Abstract
Objectives
Celiac disease (CD) is a common yet largely underdiagnosed disease. This study aimed to test the feasibility of incorporating a non-targeted CD screening in a pediatric outpatient setting and evaluate its short-term impact on children with serological evidence of disease.
Methods
Over five months, 500 children (aged 2–17 years) attending a general pediatric outpatient clinic in Gothenburg, Sweden, were enrolled and surveyed for current symptoms, quality of life, and background characteristics; 481 children were screened for tissue-transglutaminase antibodies (tTGA); repeated tTGA-positivity was defined as CD autoimmunity (CDA). Children with CDA were investigated for CD and for one year monitored for changes in symptoms, and quality of life.
Results
Eleven of 481 (2.3%) screened children had CDA. Children with CDA were younger (median 3.8 years) than those without CDA (8.8 years). No other major between-group differences were reported in background characteristics, symptoms, or quality of life. The screening was well-accepted by the families/participants. During 1-year follow-up, 8 of 11 children with CDA were diagnosed with CD. Children with screening-detected CD reported no significant changes in symptoms and quality of life and the dietary adherence rate was good.
Conclusions
Non-targeted screening for CD was feasible in a general pediatric outpatient setting. While hampered by small sample size, our results are in line with previous screening studies indicating that symptoms do not differentiate CDA from non-CDA children. Also, among an overall minimal-symptomatic group of children, diagnosing CD and installation of treatment did not significantly change their well-being during 1-year follow-up.
Introduction
Celiac disease (CD) is a chronic autoimmune disorder caused by gluten ingestion [Citation1]. The prevalence of CD is 1% worldwide [Citation2]. In children, CD diagnosis is established by either repeated CD-specific serological markers >10x upper limit of normal or by duodenal biopsy showing villous atrophy [Citation1,Citation3]. The disease is associated with, among others, impaired bone health and psychiatric disorders [Citation4–6]. Early CD diagnosis and installation of a gluten-free diet (GFD) may prevent such complications [Citation4].
Currently, serological screening for CD is advised in children with gastrointestinal symptoms and genetically high-risk groups, such as patients with type 1 diabetes [Citation7]. However, despite today’s targeted CD screening, underdiagnosis is common as the disease may cause few or no symptoms at all [Citation2]. Conversely, there is insufficient evidence on whether the benefits of universal screening outweigh potential harms [Citation8], including concerns that a CD diagnosis may not confer clear benefits in asymptomatic individuals [Citation9].
While the best approach to identify CD is not yet established, this study examines a novel, non-targeted CD screening program at a general pediatric outpatient clinic in Sweden [Citation10]. This screening approach may be viewed as a middle ground between present-day high-risk screening and universal screening for CD. Besides studies on CD screening in adult outpatient settings [Citation11–14], there are few data on the diagnostic yield and practical implementation of CD screening of children in a similar healthcare setting [Citation15].
Hence, the primary aim of this study was to test the hypothesis that it is feasible to incorporate a non-targeted CD screening in a general pediatric outpatient clinic and that such screening is well-accepted by the participants and their parents. Our secondary aim was to describe any short-term changes in the well-being of children with screening-identified CD.
Methods
Study design and setting
Tissue-transglutaminase antibody (tTGA) screening for CD was conducted at the general pediatric outpatient clinic in Hisingen, Gothenburg, Sweden, between November 4, 2019, and March 26, 2020. The clinic provides non-acute pediatric care for a geographical area of some 36,000 children. In 2020, 3559 doctor’s visits, including 113 (3.2%) related to CD follow-up, were held at the clinic; other common diagnostic groups were asthma and allergy, obesity, constipation, behavioral and mental disorders.
Briefly, children who after screening were confirmed positive for tTGA were included in a one-year structured follow-up program at Queen Silvia Children’s Hospital, Gothenburg, Sweden, for workup of CD and monitoring possible changes in their symptoms and health-related quality of life (HRQoL) from baseline. During the study period, local guidelines required all pediatric CD in Gothenburg to be diagnosed at the Queen Silvia Children’s Hospital (including non-biopsy verified CD diagnoses).
