores were estimated using multiple logistic regression with the treatment as the dependent variable and the baseline confounders (age, gender and disease activity) as independent variables. The quality of model was verified using residual analysis, deviance, Akaike information criterion, Hosmer–Lemeshow’s statistic and prediction accuracy (C-statistic). The C-statistic is a measure of balance in matched data and ranges from 0.5 to 1.0, with the minimum value indicating the PS model is perfectly balanced and has no ability to discriminate between cohorts after matching. The 95% CI (profile likelihood based) for odds ratios are reported. Jitter plot and histogram were used to visualize PS (Supplementary Figures 1 and 2). Pre- and postmatching balance in the baseline confounders were assessed using standardized differences (SDs) for each confounder individually and omnibus chi-square test for all the confounders simultaneously and C-statistic.
The normal distribution of the continuous variables was investigated by Shapiro–Wilk’s test and normal quantile plot. If data were not normally distributed, the naturally log-transformed data were used in parametric tests to reduce distribution skewness (for CRP and FC). The homogeneity of variances of continuous variables was assessed by Levene’s test. Student’s t/Welch’s t, Wilcoxon’s rank sum and Pearson’s chi-square/Fisher’s tests were used to compare unmatched baseline characteristics by cohort; whereas in the matched data, paired t, Wilcoxon’s signed rank and McNemar’s chi-square tests were used to compare baseline and follow-up characteristics for continuous and categorical variables, respectively.
Kaplan–Meier’s method and a stratified log-rank test were used for estimating the probability of remaining on treatment and testing the difference between treatment cohorts. Continuous variables are reported as mean with 95% CI, SD, median and 25th and 75th percentile (Q1, Q3). Categorical variables are summarized as absolute frequencies and proportions (percentages). The data were analyzed using R software (version 4.0.3) (R Foundation for Statistical Computing, Vienna, Austria).
Ethics statement
Data from the CREdIT registry were analyzed in accordance with the ethical principles of the Declaration of Helsinki. The project was approved by a local ISCARE ethics committee including the informed consent. Every patient who was switched from original to biosimilar SB5 was informed of the details, without any restraint, and accepted participation in this project.
Results
Demographic and baseline disease characteristics
Of 207 IBD patients treated with the originator-adalimumab and fulfilling the inclusion criteria, 175 (84.5%) had CD (including one IBD-unknown). Seventy-four of those patients were switched to SB5-adalimumab and followed up for 104 weeks between November 2018 and January 2021, whilst the remaining 101 (57.7%) continued treatment with originator-adalimumab. Excluding patients with insufficient data, 129 patients with CD (56 in the SB5-adalimimab cohort and 73 in the originator-adalimumab cohort) were available for analysis. Of these, 54 patients from each cohort could be matched pairwise for age, gender and disease activity (HBI, CRP, FC) and included in the analysis ().
Figure 1. Flowchart of the patient cohorts included in the study. an = 73 with CD, n = 1 with unclassified IBD; the patient with unclassified IBD was clinically comparable to a patient with confirmed IBD CD and did not bias the clinical outcome results. bThe missing data were mainly faecal calprotectin (n = 42). However, missing values were not significantly dependent on therapy or other variables at study entry. CD: Crohn’s disease; IBD: inflammatory bowel disease.
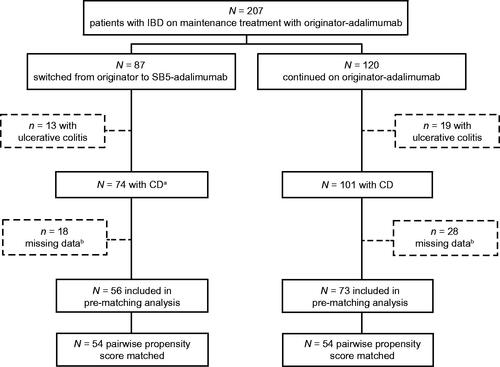
Before PS matching, the following baseline characteristics differed between originator-adalimumab and SB5-adalimumab cohorts: male gender (39.7% vs. 60.7%, SD = 0.43), proportion of patients treated with immunosuppressive therapy (42.5% vs. 25.0%, SD = 0.38) and mean (SD) FC (522 [1035] vs. 210 [298] μg/g, SD = 0.41) (). After PS matching (), baseline characteristics of the postmatched populations showed no evidence of a difference as indicated by smaller SD (i.e., <0.2) and C-statistic value of 0.578. The only variables with an SD >0.2 after PS matching were immunosuppressive therapy, Montreal classification: location, Montreal classification: behaviour, and CRP ≥5 mg/L.
