Abstract
Background and aims
Available epidemiological data on hepatocellular carcinoma (HCC) originate mainly from centre-based or disease-specific cohorts and may not reflect the general population. This population-based register study presents the incidence, aetiologies, treatments, survival, and differences related to sex or socioeconomic status in patients with HCC from Stockholm, which constitutes more than a fifth of the Swedish population.
Methods
ICD-10 codes identified incident HCC cases in the regional administrative health care database 2003–2018. Administrative coding on diseases, socioeconomic status, and dispensed drugs were used to identify risk factors and therapies. Two validation analyses 2014–2015 studied the correctness of register-based aetiologies and reasons for providing only best supportive care (BSC).
Results
We identified 2,245 incident cases of HCC. The incidence increased from 6 to 7.5 per 100,000 inhabitants over the time-period. The most common aetiologies were hepatitis C (26%), non-alcoholic fatty liver disease (22%), and alcohol-related liver disease (19%). Five-year survival probability was 79% after liver transplantation, 60% after resection, and 35% after ablation but <10% for chemoembolization, Sorafenib, and BSC. The proportion receiving any treatment increased but half of the patients only received BSC. At least 14% of potentially treatable HCC (surveillance indicated but not performed) received only BSC 2014–2015. We found no significant differences in treatments or outcomes between socioeconomic groups.
Conclusions
The incidence of HCC is rising in Stockholm, Sweden but is still low by global comparison. Near half of all patients still receive only BSC and study data suggest that surveillance practices are incomplete.
Introduction
Primary liver cancer (PLC) is estimated to be the sixth most commonly diagnosed cancer and is the third leading cause of cancer death worldwide [Citation1]. The incidence is lower in Sweden where PLC is the 13th most common cancer and the 7th leading cause of cancer-related death [Citation1]. PLC includes mostly (75–90%) hepatocellular carcinoma (HCC) but also intrahepatic cholangiocarcinoma and some other rare cancer forms [Citation2]. The epidemiological literature does not always clearly make a distinction between HCC and other types of PLC, in part because of low granularity in registers. A recent Swedish study found that ICD-10 code-based patients registers identified about 45 percent more PLC cases than the national cancer register due to under-reporting [Citation3]. The validity of ICD-codes related to liver cirrhosis was also recently reported to be high in the Swedish patient register, with positive predictive values (PPV) of >90% [Citation4].
Most HCCs occur in China and sub-Saharan Africa [Citation1,Citation2] where viral hepatitis and exposure to aflatoxin are driving factors [Citation5]. While incidence and mortality of HCC have been declining in high-risk regions in Asia, recent years have seen increases in many American and European regions [Citation6]. The evolving obesity epidemic influences HCC epidemiology in many high-income countries. Sweden has a relatively low prevalence of chronic hepatitis B/C and alcohol overconsumption [Citation7] but an increasing prevalence of obesity and its complications [Citation8]. Epidemiological information about HCC in Sweden is scarce. What we know is based on public databases [Citation9], centre-based cohorts [Citation10–12], or disease-based cohorts, such as for hepatitis B [Citation13], hepatitis C [Citation14], and non-alcoholic fatty liver disease (NAFLD) [Citation15,Citation16]. No detailed register-based epidemiological information that exclusively focusses on HCC rather than PLC is available. The potential influence of socioeconomic status on therapy and the outcome of HCC has never been studied in Sweden.
We aimed to narrow the Swedish knowledge gap by studying epidemiology, underlying aetiologies, management, and outcome of HCC in Region Stockholm. With two clinical validation cohorts, we estimated: (1) the positive and negative predictive values of register-based identification of aetiologies including NAFLD, which by necessity must partly be inferred from information about obesity, diabetes, and hyperlipidaemia, and (2) the reasons why many patients only receive best supportive care (BSC) and whether late diagnosis due to incomplete surveillance practice is a common reason.