Study sample
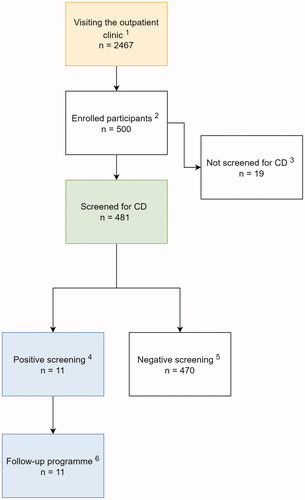
Children aged 2 to 17 years, who visited the clinic for whatever reason during the study period, were eligible to participate (, Flowchart). Patients were recruited by employees at the clinic and dedicated research personnel. Children with pre-existing CD diagnosis or type 1 diabetes (i.e., who are routinely screened for CD) were excluded from serological testing but could participate in study surveys. Swedish language proficiency was required to understand the study surveys and give informed consent.
Figure 1. Flowchart of study formation. 1In total, 2467 children visited the outpatient clinic between November 2019 and March 2020. 2There were 500 children enrolled aged 2–17 years old. 3Nineteen children with established celiac disease (CD) were excluded from the serological testing and participated with self-reported questionnaires. 4A concentration of IgA-tTGA ≥7.0 U/mL or IgG-TGA ≥7.0 U/mL. 5A concentration of IgA-tTGA <7.0 U/mL or of IgG-TGA <7.0 U/mL. 6One-year follow-up consisted of four visits at Queen Silvia Children’s Hospital in Gothenburg. Serological evidence of CD in 11 children (six boys and five girls).
Serological screening
Participating children were screened for CD using IgA-tTGA measurement by Fluorescence Enzyme Immunoassay (EliA Celikey, Thermo Fisher Scientific, Sweden) at Sahlgrenska University Hospital, Gothenburg, Sweden. Children indicated to have low IgA (<0.07 g/L) were tested for IgG-tTGA. Concentrations of IgA/IgG-tTGA ≥7 U/ml were considered positive [Citation16]. Children positive for tTGA were re-tested two weeks later for confirmation.
Follow-up
Children with confirmed tTGA positivity were included in a one-year structured follow-up program for workup of CD lead by last author KM. The diagnostic process of CD was informed by national guidelines for CD (described below); we did not practice a ‘watch and wait’ approach to the children with positive tTGA screening. During follow-up, we repeatedly surveyed symptoms and HRQoL. Once CD was diagnosed, we monitored the adherence to the GFD. Supplementary Figure 1 depicts an overview of the collected data. The used questionnaires are described below.
Definition of outcomes
The primary outcome of the study was CD autoimmunity (CDA) defined as repeated tTGA positivity. Children screened negative for tTGA were referred to as non-CDA. The secondary outcome of our study was a clinical diagnosis of CD at the end of a one-year follow-up. Diagnosis of CD was made according to current Swedish national diagnostic guidelines [Citation17]. Accordingly, the diagnosis of CD required either confirmation of small-intestinal villous atrophy or, in a non-biopsy approach, repeated tTGA levels above ten times the upper limit of normal (i.e., ≥70 U/ml) [Citation7]. Although the national guidelines for CD are largely compliant with the revised ESPGHAN criteria [Citation7], they are adopted to the unavailability of endomysial antibody test in Sweden.
Questionnaire data
At enrollment, we retrieved questionnaire data on socio-demographic characteristics, past medical history of the participant and family and the reason for the visit (i.e., presenting complaint). Data were categorized as shown in . We surveyed the overall acceptance of the study, and children aged ≥8 years reported the pain from blood draw using a visual analog scale (VAS) ranging from 0 (‘no pain at all’) to 100 (‘pain as bad as it could be’). Children aged ≥12 years (or ≥8 years for specific questionnaires [Citation18–20] mainly answered the questionnaires themselves and otherwise with the support of their parents.
Table 1. Background characteristics of children with celiac disease autoimmunity (CDA), non-CDA and preexisting celiac disease (CD). Reported numbers, percentages and medians/means relate to non-missing valuesa.
The following questionnaires were used in the study:
Gastrointestinal symptom rating scale (GSRS)
The 15-item GSRS questionnaire assesses the occurrence of gastrointestinal symptoms in the past week within the following five domains: ‘Diarrhea’, ‘Indigestion’, ‘Constipation’, ‘Abdominal pain’ and ‘Reflux’. Each response ranges from 1 (no symptom) to 7 (most severe) forming sub-scores for each domain and a total score where higher scores indicate more severe symptoms [Citation21,Citation22]. While the GSRS was not specifically designed for CD it has been used in CD research [Citation23,Citation24], foremost on adult patients, and more occasionally in children and adolecents [Citation25–27].