Table 1. Demographic and baseline clinical characteristics: prematched originator-adalimumab and SB5-adalimumab cohorts.
Table 2. Demographic and baseline clinical characteristics: propensity score-matched originator-adalimumab and SB5-adalimumab cohorts.
Disease activity in the matched cohorts
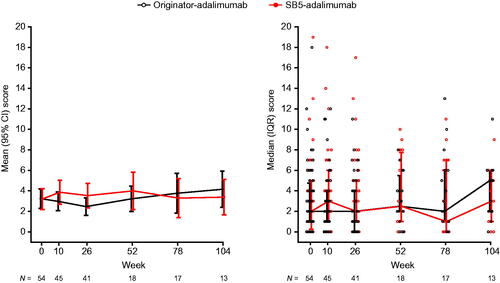
At week 0 (baseline), mean (SD) HBI score was similar in the originator-adalimumab (3.2 [3.5]) and SB5-adalimumab (3.2 [3.7]) cohorts (difference [95% CI] between cohorts 0.04 [−1.3, 1.4]; n = 54) ( and Supplementary Table 2). At week 26, mean (SD) HBI score was 2.5 (2.7) for originator-adalimumab and 3.5 (3.8) for SB5-adalimumab (difference [95% CI] between cohorts −1.1 [−2.6, 0.5]; n = 41). At week 52, mean (SD) HBI score was 3.2 (2.5) for originator-adalimumab and 4.0 (3.6) for SB5-adalimumab (difference [95% CI] between cohorts −0.8 [−2.8, 1.3]; n = 18). Mean HBI scores in both treatment cohorts were <5 at each timepoint, and the differences between treatment cohorts were not clinically meaningful. Sample size for weeks 78 and 104 are small and therefore only presented in the figures and in Supplementary Table 2.
Figure 2. The Harvey-Bradshaw index disease scores over time (propensity score-matched population). IQR: interquartile range.
Week 0 (baseline) mean (SD) CRP levels were similar in the matched cohorts: 4.5 (6.1) in the originator-adalimumab cohort and 3.8 (10.5) in the SB5-adalimumab cohort (difference [95% CI] 0.7 [−2.8, 4.1]). At week 26, mean (SD) CRP levels were 3.0 (2.9) in the originator-adalimumab cohort and 4.8 (6.7) in SB5-adalimumab patients (difference [95% CI] −1.7 [−3.6, 0.2]). At week 52, mean (SD) CRP levels were 2.9 (3.7) in the originator-adalimumab cohort and 2.8 (2.4) in SB5-adalimumab patients (difference [95% CI] 0.1 [−1.6, 1.7]). As the data were not normally distributed, a log transformation was applied to the data. In both cases, no clinically meaningful differences were observed between the cohorts through week 52 ( and Supplementary Table 3).
Figure 3. C-reactive protein levels over time (propensity score-matched population). The left graph shows mean with 95% CI of log-transformed data and the right graph shows the median with IQR. IQR: interquartile range.
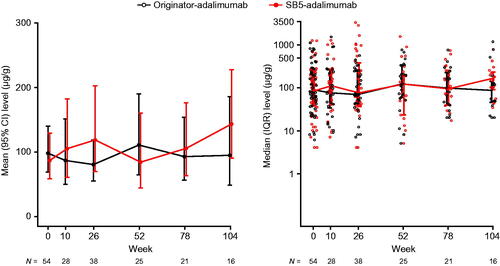
Week 0 (baseline) mean (SD) FC levels were similar in the matched cohorts: 203 (251) in the originator-adalimumab cohort and 214 (302) in the SB5-adalimumab cohort (difference [95% CI] −10 [−105, 84]). At week 26, mean (SD) FC levels were 140 (139) in the originator-adalimumab cohort and 410 (743) in SB5-adalimumab patients (significant difference [95% CI] −271 [−504, −36]). At week 52, mean (SD) FC levels were 201 (201) in the originator-adalimumab cohort and 224 (350) in SB5-adalimumab patients (difference [95% CI] −24 [−177, 130]). As the data were not normally distributed, a log transformation was applied to the data. No clinically meaningful differences were observed between the cohorts through week 52 ( and Supplementary Table 4).