Patients and methods
Data sources
Region Stockholm, Sweden, comprised ∼2.3 million inhabitants in 2018 and thus more than 22% of the population of Sweden (10.1 million). The administrative health care database of Region Stockholm contains both inpatient care and outpatient consultations. All pharmacy dispensed drugs are included in the database since July 2010.
Health care in Sweden is tax-funded with largely activity-based compensation, which ensures virtually complete registration. Registry-based research in Sweden is facilitated by the Swedish personal identity number (PIN) which is a unique personal identifier, maintained by the National Tax Board since 1947 for all individuals who reside in Sweden [Citation17].
Study population
All individuals residing in Region Stockholm that received a first-time diagnosis of HCC (ICD-10 C22.0) 2003–2018 were included in the study. Individuals diagnosed with HCC who migrated out of Region Stockholm were excluded for follow-up completeness and reliable outcome assessment.
Variables
ICD-10 codes for comorbidities present at or before HCC diagnosis were collected for each patient with HCC to identify underlying aetiologies of HCC. Definitions of specific risk factors for HCC and ICD-10 codes used to identify these are presented in Supplementary Table 1. Every patient was only attributed one risk factor, according to a ranking system of risk factors for HCC presented in Supplementary Table 1, and each patient is therefore only included once. Primary liver disease, such as primary sclerosing cholangitis (PSC), autoimmune hepatitis (AIH), and Budd-Chiari syndrome was given the highest rank, followed by Hepatitis B and then Hepatitis C, which included unspecified chronic viral hepatitis. The underlying aetiology was defined as alcohol-related liver disease (ARLD) if an individual had either (a) an ICD-10 diagnosis of ARLD or (b) a diagnosis of alcohol abuse, and (c) no other liver diagnosis. The underlying aetiology was defined as non-alcoholic fatty liver disease (NAFLD) if no other aetiologies were identified and the patient had either (a) a diagnosis of fatty liver disease, (b) diabetes mellitus, (c) treatment for diabetes mellitus, (d) obesity, or (e) hyperlipidaemia. Individuals were classified as having cryptogenic cirrhosis if they had the code for cirrhosis (ICD-10 K74.6) but no other identified risk factor or liver disease.
We limited the analyses of treatments to 2010–2018 to reflect current practices and to allow for data on sorafenib, introduced in Sweden in 2007, to be included. Patients who received sorafenib at any time were defined as treated. The dataset did not include information on the dosage or duration of sorafenib therapy. Individuals who received more than one treatment modality were analysed according to the treatment with the highest rank. Individuals were thus included only once in the treatment analyses and identified by, e.g., resection even if additional therapies, such as trans-arterial chemoembolization (TACE) or sorafenib were later given. See Supplementary Table 2 for ICD-10 therapy codes and ranking of treatments. All therapy data in the study are based on actual applied treatments. Active HCC treatments were centralized to Karolinska University Hospital in Region Stockholm in 2005 and have remained so except for ablation, which is performed in an affiliated hospital. The preferred method for TACE was changed from lipiodol-based to drug-eluting beads in 2010 [Citation18]. The proportion of patients discussed at the regional multidisciplinary treatment conference (MDT) was also analysed.
Data extraction from the regional health care data register
All data were extracted electronically. Included individuals were pseudonymized by non-identifiable consecutive case numbers. For two validation analyses, all individuals with a first-time HCC diagnosis 2014–2015 were de-anonymized and re-identified with their personal unique PIN.
Validation analyses
In a first analysis, we validated the underlying aetiologies of HCC that were identified or inferred from comorbidities in the regional health care database. We used a clinical HCC database at Karolinska University Hospital for reference. This database includes data from medical charts on all HCC cases that have been managed in the Karolinska Hepatology Department [Citation15]. Validation was performed by cross-checking the diagnosis made in the regional health care database to the HCC database, and both the positive predictive value, PPV (the diagnosis was correct), and negative predictive value, NPV (absence of the diagnosis was correct) were calculated.