Celiac disease symptom index (CSI)
This 16-item disease-specific questionnaire evaluates CD-related symptoms using a 5-point Likert scale. In this study, four questions were selected from CSI to capture extra-intestinal symptoms such as headache, energy level, appetite, and overall health [Citation28]. The CSI has been validated in adults [Citation28], but not in children.
Kidscreen-27
KIDSCREEN-27 is a generic, self-reported, HRQoL instrument that has been extensively validated for children 8–18 years of various countries, including Sweden [Citation18]. The questionnaire is grouped into five dimensions including ‘Physical Wellbeing’ (covering physical activity and energy), ‘Psychological Wellbeing’ (emotional balance and life satisfaction), ‘Autonomy & Parent Relations’ (relationship with parents and having age-appropriate freedom), ‘Social Support & Peers’ (friendships), and ‘School Environment’ (cognition and feelings about school). Each item was scored on a 5-point scale and summed by each dimension using a Rasch model [Citation29,Citation30]. As previously described [Citation18], sub-scores were then transformed using country-specific T-values yielding a mean score of 50 (standard deviation [SD] 10) which defines normality for children 8–18 years (higher score = better HRQoL).
Celiac disease DUX (CDDUX)
This is a 12-item CD-specific HRQoL questionnaire using emoji-like smileys responses, ranging from 1 = ‘very sad face’ to 5 = ‘very happy face’. The questionnaire has three dimensions: ‘Communication’ (including feelings when talking about the disease), ‘Diet’ (feelings about following a lifelong diet), and ‘Having CD’ (feelings when offered gluten-containing food). A higher score indicates better health [Citation20,Citation31]. The instrument has been validated for 8–18-year-olds [Citation20,Citation31].
Celiac dietary adherence test (CDAT)
We used the Swedish version of CDAT (CDAT-SWE) to assess GFD adherence of children diagnosed with CD [Citation19,Citation32]. The questionnaire consists of seven items on a 5-point Likert scale from which an additive score from 7 to 35 was calculated. Scores <13 indicate very good adherence, >17 indicate poor adherence, and scores of 13–17 are inconclusive [Citation32]. The test has been validated for adults [Citation32], but also used in children aged ≥12 years [Citation19].
Statistical analyses
We estimated differences in baseline characteristics, surveyed symptoms, and HRQoL between children with vs. without CDA using Fisher’s exact test, Chi-Square test, Mann-Whitney U-test, and Wilcoxon Signed-Rank test, as appropriate. We report means/medians, numbers and percentages of non-missing values. Missingness related to incomplete answered questionnaires. All significance tests were two-sided and conducted at the 5% significance level. SAS Version 9.4, (SAS Institute, Cary, NC, USA) was used for the statistical analyses. See Supplementary material, Power analysis.
Post-hoc analysis
In a post-hoc analysis, we used ANCOVA Tukey to adjust for child’s age for differences in extra-intestinal manifestations of CSI, GSRS and KIDSCREEN-27 sub-scores of children with vs without CDA.
Ethics
This study was approved by the Swedish Ethical Review Authority (Dnr 2019-01950).
Informed consent was received from all participants.
Results
A total of 2467 children visited the outpatient clinic during the study period, out of whom approximately 2000, aged 2–17 years, were approached and 500 enrolled in the study (the exact number of children approached, and reason for non-participation was not documented). A total of 481 children were serologically screened for CD; the remaining 19 participants had a pre-existing CD diagnosis and were hence not screened (, Flowchart). Three children (0.6%) had total IgA levels <0.07 g/L and were tested for IgG-tTGA. The majority preferred capillary blood sampling (64%) rather than venous blood sampling. The mean VAS pain score at blood draw was 26 (SD 21) (median 20, range 0–100). Participants reported being overall satisfied with their participation in the study (See Supplementary Table 1).