Figure 4. Faecal calprotectin levels over time (propensity score-matched population). The left graph shows mean with 95% CI of logtransformed data and the right graph shows the median with IQR. IQR: interquartile range.
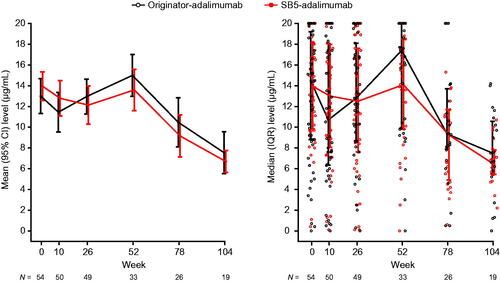
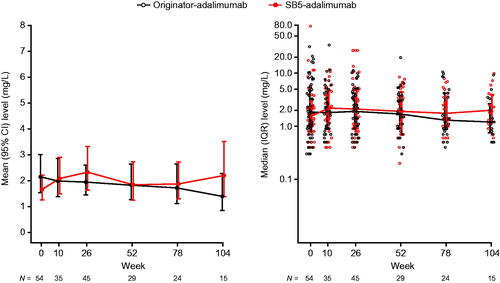
Week 0 (baseline) mean (SD) drug concentrations were similar in the matched cohorts: 12.9 (6.2) in the originator-adalimumab cohort and 13.9 (5.0) in the SB5-adalimumab cohort (difference [95% CI] between cohorts −1.0 [−3.3, 1.3]). At week 26, mean (SD) drug concentrations were 12.9 (5.8) for originator-adalimumab and 12.1 (6.4) for SB5-adalimumab (difference [95% CI] between cohorts 0.8 [−1.7, 3.4]). At week 52, mean (SD) drug concentrations were 14.9 (5.7) for originator-adalimumab and 13.5 (5.6) for SB5-adalimumab (difference [95% CI] between cohorts 1.4 [−1.0, 3.8]). No clinically meaningful differences were noted between the cohorts to week 52 ( and Supplementary Table 5).
Treatment persistence
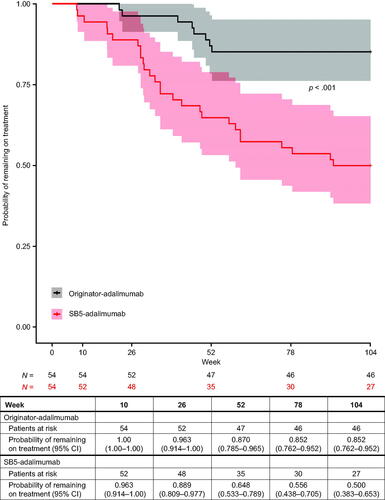
Kaplan–Meier’s estimates (95% CI) of treatment persistence for originator-adalimumab versus SB5-adalimumab were 0.96 (0.91–1.00) versus 0.89 (0.81–0.98) at week 26, 0.87 (0.79–0.97) versus 0.65 (0.53–0.79) at week 52 and 0.85 (0.76–0.95) versus 0.50 (0.38–0.65) at week 104. Over the entire follow-up period (up to 104 weeks), persistence on SB5-adalimumab therapy was statistically significantly lower versus originator-adalimumab (stratified log-rank p< .001) ().
Reasons for treatment discontinuation
Reasons reported for discontinuation of therapy include: adverse event, 23 (43%) and three (6%) patients in the SB5 and originator cohorts, respectively; loss of response, two (4%) and three (6%) patients in the SB5 and originator cohorts, respectively; and in both cohorts, one patient (2%) withdrew due to safety concerns and one (2%) was lost to follow-up ().
Table 3. Reasons for treatment discontinuation in total and propensity score-matched population.
Adverse events leading to treatment discontinuation included: local skin reaction or pain in 16 (30%) of the SB5 cohort; skin events in four (7%) and one (2%) of the SB5 and originator cohort, respectively; allergy in one patient in the originator cohort; infection in one patient in each cohort. There was no further information for two patients in the SB5 cohort ().
Discussion
SB5-adalimimab and originator-adalimumab have comparable pharmacokinetics, immunogenicity, biological activity and quality attributes [Citation6–9]. Evidence of long-term clinical safety and effectiveness for SB5-adalimumab compared with originator-adalimumab in patients with rheumatoid arthritis is strong [Citation9,Citation15], while evidence in patients with IBD is more limited by sample size or reported treatment duration [Citation16–20]. Long-term safety and efficacy/effectiveness data with biosimilars are of importance to provide clinicians with confidence in using biosimilars in IBD, as well as in rheumatology and dermatology indications.