In a second validation analysis, we manually reviewed medical records for patients who received only BSC 2014–2015. We limited the review to all individuals who had their medical records within the electronic healthcare chart system TakeCare™ (https://profdoccare.se). This system has gradually been implemented since 2004 and now covers most healthcare provided in Region Stockholm. We explored whether HCC surveillance had been implemented, reasons for patients not being part of a surveillance program, and reasons for not applying active HCC therapy. The charts were reviewed by two of the study authors (SN and SW) who had to have a consensus on each case. Surveillance was assessed based on EASL criteria for HCC surveillance [Citation2] and deemed implemented if a liver ultrasound was performed every 6–9 months. The purpose of this second clinical in-depth review was to analyse reasons for only receiving BSC, specifically to see if surveillance practices were incomplete and contributing to late diagnosis and thus potential loss of opportunities for active or even curative HCC treatment.
Survival outcomes
The starting point for the survival analyses was the first date of an HCC diagnosis in the register. Overall survival (OS) was defined as the time from the starting point to the date of death or the last date for survival status update (Nov 28, 2019).
Socioeconomic analysis
Data on socioeconomic status were extracted to analyse any differences between different socioeconomic groups regarding aetiology patterns, access to treatment, and outcome. The Region Stockholm uses the Mosaic system, which is based on the proposition that geographical areas share common patterns due to residential segregation. It uses multivariate modelling utilizing over 400 variables to group postal codes into different types. The Swedish population is divided into 171 subtypes and 74,000 different postal areas with a geographic area of 125 × 125 meters containing ∼121 persons or 62 families. In Stockholm, the Mosaic system includes socioeconomic information from regular public health surveys conducted by Region Stockholm, such as the survey from 2014 [Citation19] published by Karolinska public health academy using the Mosaic system. The Mosaic system was developed by Webber et al at Kings College University, London. It is based on data from 29 different countries and has been shown to be useful for the classification of cohorts in epidemiologic research [Citation20]. In Region Stockholm, the different clusters are aggregated into broader socioeconomic groups in three levels, i.e., high, middle, or low creating three broader socioeconomic groups in the analyses: high education and high income; intermediate education and intermediate income; and low education and low income.
Statistical analyses
Datasets were stratified for age, sex, time period, and socioeconomic status. Categorical variables are summarized as counts and percentages. The incidence rate per 100,000 inhabitants was calculated without adjusting for potential differences in age distributions. A best-fit linear trendline was used to illustrate the change in incidence during the study period. Kaplan-Meier curves were plotted to describe overall survival by categorical groups. Median, one and five-year survival rates were calculated and compared with log-rank statistics and Chi-square tests. Results are presented with 95% confidence intervals (CI). Group-wise differences were analysed with Chi-square tests. A p-value <.05 was considered statistically significant. The SAS 9.4 software was used for statistical analyses.
The study was approved by the regional ethics review board in Stockholm (Dnr: 2018/149-31). Informed consent was waived by the committee because of the retrospective nature of the data collection process and because there was no direct contact with any of the patients.
Results
Characteristics of the study population
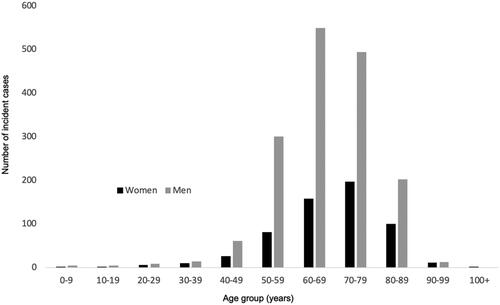
Two thousand two hundred and forty-five individuals (26% female) constituted our study population (). An additional 48 (2%) incident cases were excluded as they had emigrated out of the Stockholm region. The incidence of HCC increased during the study period from ∼6 to 7.5 per 100,000 inhabitants (). 93% of the individuals were diagnosed after age 50, with peaks in the 7th (men) and 8th (women) decades of life (). The median age at diagnosis was 68 years.
Figure 1. Total incidence of HCC per 100,000 inhabitants in Region Stockholm 2003–2018. The dotted line represents linear trend.