Screening-detected celiac disease autoimmunity and pre-existing celiac disease
Eleven screened children had persistent tTGA-positivity (i.e., CDA) and 470 children were tTGA negative (non-CDA). No children had transient tTGA positivity at screening (i.e., one positive and one negative test). Most of the children with a pre-existing CD diagnosis (n = 14/19, 74%) reported that their diagnostic workup had been initiated due to symptoms of disease which, on average, had been present one year before diagnosis. Almost half of the children had a biopsy-verified CD diagnosis (n = 9/19, 47%), and the remaining a serology-based, diagnosis.
Background characteristics at screening
The median age was 8.8 years (range, 2.0–17.9) for all participants, 3.8 years (3.1–14.6) for children with CDA, and 8.8 years (2.0–17.9) for children without CDA (p < .01). Half of the participants were girls and there was comparable ethnicity, autoimmune heredity, parental educational level, and parental employment rate between children with CDA and non-CDA (). Overall, asthma and allergy (35%) followed by gastrointestinal symptoms (23%) were the most common reasons (i.e., presenting complaint) for attending the pediatric outpatient clinic, with no major differences reported between children with vs. without CDA (, all p > .20). Only three of eleven children with CDA reported gastrointestinal symptoms as their main reason for the visit. Most of the participants (60%) had two or more previous visits over the last 12 months; all children with screening-identified CDA had during the same period at least one previous visit ().
Gastrointestinal and extra-intestinal symptoms at screening
Generally, the participants experienced minor gastrointestinal symptoms that were similar both in overall severity, and across the five dimensions of GSRS for children with vs. without CDA (all p > .10). Also surveyed extra-intestinal symptoms were largely similar between groups (). Most children reported their overall health as ‘good’ or ‘excellent’ (CDA, n = 7/11 [63%], non-CDA, n = 351/460 [77%]; ). P-values adjusted for age were largely unchanged and remained non-significant for differences in GSRS sub-scores and extra-intestinal symptoms of CDA vs non-CDA children (all p ≥ .34).
Table 2. Gastrointestinal and extra-intestinal symptoms in children with celiac disease autoimmunity (CDA), non-CDA and preexisting celiac disease (CD). Reported numbers, percentages and means relate to non-missing valuesa.
Health-related quality of life at screening
Using the generic HRQoL-questionnaire KIDSCREEN-27, overall HRQoL was similar for children with vs. without CDA (median total score, CDA = 119, and non-CDA = 114; p = .44). The sub-scores of all five domains had T-values around or above 50, indicating a good HRQoL () with no significant differences between CDA and non-CDA children in any of the five dimensions. For instance, the sub-score of psychological well-being was 51.8 in children with CDA and 51.6 in children without CDA (p = .82). Age-adjusted P-values remained essentially unchanged and non-significant for differences in KIDSCREEN-27 sub-scores of CDA vs non-CDA children (e.g., p = .85 for sub-score of psychological well-being).
Table 3. Health-related quality of life (HRQoL) using KIDSCREEN-27 in children with celiac disease autoimmunity (CDA), non-CDA and preexisting celiac disease (CD). Reported means/medians relate to non-missing valuesa.
One-year follow-up program: celiac investigation and monitoring of well-being
Of 11 children with serological evidence of CD, eight were diagnosed with CD: four had repeated tTGA test above ten times the upper limit of normal, and four had villous atrophy (i.e., Marsh 3) at small-intestinal biopsy. Three children were not diagnosed with CD, two because of spontaneous normalization of tTGA during follow-up, and one child, who remained tTGA positive, had normal mucosa at small-intestinal biopsy; none of the children were reported to have lowered their gluten intake during CD investigation. Supplementary Table 2 details clinical data from the CD investigation of children with CDA. Three out of eight children diagnosed with CD had prior to screening reported symptoms or signs which according to ESPGHAN guidelines should have prompt testing for CD [Citation7]. Participants with CDA were overall satisfied with study participation (data not shown).
During the one-year follow-up, children with screening-detected CDA experienced no major changes in symptoms compared to baseline (See Supplementary Table 3). Furthermore, the children reported during follow-up no significant changes in their overall and dimensional sub-scores of HRQoL as measured by KIDSCREEN-27 (Change in overall median score 1.0 [−21;28], p = .75; Supplementary Table 3). At the end of one-year follow-up, children diagnosed with CD had a mean CDDUX (CD-specific) HRQoL-score of 3 (indicated by a ‘neutral face’-emoji). However, four of the children reported difficulties (‘sad’ or ‘very sad faces’) specifically for ‘not being able to eat like other people’ and ‘thinking about gluten-containing food’ (Supplementary Figure 2). The mean CDAT adherence score after CD diagnosis was 10 (range, 6–15), indicating ‘very good adherence’.