In this study, a cohort of patients from the CREdIT registry treated with originator-adalimumab who were switched to SB5-adalimumab for nonmedical, cost-associated reasons was compared with a cohort of patients who continued to be treated with originator-adalimumab. The cohorts were PS matched to ensure the comparability of the treatment cohorts and to mitigate the risk of a selection bias.
No clinically meaningful differences were noted between the adalimumab treatment cohorts for HBI scores, CRP levels or FC levels from baseline through week 52, indicating similar efficacy for IBD treatment in both cohorts. Consistent with results from this study, results from an interim analysis of the PROPER study indicated no clinically meaningful changes in a cohort of patients with IBD whose treatment was switched from originator-adalimumab to SB5, followed for 48 weeks post-switch [Citation21]. A previous 1-year study of patients with IBD who started with SB5 as their first adalimumab treatment and a small cohort of patients who switched from originator-adalimumab to SB5-adalimumab had similar remission rates between the two cohorts [Citation22].
Importantly, drug concentrations in this study did not differ between treatment cohorts from baseline through week 52, indicating similar biological availability in both cohorts of patients. Drug concentrations in both cohorts followed an unusual U-shaped pattern rather than the expected constant levels, with a dip near weeks 10 and 26. This may be explained by a change in the laboratory conducting the trough assays during the follow-up of both treatment cohorts. As the fluctuations were similar for both therapies, a systemic change in the assay procedure is probably the cause, rather than true variability. There were no differences in adalimumab serum concentrations from baseline, 3 months and 6 months in either SB5-treated cohort after switching [Citation22].
Treatment persistence was similar up to week 26, after which the Kaplan–Meier curves separate. Persistence on SB5-adalimumab was lower than on originator-adalimumab from week 52 to week 104. The reason for this separation beyond week 26 is not certain, although the main reason for early treatment discontinuation of SB5-adalimumab was reported to be local injection reaction/pain, observed in 20 out of 54 patients (37%). At baseline (week 0), patients received a 2–3-month supply of the study treatment and returned for a checkin at week 10 and week 26. The week 26 visit focused on the control of the patients’ disease and their satisfaction with the medication. Specifically, patients were asked about their desire to remain on or switch treatment at the week 26 appointment, which may have resulted in an increase in treatment-modifying decisions at this timepoint. Perceived differences in injection experience between the two products may have contributed to patients opting to change therapy at week 26.
Although there is no direct evidence for nocebo effect, a nocebo effect could be considered as a potential factor, as has been noted in connection with biosimilars. A nocebo effect is the opposite of a placebo effect, whereby patient expectations lead to a negative outcome of a medical treatment, in the absence of clinical evidence [Citation23]. Nocebo effects may occur in biosimilars, requiring a carefully managed multidisciplinary effort involving proper patient education and healthcare worker training [Citation24]. The change from originator to biosimilar in this study was a cost-driven, nonmedical switch, which may have suggested to patients who were insufficiently educated on biosimilars that the biosimilar was not as effective as the originator. Nonmedical switch patients may represent a more sensitive and fragile population for nocebo effects. It is possible that the nocebo effect was a part of the reason for back switch, in the sense that suboptimal education of medical staff and patients around use of biosimilars, was a major driver for therapy discontinuation in patients with SB5-adalimumab after week 26. This may be because not every doctor was convinced about drug interchangeability and their reluctance might have seemed suspicious to some patients. However, we believe that the main driver for the back switch from SB5-adalimumab to originator-adalimumab was probably local skin reaction. In most cases, there was not a local skin reaction, but instead painful application, for which we do not have any objective data and it is instead a subjective feeling. Despite the differences in the treatment persistence, there were no differences in more objective clinical parameters, such as HBI, CRP and FC levels, or other significant side effects seen in the switched cohort over the whole study duration. Instead, the reasons for treatment discontinuation were largely patient-reported or patient decisions.
The mode of administration of adalimumab was different between the two treatment cohorts, with most patients receiving the originator via prefilled syringe injection and receiving SB5 via pen. This may have also had some impact on treatment persistence; 37% of patients treated with SB5-adalimumab withdrew due to skin irritation and local reactions/pain, compared with 2% in the originator-adalimumab cohort, and yet local skin reactions are typically very rare (3%) [Citation9]. Most of the SB5-adalimumab-treated patients with these adverse events switched back to originator-adalimumab.