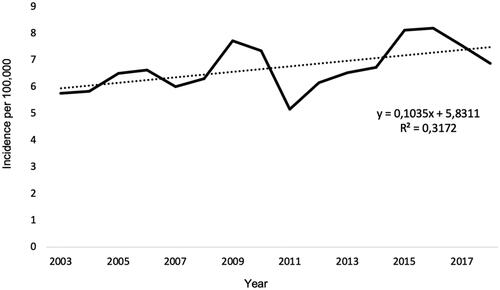
Table 1. Characteristics of the study population.
Underlying risk factors for HCC
Risk factors representing underlying aetiologies were found in 83% of patients with HCC. No aetiology was identified in 24% of women and in 11% of men. The most common aetiologies were HCV (26%), NAFLD (22%), and ARLD (19%) (). These three aetiologies also increased the most in absolute and relative numbers during the study period (data not shown). The proportion of patients without an identified aetiology decreased from 20 to 7% during the study period. Diabetes and/or obesity were mostly used for identifying NAFLD, but in 27 (5%) the diagnosis was based on hyperlipidaemia.
Validation of aetiologies 2014–2015
Among 329 cases found in the regional health care database 2014–2015, 201 (61%) were also included with complete clinical information in the local HCC database at the Hepatology Department, Karolinska University Hospital. Concordance between identified risk factors, such as HCV, NAFLD, and ARLD in the regional register and the clinical registers of patients with HCC were analysed and illustrated by positive predictive value, PPV, and negative predictive value, NPV. See for results. The concordance was near perfect for rare liver diseases (PPV 100% and NPV 99%). We found reasonably good concordance for NAFLD (PPV 83%, NPV 91%) and ARLD (PPV 87%, NPV 96%), aetiologies that are often not clearly registered in clinical practice. The poorest concordance was found in the group without an identified underlying aetiology for HCC (PPV 48%, NPV 97%).
Table 2. Analysis of concordance between risk factors identified in the regional health care data register in Region Stockholm and in the clinical HCC register at Karolinska University Hospital 2014–2015.
Management of HCC
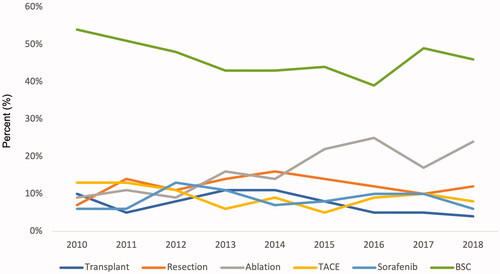
The proportion of incident cases discussed at the regional multidisciplinary therapy conference increased from 86% in 2010–2011 to 98% in 2017–2018. In total, 36% received a potentially curative treatment (transplantation, resection, or ablation), and this proportion increased from 26 to 40% between 2010 and 2018, mostly due to increasing rates of ablation therapy (9% in 2010, 24% in 2018). In total, 18% received palliative treatment (TACE or sorafenib), and 52% had no active HCC treatment (BSC). The proportion of patients that did not receive any treatment except best supportive care decreased from 54% in 2010 to 46% in 2018 ().
Investigation of reasons for best supportive care 2014–2015
We identified 143 (43%) individuals who received only BSC 2014–2015. Complete clinical information was available for 111 (78%) of these in the electronic medical chart system. A definite HCC diagnosis and no active treatment could be verified for all.
Among these 111, 85 (77%) were deemed not to fulfill standard criteria for biannual HCC ultrasound surveillance since they had no previously known liver cirrhosis or a low-performance status [Citation2]. Among 26 (23%) for whom surveillance was regarded indicated, 6 (23%) individuals had chosen not to be under surveillance, and surveillance was never initiated for 15 (58%) individuals despite being indicated (as suggested by a review of their medical charts). Five (19%) were under surveillance but received only BSC as poor performance status or advanced HCC prevented active treatment.