Discussion
This study shows that screening for CD is feasible in a general pediatric outpatient setting. We found that 11 of 481 (2.3%) screened children had serological evidence of CD, out of whom eight fulfilled diagnostic criteria for CD during follow-up. No major differences in symptoms and HRQoL were observed between children with vs. without serological positivity at screening, nor did we observe major changes in symptoms and HRQoL among screening-positive children after CD diagnosis and installation of GFD. Notably, only three out of eight children with screening-identified CD met current criteria for CD case finding (e.g., gastrointestinal symptoms).
To our knowledge, this is one of the first European screening programs for CD incorporated into a pediatric outpatient clinic. Compared to universal, population-based screening, our approach benefits from an existing infrastructure for a blood draw, analyses, and follow-up of screening results. There are also ethical differences between universal screening and screening of patients seeking healthcare who already expect workup for their symptoms or medical condition [Citation9]. Aptly called ‘the great pretender’, CD may show up masked as many childhood conditions. On the other hand, at best, our approach would only identify those who seek healthcare. Furthermore, in settings without accessible healthcare, such an approach may cause ethical concerns as it may bring more benefits for those with better socio-economic status. From an ethical perspective it is also noteworthy that three out of eleven screening-positive children in this study were eventually not diagnosed and thus had an unnecessary workup.
Strengths and limitations
A strength of this study derives from using validated and extensively researched questionnaires, such as the generic HRQoL-questionnaire KIDSCREEN-27 [Citation18]. Validated questionnaires ensure high-quality data that are comparable, both longitudinally for a specific participant and across studies. However, as CSI and GSRS have only been validated in adults [Citation23,Citation24,Citation28], their use in children should be more cautiously interpreted. Our detailed data on background characteristics are additional strengths. Finally, a structured one-year follow-up of screening-positive children allowed us to establish the diagnosis of CD. While we described major short-term changes in the well-being during workup and post-diagnosis, it was beyond the scope of this study to determine the effect of CD diagnosis and GFD treatment on the asymptomatic child in general.
A limitation of this study was its small sample size (n = 500); due to internal attrition (incomplete answered questionnaires) the actual number of included participants was in some analyses even lower. Our sample size meant a risk of committing a type-2 error (i.e., to erroneously accept a false null hypothesis). While our results did not indicate major differences in symptoms and HRQoL between children with vs. without screening-detected CDA, our limited sample size and wide age distribution prevents us from ruling out such differences in specific age-groups of children [Citation18,Citation20,Citation33].
In this study CD was diagnosed based on national diagnostic guidelines. In contrast with ESPGHAN 2020 criteria requiring positive endomysial antibodies for a non-biopsy CD diagnosis [Citation7], the Swedish guidelines [Citation17], due to an unavailability of endomysial antibody tests, allow for a non-biopsy CD diagnosis using repeated tTGA tests above ten times the upper limit of normal. While some reports have indicated that a tTGA-based strategy alone may be predictive of CD even without biopsy [Citation34], we acknowledge that serology strategies beyond ESPGHAN 2020 need to be more carefully evaluated to be fully evidence based [Citation35].
Lastly, even though the participants generally reported few worries regarding screening, and were satisfied with screening and follow-up, only around one in four of those invited for screening agreed to participate in the study. Although the reason for non-participation was not documented, the low participation rate may indicate a lack of awareness of pauci-symptomatic CD and the potential benefits of early detection. It is also possible that a simplified screening process, e.g., expedited consent and information retrieval, could increase the participation rate. Due to lack of data, it is unknow if patient characteristics differed between those included vs. not included in the study. For example, we cannot rule out that the genetic risk of CD (e.g., CD hereditability) may have been higher among those included vs. not included in the study.
Interpretation of findings and previous literature
In this study, 2.3% (n = 11/481) of children had serological evidence of CD, a prevalence which is similar to the 2.1% undiagnosed CD reported in a population-based study of Swedish 12-year-olds born during the so-called ‘Swedish CD epidemic’ (1984–1996), characterized by a high incidence of CD in early life [Citation2]. The prevalence of undetected CD has seemed to be somewhat lower (1.6%) in Swedish children born after the ‘epidemic’ (1997) [Citation36].