Several limitations should be considered when interpreting the study results, inherent in the retrospective, observational nature of the data extracted from the CREdIT registry database. Missing values limited some analyses. The total sample size is relatively small. No formal sample size was calculated, given that available data were already captured on the CREdIT registry; therefore, the sample size may not be sufficiently large to reveal all differences between the treatment cohorts.
Conclusions
The results from this long-term study of CD patients following nonmedical switch from originator-adalimumab to SB5-adalimumab demonstrate that biosimilar SB5 had the same therapeutic effects in terms of clinical disease activity, biological parameters and pharmacokinetics profile at week 52 as the originator-adalimumab. Persistence on SB5-adalimumab therapy was significantly lower than on originator-adalimumab. A clear separation in persistence is observed beyond week 26. This difference appears to be due to reported local skin reactions/pain; reporting may have been influenced by perceived differences between originator and biosimilar products. A multidisciplinary patient and healthcare provider education effort may be needed to counteract the decrease in persistence. Focus on reassurance of quality, safety and effectiveness of biosimilars, along with proper training in use of different devices, would be expected to reduce nocebo leading to discontinuation of effective therapies. This would lead to full realization of the potential cost and access advantages to healthcare services of using biosimilars for treating patients with IBD.
Authors contributions
Milan Lukas contributed to the study design, data analysis, investigator or patient recruitment, and interpretation and writing of the first draft. M. Kolar contributed to the study design, data analysis, investigator or patient recruitment, data collection and interpretation and writing of the first draft. J. Reissigova contributed to statistical analysis, and interpretation and writing of the first draft. D. Duricova contributed to the study design, data analysis and investigator or patient recruitment. N. Machkova and V. Hruba contributed to the investigator or patient recruitment, and data collection. Martin Lukas contributed to the study design, data analysis, investigator or patient recruitment, and interpretation and writing of the first draft. M. Vasatko contributed to the investigator or patient recruitment, and data collection. J. Jirsa and K. Pudilova contributed to the data collection. K. Malickova contributed to the study design, data analysis and data collection.
| Abbreviations | ||
| CD | = | Crohn’s disease |
| CI | = | confidence interval |
| CRP | = | C-reactive protein |
| EMA | = | European Medicines Agency |
| FC | = | faecal calprotectin |
| FDA | = | US Food and Drug Administration |
| HBI | = | Harvey-Bradshaw index |
| IBD | = | inflammatory bowel disease |
| IQR | = | interquartile range |
| PS | = | propensity score |
| SD | = | standardized difference |
Supplemental Material
Download PDF (514 KB)Acknowledgements
The authors thank the ISCARE study participants. Writing and editorial support for the preparation of this manuscript was provided by Excel Medical Affairs (Fairfield, CT); funding was provided by Biogen International GmbH (Baar, CH). Biogen provided funding for medical writing support in the development of this paper; Karen Spach, PhD, wrote the first draft of the manuscript based on input from authors and Nathaniel Hoover from Excel Medical Affairs (Fairfield, CT) copyedited and styled the manuscript per journal requirements. Biogen reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper and provided their final approval of all content for submission.
Disclosure statement
Milan Lukas reports receiving support for consultancies/advisory panels from Pfizer, Takeda and Janssen and for speakers’ bureaus from Celltrion. M. Kolar, J. Reissigova, D. Duricova, N. Machkova, V. Hruba, Martin Lukas, M. Vasatko, J. Jirsa, K. Pudilova and K. Malickova report nothing to disclose.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- European Medicines Agency. Summary of product characteristics. Imraldi 40 mg solution for injection in pre-filled syringe; 2021 [cited 2021 Jul 20]. Available from: https://www.ema.europa.eu/en/documents/product-information/imraldi-epar-product-information_en.pdf
- Samsung Bioepis. Highlights of prescribing information. Hadlima (adalimumab-bwwd) injection, for subcutaneous use); 2021 [cited 2021 Jul 20]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761059s000lbl.pdf
- Argollo M, Fiorino G, Gilardi D, et al. Biosimilars of adalimumab in inflammatory bowel disease: are we ready for that? Curr Pharm Des. 2019;25(1):7–12.
- European Medicines Agency. Committee for the Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products; 2020 [cited 2020 Nov 9]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf
- US Department of Health and Human Services. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry; 2021 [cited 2021 Jul 20]. Available from: https://www.fda.gov/media/82647/download
- Lee JJ, Yang J, Lee C, et al. Demonstration of functional similarity of a biosimilar adalimumab SB5 to Humira®. Biologicals. 2019;58:7–15.