Among the 21 patients who could have been but were not included in surveillance, 19 (90%) (17% of the 111 who received BSC only) had too advanced disease (Barcelona Clinic Liver cancer, BCLC, stage D) at HCC diagnosis to be eligible for active treatment. Two (10%) were offered sorafenib but declined. Nineteen (90%) thus received only BSC among 21 patients for whom surveillance was indicated but never initiated. In the 2014–2015 validation cohort, 14% with potentially treatable HCC thus received only BSC during a two-year period (2014–2015) in the Stockholm region.
Survival analyses
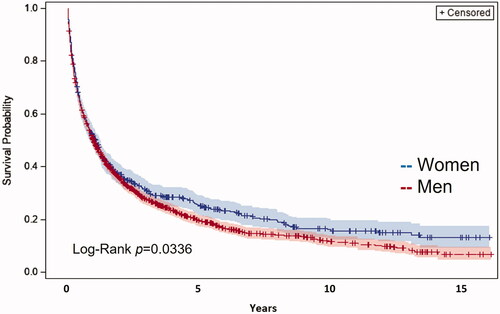
Overall survival (OS) stratified by sex is shown in . Median OS was significantly better for women, 396 days (95% CI, 313–476) compared to 368 days for men (95% CI, 336–416, p = .03). The median overall survival increased during the study period from 281 days (95% CI, 209–349) in 2003–2006 to 509 days (95% CI, 423–618) year 2015–2018 (p < .001).
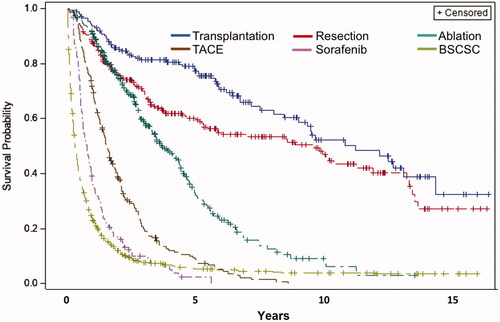
OS stratified for different treatments is shown in . As expected, the potentially curative treatment alternatives (transplantation, resection, and ablation) had better outcomes than palliative treatments (TACE and sorafenib) and best supportive care. The one-year and five-year survival probabilities were 94 and 79% for transplantation, 87 and 60% for resection, 91 and 35% for ablation, 71 and 9% for TACE, 36 and 2% for Sorafenib. BSC had a 23% one-year survival and a 6% five-year survival. Median survivals for the different treatments were: transplantation 11.5 years (95% CI, 9.3–14.8 years), resection 9.9 years (96% CI, 5.6–12.2 years), ablation 3.8 years (95% CI, 3.4–4.5 years), TACE 1.6 years (95% CI, 1.3–1.8 years), sorafenib 0.8 years (95% CI, 0.6–0.9 years) and BSC 0.3 years (95% CI, 0.3–0.4) p < .0001.
Patients with rare liver diseases and viral hepatitis as HCC aetiologies had the best outcome with one-year and five-year OS of around 60 and 30%, respectively. Patients with ARLD, NAFLD, cryptogenic cirrhosis, and no identified aetiology had worse outcomes with 1-year and 5-year OS of around 50 and 20%, respectively. Patients with cryptogenic cirrhosis had a five-year OS of only 6% (data not shown).
Socioeconomic analyses
The distribution in the Region Stockholm of the three broader socioeconomic groups (high education and income; intermediate education and income; and low education and income) was 30, 40, and 30%. The corresponding distribution in the HCC cohort 2003–2018 was 22, 36, and 42% (missing data for 2%) (p = .03), indicating a higher HCC incidence in the low education and income group. The different risk factors for HCC were evenly distributed between socioeconomic groups but the low education and income group had somewhat higher rates of HBV and HCV and somewhat lower rates of ARLD (data not shown). We found no significant differences in treatment allocations (grouped as curative, non-curative, and BSC), or in OS probability (low vs. intermediate: p = .93, Intermediate vs. high: p = .94, low vs. high: p = .91) between socioeconomic groups.