While firm conclusions are hampered by our small sample size, one could have expected a higher underlying CD prevalence in our healthcare setting, where children with CD-related autoimmune conditions (e.g., thyroiditis [Citation37] should be more prevalent compared to the average Swedish population. In adults, the prevalence of undiagnosed CD in primary care settings have been reported to be between 1% (USA and United Kingdom) and 2% (Finland) [Citation11,Citation13,Citation14], and around 3% in secondary care settings [Citation12].
Gastrointestinal symptoms
Overall, participants experienced few gastrointestinal symptoms, although one in four reported such symptoms as their presenting complaint to seek outpatient care. The mean GSRS score was very similar between the children with CDA and without CDA. This finding aligns with previous pediatric studies suggesting that symptoms, including gastrointestinal manifestations, are poor predictors of undiagnosed CD [Citation2,Citation38–40]. Similar findings have been reported in adults with screening-detected CD [Citation11,Citation14].
The overall few symptoms reported at the time of screening may, as indicated by previous works [Citation41], also explain why we did not detect major changes in gastrointestinal or extra-intestinal symptoms related to CD during follow-up.
Health-related quality of life
In our study, the HRQoL measured by the KIDSCREEN-27 questionnaire was similar in children with and without CDA/CD. These results are in accordance with several previous studies indicating that individuals with few symptoms have not or only slightly decreased HRQoL [Citation42–44]. While a formal interaction analysis between HRQoL and symptom score was outside the scope of this study, it is conceivable that the similar HRQoL of CDA and non-CDA groups of children might be explained by the overall few symptoms of this population. Some children may even be accustomed to their condition and not realizing that they have symptoms [Citation42,Citation45].
While some follow-up studies after CD screening have shown an improvement of HRQoL, particularly for children who were symptomatic at the time of diagnosis [Citation44], others have instead shown a reduced HRQoL after diagnosis, follow-up, and installation of GFD [Citation20,Citation46]. Yet other studies have [Citation43,Citation47], similar to our results, found no change on HRQoL during follow-up after CD screening. Similar to above, it is possible that a change in HRQoL after CD screening is directly related to symptom severity at CD diagnosis [Citation44].
Finally, we found children with screening-detected CD to have a good adherence to GFD. Although our data were based on few children, similar findings have been noted in other screening studies for CD [Citation24,Citation45,Citation48].
Conclusions
This study showed that non-targeted screening for CD was feasible in a general pediatric outpatient setting. While hampered by a small sample size, our results are in line with previous studies indicating that symptoms do not differentiate CDA from non-CDA children. Also, among an overall minimal-symptomatic group of children, diagnosing CD and installation of treatment did not significantly change their well-being during one-year follow-up. Therefore, larger-scale studies are needed to determine the diagnostic yield, benefits and cost-effectiveness of CD screening more precisely within real-world clinical settings.
Author contributions
Karl Mårild conceptualized and designed the study as well as acquired the data. Statistics were done by Henrik Albrektsson. Sunna Gunnarsdottir wrote the first version of the manuscript. Karl Mårild, Jenny M. Kindblom, Nicolae Miron, Julia Frydebo and Ketil Størdal critically revised the manuscript. Karl Mårild supervised the project and takes responsibility for the integrity of the data. All authors contributed to the interpretation of the data and approved the final manuscript as submitted.
Supplemental Material
Download PDF (620.9 KB)Acknowledgments
The screening approach was developed according to advice from Dr Marian Rewers who has initiated the Autoimmunity Screening for Kids (ASK) program at Barbara Davis Center for Diabetes, CO, USA. The authors are grateful to all families participating in this study and for the support from the personnel at the Hisingen general pediatric outpatient clinic in Gothenburg, Sweden.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
According to the Ethical approval for this study data cannot be shared. www.clinicaltrial.gov, identification number: NCT03966625.
Additional information
Funding
References
- Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391(10115):70–81.
- Myléus A, Ivarsson A, Webb C, et al. Celiac disease revealed in 3% of swedish 12-year-olds born during an epidemic. J Pediatr Gastroenterol Nutr. 2009;49(2):170–176.
- Popp A, Kivelä L, Fuchs V, et al. Diagnosing celiac disease: towards Wide-Scale screening and Serology-Based criteria? Gastroenterol Res Pract. 2019;2019:2916024.