- Lee N, Lee JJ, Yang H, et al. Evaluation of similar quality attribute characteristics in SB5 and reference product of adalimumab. MAbs. 2019;11(1):129–144.
- Shin D, Lee Y, Kim H, et al. A randomized phase I comparative pharmacokinetic study comparing SB5 with reference adalimumab in healthy volunteers. J Clin Pharm Ther. 2017;42(6):672–678.
- Weinblatt ME, Baranauskaite A, Niebrzydowski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2018;70(1):40–48.
- European Medicines Agency. Summary of product characteristics. Humira 20 mg solution for injection in pre-filled syringe; 2021 [cited 2021 Jul 20]. Available from: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf
- Abbot Laboratories. Highlights of prescribing information. Humira (adalimumab) injection, solution for subcutaneous use; 2021 [cited 2021 Jul 20]. Available from: https://www.rxabbvie.com/pdf/humira.pdf
- Lukas M, Malickova K, Kolar M, et al. Switching from originator adalimumab to the biosimilar SB5 in patients with inflammatory bowel disease: short-term experience from a single tertiary clinical centre. J Crohns Colitis. 2020;14(7):915–919.
- European Medicines Agency. The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) guide on methodological standards in pharmacoepidemiology (revision 8). Available from: http://www.encepp.eu/standards_and_guidances/documents/GuideMethodRev8.pdf
- Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques; 2021 [cited 2021 Jul 20]. Available from: https://support.sas.com/resources/papers/proceedings/proceedings/sugi26/p214-26.pdf
- Di Cesare A, Tronconi G, Fastame TM, et al. SB5 adalimumab biosimilar in the treatment of psoriasis and psoriatic arthritis. Dermatol Ther. 2020;33:e13435.
- Dragoni G, Pieraccini A, Bagnoli S, et al. Maintenance of clinical remission with SB5 biosimilar after switch from adalimumab originator: real-life experience of a tertiary referral centre. Presented at: European Crohn's and Colitis Organisation (ECCO); 2020. Virtual.
- Padilla Suarez C, Webb K, Persad N, et al. Long-term follow-up of switching from original adalimumab to adalimumab biosimilar: real-world data in IBD. Presented at: European Crohn's and Colitis Organisation (ECCO); 2020. Virtual.
- Young D, Freudensprung U, Harris C, et al. IBD reference and biosimilar adalimumab CroSS over study (iBaSS): design considerations and methodology. Presented at: European Crohn's and Colitis Organisation (ECCO); 2020. Virtual.
- Rosembert D, Malaviya A, How J, et al. Different failure rates after non-medical switching of 744 patients from adalimumab originator to 2 different adalimumab biosimilars at Cambridge University Hospitals, UK: real-world experience. Presented at: European Crohn's and Colitis Organisation (ECCO); 2020. Virtual.
- Müller-Ladner U, Gaffney K, Jadon D, et al. The PROPER study: results of the first interim analysis of a pan-EU real-world study of SB5 biosimilar following transition from reference adalimumab in patients with rheumatoid arthritis, axial spondyloarthritis or psoriatic arthritis. Arthritis Rheumatol. 2020;72(suppl 10):Abstract 0805.
- Dignass AU, Gisbert JP, Freudensprung U, et al. The PROPER study: interim analysis of a pan-European real-world study of SB5 adalimumab biosimilar after transition from reference adalimumab in patients with Crohn’s disease. Presented at: European Crohn's and Colitis Organisation (ECCO); 2021. Virtual.
- Tapete G, Bertani L, Pieraccini A, et al. Effectiveness and safety of nonmedical switch from adalimumab originator to SB5 biosimilar in patients with inflammatory bowel diseases: twelve-month follow-up from the TABLET registry. Inflamm Bowel Dis. 2022;28(1):62–69.
- Pouillon L, Danese S, Hart A, et al. Consensus report: clinical recommendations for the prevention and management of the nocebo effect in biosimilar-treated IBD patients. Aliment Pharmacol Ther. 2019;49(9):1181–1187.
- D’Amico F, Pouillon L, Argollo M, et al. Multidisciplinary management of the nocebo effect in biosimilar-treated IBD patients: results of a workshop from the NOCE-BIO consensus group. Dig Liver Dis. 2020;52(2):138–142.