Discussion
The incidence and the etiologic panorama of HCC in our study reflect the epidemiology of causative risk factors in Sweden. Instead of HBV and aflatoxin, which dominate globally, HCV, ARLD, and NAFLD were the most common identified risk factors. That HCC in Sweden is the 13th rather than the 6th most common cancer form, as seen globally [Citation1], is most likely explained by low HBV prevalence (<2%) in Sweden [Citation11]. HBV is estimated to be the cause in 54% of HCC cases globally [Citation21], in about 20% in the western world [Citation21], but only in 9% of our cases.
The prevalence of chronic HCV is also low at about 0.5% [Citation22]. As the prevalence of HCV-related cirrhosis decreases, HCC caused by HCV is expected to decrease. Indeed, HCC caused by HCV increased from 2003 to 2016 in absolute and relative numbers but decreased in 2017–2018. Overall, HCV was the most common aetiology during the study period in 26% of the patients.
The evolving obesity and NAFLD epidemics in the world have influenced the landscape of HCC also in Sweden, even if the estimated obesity prevalence in Sweden of 15% of the adult population [Citation23] is towards the lower end in an international comparison. NAFLD as the aetiology for HCC continuously increased during the study period. We found NAFLD to overall be the second leading cause of HCC, responsible for 22% of incident HCC overall and for 26% in women.
Studying NAFLD poses difficulties in registry research. NAFLD appears to be underdiagnosed or at least not coded as such [Citation24]. Only one patient of the NAFLD-related HCC cases had been diagnosed with fatty liver disease (ICD-10 K76.0). With a search strategy that integrated associated comorbidities, such as diabetes mellitus and obesity, and excluded other liver diseases, the NAFLD group increased to 22%. This proportion is more in line with both international and earlier Swedish reports [Citation25]. This, together with the reasonably good concordance between the register and validation cohorts (PPV 83%, NPV 91%), suggests that the search strategy we used was reasonable.
Despite a decline in the mean alcohol consumption in Sweden during the last decade [Citation26], ARLD was the third most common aetiology for HCC, responsible for 19% overall, and 10% in women. The true proportion is probably higher as suggested by the validation sub-study in which 54% of the patients identified as cryptogenic cirrhosis in the database rather had ARLD in the clinical validation 2014–2015.
Many had ICD-10 codes suggesting two or more aetiologies/risk factors. Our choice to rank viral disease higher than ARLD, even if ICD-10 codes for both were present, also influenced the results. We chose to give easily definable diagnoses in the ICD-system, such as primary liver diseases, HBV, and HCV a higher rank.
The group without an identified aetiology decreased during the study period. This most likely reflects improved diagnosis or coding practices. In the validation cohort 2014–2015, the absence of a risk factor for HCC in the register had a relatively poor PPV (48%) but almost perfect NPV (97%). We noticed a sex difference in this group, as 24% of the women but only 11% of the men did not have an identified risk factor. This could lead to inequality regarding treatment since surveillance is only initiated if a risk factor for HCC is known. We did, however, not see any significant differences regarding allocated treatment between genders and women also had a better overall survival ().
Adherence to a general recommendation that all new cases of HCC should be evaluated at the regional multidisciplinary conference gradually increased during the study period. We found that 98% of all cases had been discussed at the conference at the end of the study period. We did not analyse the characteristics of the small proportion of patients that did not reach the conference and therefore do not know whether they differed regarding, e.g., advanced age or disease state.
The guiding treatment algorithm in Region Stockholm is closely related to the Barcelona Clinic Liver Cancer (BCLC) algorithm and largely follows international guidelines [Citation2,Citation11]. Consequently, the survival outcomes do not differ much from previous international reports. The 79% five-year survival after liver transplantation in our cohort 2003–2018 is, however, surprisingly high. Of note, we found a minimal difference between the survival curves for sorafenib and BSC (), which appeared to originate early after diagnosis. The lack of clinical information prevents analysis of whether this reflects a difference in patient selection.