- Kivelä L, Kurppa K. Screening for coeliac disease in children. Acta Paediatr. 2018;107(11):1879–1887.
- Björck S, Brundin C, Karlsson M, et al. Reduced bone mineral density in children with screening-detected celiac disease. J Pediatr Gastroenterol Nutr. 2017;65(5):526–532.
- Lebwohl B, Haggård L, Emilsson L, et al. Psychiatric disorders in patients with a diagnosis of celiac disease during childhood from 1973 to 2016. Clin Gastroenterol Hepatol. 2021;19(10):2093–2101.e13.
- Husby S, Koletzko S, Korponay-Szabó I, et al. European society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70(1):141–156.
- Bibbins-Domingo K, Grossman DC, Curry SJ, US Preventive Services Task Force, et al. Screening for celiac disease: US preventive services task force recommendation statement. JAMA - J Am Med Assoc. 2017;317(12):1252–1257.
- Ludvigsson JF, Card TR, Kaukinen K, et al. Screening for celiac disease in the general population and in high-risk groups. United European Gastroenterol J. 2015;3(2):106–120.
- Anell A, Glenngård AH, Merkur S. Sweden health system review. Health Syst Transit. 2012;14(5):1–159.
- Katz KD, Rashtak S, Lahr BD, et al. Screening for celiac disease in a North American population: Sequential serology and gastrointestinal symptoms. Am J Gastroenterol. 2011;106(7):1333–1339.
- Mooney PD, Leeds JS, Libzo N, et al. Case-finding for coeliac disease in secondary care: a prospective multicentre UK study. Dig Liver Dis. 2014;46(1):32–35.
- Sanders DS, Patel D, Stephenson TJ, et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol. 2003;15(4):407–413.
- Tikkakoski S, Savilahti E, Kolho KL. Undiagnosed coeliac disease and nutritional deficiencies in adults screened in primary health care. Scand J Gastroenterol. 2007;42(1):60–65.
- Gesualdo PD, Bautista KA, Waugh KC, et al. Feasibility of screening for T1D and celiac disease in a pediatric clinic setting. Pediatr Diabetes. 2016;17(6):441–448.
- Werkstetter KJ, Korponay-Szabó IR, Popp A, ProCeDE study group, et al. Accuracy in diagnosis of celiac disease without biopsies in Clinical Practice. Gastroenterology. 2017;153(4):924–935.
- National guideline on celiac disease, version 1.0. (Swedish: Nationellt vårdprogram för celiaki version 1.0). 2020. Available from: https://gastro.barnlakarforeningen.se/vardprogram-2/
- Ravens-Sieberer U, Auquier P, Erhart M, European KIDSCREEN Group, et al. The KIDSCREEN-27 quality of life measure for children and adolescents: Psychometric results from a cross-cultural survey in 13 european countries. Qual Life Res. 2007;16(8):1347–1356.
- Johansson K, Norström F, Nordyke K, et al. Celiac dietary adherence test simplifies determining adherence to a gluten-free diet in swedish adolescents. J Pediatr Gastroenterol Nutr. 2019;69(5):575–580.
- Van Doorn RK, Winkler LMF, Zwinderman KH, Mearin ML, et al. CDDUX: a disease-specific health-related quality-of-life questionnaire for children with celiac disease. J Pediatr Gastroenterol Nutr. 2008;47(2):147–152.
- Svedlund J, Sjodin I, Dotevall G. GSRS-A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33(2):129–134.
- Kulich KR, Madisch A, Pacini F, et al. Reliability and validity of the gastrointestinal symptom rating scale (GSRS) and quality of life in reflux and dyspepsia (QOLRAD) questionnaire in dyspepsia: a six-country study. Health Qual Life Outcomes. 2008;6:12.
- Canestaro WJ, Edwards TC, Patrick DL. Systematic review: patient-reported outcome measures in coeliac disease for regulatory submissions. Aliment Pharmacol Ther. 2016;44(4):313–331.
- Kurppa K, Paavola A, Collin P, et al. Benefits of a gluten-free diet for asymptomatic patients with serologic markers of celiac disease. Gastroenterology. 2014;147(3):610–617.e1.