HCC surveillance is associated with early diagnosis and improved survival [Citation27]. The large proportion (52%) that received no active HCC treatment suggests that there is considerable room for improvement regarding adherence to surveillance recommendations. Surveillance practices, such as rates and frequencies of ultrasound surveillance were not possible to ascertain as radiology performed in hospitals are not included in the regional health care data register. We could not investigate what proportion of patients in Region Stockholm with an indication for surveillance were indeed included in surveillance, what proportion of all HCC cases were detected in surveillance, and whether these proportions changed from 2003 to 2018. However, we analysed radiological surveillance for HCC cases in 2014–2015 who received only best supportive care. This analysis suggested that at least 14% (15 of 111) of potentially treatable HCC received only BSC. We found it encouraging that the proportion of patients that received active HCC treatment almost doubled from 2010 to 2018 ().
The relative distribution between socioeconomic groups differed between the HCC cohort (42% low education and income) and the general population (30% low education and income). Viral hepatitis was a more common risk factor for HCC (41%) in the low education and income group. Immigration from countries with a higher prevalence of HBV and HCV may partly explain this difference. We, however, did not note any differences in treatments or survival between socioeconomic groups.
The Swedish health care system is tax-funded and all care providers in Region Stockholm report to the regional health care database. This study, therefore, has virtually complete coverage, which enables reliable epidemiological analyses. All HCC therapy is provided within the Stockholm Region and only patients residing in Stockholm were included in the study. The risk for referral bias is therefore negligible. We do acknowledge some other limitations. The study collected no clinical information on the severity of liver disease or extent of tumour growth, which both influence therapy choices. Quantitative risk factors, such as BMI, smoking, and alcohol consumption were not available for analysis. Register-based research carries a risk of misclassification bias. Manual reviews of patient charts for HCC cases 2014–2015 were done to estimate the extent of such bias. We found perfect concordance for rare aetiologies and reasonably good concordance for NAFLD and ARLD (), aetiologies for which the risk of misclassification is expected to be significant. The concordance was lower for cryptogenic cirrhosis and for those with no identified risk factor. For follow-up completeness and reliable outcome assessment, we excluded individuals who migrated out of Stockholm after HCC diagnosis. This introduced potential bias but the low proportion (n = 48, 2%) limited the impact on our analyses.
To conclude, in this registry-based regional study of more than a fifth of Sweden’s total population with detailed data, HCC incidence increased from 6 to 7.5/100,000 during the study period. HCV, NAFLD, and ARLD were the three most common aetiologies. Access to treatment and outcomes did not differ between socioeconomic groups. The proportion of patients that was discussed at the regional multidisciplinary therapy conference increased and the proportion of patients without an identified aetiology decreased. It is unsatisfactory that almost half of the patients still receive only BSC and that the 2014–2015 sub-study suggested that one in seven could potentially have been subject to active treatment if surveillance practices had been fully implemented. Study results suggest a continuing problem with incomplete surveillance practices.
Ethical approval
The study was approved by the regional ethics review board in Stockholm (Dnr: 2018/149-31). Informed consent was waived by the committee because of the retrospective nature of the data collection process and because there was no direct contact with any of the patients.
Author contributions
SW conceptualized the study. GL, SN, and BB extracted and assembled the data. GL, SN, and SW analysed the data. SN and SW wrote the manuscript. All authors reviewed the manuscript and approved the final version.
| Abbreviations | ||
| PLC | = | primary liver cancer |
| HCC | = | hepatocellular carcinoma |
| ICD-10 | = | 10th revision of the International Statistical Classification of Diseases and Related Health Problems |
| OS | = | overall survival |
| TACE | = | transarterial chemoembolization |
| HBV | = | hepatitis B virus |
| HCV | = | hepatitis C virus |
| NAFLD | = | non-alcoholic fatty liver disease |
| ARLD | = | alcohol-related liver disease |
| BSC | = | best supportive care |
| MDT conference | = | multidisciplinary treatment conference |
Supplemental Material
Download MS Word (1.4 MB)Disclosure statement
The authors declare no conflicts of interest.
Data availability statement
The data that support the findings in this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Torner A, Stokkeland K, Svensson A, et al. The underreporting of hepatocellular carcinoma to the cancer register and a log-linear model to estimate a more correct incidence. Hepatology. 2017;65(3):885–892.
- Bengtsson B, Askling J, Ludvigsson JF, et al. Validity of administrative codes associated with cirrhosis in Sweden. Scand J Gastroenterol. 2020;55(10):1205–1210.
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171.
- Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156(2):477–491.e1.
- Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735.
- Neovius K, Johansson K, Kark M, et al. Trends in self-reported BMI and prevalence of obesity 2002–10 in Stockholm county, Sweden. Eur J Public Health. 2013;23(2):312–315.
- National Board of Health and Welfare's Cancer Register [Internet]; 2020 [cited 2021 Sept 15]. Available from: https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/swedish-cancer-register/
- Edenvik P, Davidsdottir L, Oksanen A, et al. Application of hepatocellular carcinoma surveillance in a European setting. What can we learn from clinical practice? Liver Int. 2015;35(7):1862–1871.
- Henriksson M, Bjornsson B, Sternby Eilard M, et al. Treatment patterns and survival in patients with hepatocellular carcinoma in the Swedish National Registry SweLiv. BJS Open. 2020;4(1):109–117.
- Nilsson E, Anderson H, Sargenti K, et al. Risk and outcome of hepatocellular carcinoma in liver cirrhosis in Southern Sweden: a population-based study. Scand J Gastroenterol. 2019;54(8):1027–1032.
- Davidsdottir L, Duberg AS, Torner A, et al. Hepatocellular carcinoma in individuals with HBV infection or HBV-HCV co-infection in a low endemic country. Scand J Gastroenterol. 2010;45(7–8):944–952.
- Strauss R, Torner A, Duberg AS, et al. Hepatocellular carcinoma and other primary liver cancers in hepatitis C patients in Sweden – a low endemic country. J Viral Hepat. 2008;15(7):531–537.
- Bengtsson B, Stål P, Wahlin S, et al. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019;39(6):1098–1108.
- Simon TG, Roelstraete B, Sharma R, et al. Cancer risk in patients with biopsy-confirmed nonalcoholic fatty liver disease: a population-based cohort study. Hepatology. 2021;74(5):2410–2423.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667.
- Karalli A, Teiler J, Haji M, et al. Comparison of lipiodol infusion and drug-eluting beads transarterial chemoembolization of hepatocellular carcinoma in a real-life setting. Scand J Gastroenterol. 2019;54(7):905–912.
- Center of Epidemiology and Community Medicine in Region Stockholm. Living conditions, living habits and health in Stockholm County; 2014 [cited 2021 Sept 15]. Available from: http://dok.slso.sll.se/CES/FHG/Jamlik_halsa/Rapporter/livsvillkor-levnadsvanor-halsa.2014_3.2014.pdf
- Douglas L, Szatkowski L. Socioeconomic variations in access to smoking cessation interventions in UK primary care: insights using the mosaic classification in a large dataset of primary care records. BMC Public Health. 2013;13:546.
- de Martel C, Georges D, Bray F, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–e190.
- Nordvik M, Axelsson M, Berglund T, et al. Estimation of the number of individuals living with hepatitis C-infection in Sweden: Monica K. Nordvik. Eur J Public Health. 2016;26(suppl_1). DOI: 10.1093/eurpub/ckw174.206
- Public health agency of Sweden. Overweight and obesity; 2018 [cited 2021 Sept 15]. Available from: https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/living-conditions-and-lifestyle/obesity/
- Alexander M, Loomis AK, van der Lei J, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17(1):95.
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.
- Trolldal L. CAN report; Alcohol consumption i Sweden 2016; 2017 [cited 2021 Sep 15]. Available from: http://www.can.se/contentassets/3ecc5b9f2dd849bd992e23094b5a8473/alkoholkonsumtionen-i-sverige-2016.pdf
- Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099–1106.e1.