- Lionetti E, Gatti S, Galeazzi T, et al. Safety of oats in children with celiac disease: a Double-Blind, randomized, Placebo-Controlled trial. J Pediatr. 2018;194:116–122.e2.
- Bonnert M, Olén O, Lalouni M, et al. Internet-Delivered cognitive behavior therapy for adolescents with irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol. 2017;112(1):152–162.
- Harris C, Card B. A pilot study to evaluate nutritional influences on gastrointestinal symptoms and behavior patterns in children with autism spectrum disorder. Complement Ther Med. 2012;20(6):437–440.
- Leffler DA, Dennis M, Edwards George J, et al. A validated Disease-Specific symptom index for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7(12):1328–1334.e3.
- Dabaghi S, Esmaielzadeh F, Rohani C. Application of rasch analysis for development and psychometric properties of Adolescents' Quality of Life Instruments: A Systematic Review. Adolesc Health Med Ther. 2020;11:173–197.
- Ravens-Sieberer U. The kidscreen questionnaires: quality of life questionnaires for children and adolescents. Lengerich: Pabst Science Publishers; 2006.
- Meyer S, Shani M. Structural validation and dyadic child-parent measurement invariance of the celiac disease quality of life questionnaire. Eur J Gastroenterol Hepatol Hepatol. 2021;34(1):39–47.
- Leffler DA, Dennis M, Edwards George JB, et al. A simple validated Gluten-Free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7(5):530–536.e2.
- Fasano A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology. 2005;128(4):S68–S73.
- Wolf J, Petroff D, Richter T, et al. Validation of Antibody-Based strategies for diagnosis of pediatric celiac disease without biopsy. Gastroenterology. 2017;153(2):410–419.e17.
- Wessels M, Auricchio R, Dolinsek J, et al. Review on pediatric coeliac disease from a clinical perspective. Eur J Pediatr. 2022.
- Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131(3):e687–e694.
- ElfströM P, Montgomery SM, KäMpe O, et al. Risk of thyroid disease in individuals with celiac disease. J Clin Endocrinol Metab. 2008;93(10):3915–3921.
- Agardh D, Lee HS, Kurppa K, for the TEDDY Study Group, et al. Clinical features of celiac disease: a prospective birth cohort. Pediatrics. 2015;135(4):627–634.
- Rosén A, Sandström O, Carlsson A, et al. Usefulness of symptoms to screen for celiac disease. Pediatrics. 2014;133(2):211–218.
- Jansen M, van Zelm M, Groeneweg M, et al. The identification of celiac disease in asymptomatic children: the generation R study. J Gastroenterol. 2018;53(3):377–386.
- Mustalahti K, Lohiniemi S, Collin P, et al. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff Clin Pract. 2002;5(3):105–113.
- Myléus A, Petersen S, Carlsson A, et al. Health-related quality of life is not impaired in children with undetected as well as diagnosed celiac disease: a large population based cross-sectional study. BMC Public Health. 2014;14(1):1–9.
- Nordyke K, Norström F, Lindholm L, et al. Health-related quality of life in adolescents with screening-detected celiac disease, before and one year after diagnosis and initiation of gluten-free diet, a prospective nested case-referent study. BMC Public Health. 2013;13(1):142.
- Van Koppen EJ, Schweizer JJ, Csizmadia CGDS, et al. Long-term health and quality-of-life consequences of mass screening for childhood celiac disease: a 10-year follow-up study. Pediatrics. 2009;123(4):e582–e588.
- Rosén A, Ivarsson A, Nordyke K, et al. Balancing health benefits and social sacrifices: a qualitative study of how screening-detected celiac disease impacts adolescents’ quality of life. BMC Pediatr. 2011;11:32.
- Barratt SM, Leeds JS, Sanders DS. Quality of life in coeliac disease is determined by perceived degree of difficulty adhering to a gluten-free diet, not the level of dietary adherence ultimately achieved. J Gastrointest Liver Dis. 2011;20(3):241–245.
- Johnston SD, Rodgers C, Watson RGP. Quality of life in screen-detected and typical coeliac disease and the effect of excluding dietary gluten. Eur J Gastroenterol Hepatol. 2004;16(12):1281–1286.
- Webb C, Myléus A, Norström F, et al. High adherence to a gluten-free diet in adolescents with screening-detected celiac disease. J Pediatr Gastroenterol Nutr. 2015;60(1):54–59.
