Abstract
Background
Esophageal adenocarcinoma (EAC) is the sixth leading cause of cancer-related death worldwide. It develops through Barrett’s metaplasia – dysplasia sequence. However, the effectiveness of endoscopic surveillance is limited, since diagnosis of low-grade dysplasia (LGD) is known to be challenging for pathologists. Our aim was to compare the risk of Barrett’s progression based on diagnoses of general and expert gastrointestinal (GI) pathologists in a population-based cohort.
Methods
A total of 60 patients with non-dysplastic metaplasia (BE) or LGD progressing to high grade dysplasia (HGD) or EAC during follow-up could be identified in the population. For comparison, series representing non-progressive BE (n = 56) and LGD cases (n = 54), matched for age, gender, and length of follow-up were collected. All available original HE stained slides (n = 292) were blindly re-evaluated by two experienced GI pathologists and patient groups of progressive non-progressive BE and LGD were formed according to revised diagnoses.
Results
Original diagnosis for each sample was changed in 25% of BE, 59% of LGD, and 33% of HGD diagnoses. Of the original LGD diagnoses, 53% were downgraded to BE or indefinite for dysplasia (ID). Of LGD diagnoses made by an expert GI pathologist, 61% were in the progressive LGD group, whereas only 42% of general pathologists’ LGD diagnoses were in the progressive LGD group.
Conclusion
Based on this retrospective case-control study, LGD is strongly over-diagnosed among general pathologists. LGD diagnosed by expert GI pathologists predicts progressive disease. Recommendation for consensus diagnosis by expert GI pathologists is justified also in the Finnish population-based setting.
Introduction
Barrett’s esophagus (BE) is the main risk factor for esophageal adenocarcinoma (EAC), a sixth leading cause of cancer-related death worldwide [Citation1]. BE is considered to result from gastroesophageal reflux disease with chronic tissue injury and inflammation due to gastric acid content irritation [Citation2]. In BE, the normal esophageal stratified squamous epithelium is replaced by intestinal-type mucinous simple columnar epithelium [Citation2]. The incidence of BE is increasing as a consequence of obesity-related reflux disease [Citation1].
EAC develops through Barrett’s metaplasia – dysplasia sequence [Citation3]. Diagnosis of BE-associated low-grade dysplasia (LGD) is challenging for pathologists [Citation2]. For instance, in studies where at least two expert GI pathologists re-evaluated tissue samples, 73–85% of originally diagnosed LGDs were downgraded to non-dysplastic metaplasia or indefinite for dysplasia [Citation4]. More importantly, the consensus diagnosis by the experts resulted in approximately 33% progression rate during a 5-year follow-up, whereas the downgraded consensus of LGD to indefinite for dysplasia (ID) or non-dysplastic metaplasia 5-year progression rate was under 3% [Citation5,Citation6]. There is no evidence supporting routine follow-up of BE patients. However, all national guidelines recommend endoscopic surveillance every 6–12 months if BE has progressed to LGD [Citation7], and in cases with persisting LGD, treatment with radiofrequency ablation is recommended [Citation8].
The aim of the current study was to compare Barrett’s metaplasia and dysplasia diagnoses between general and expert GI pathologists, and the risk of progression to high-grade dysplasia (HGD) or EAC. Furthermore, we aimed to report the results of the executed endoscopic follow-up for BE and LGD patients in the Northern- and Central Finland populations.
Materials and methods
Study design
This study was a retrospective case-control study derived from the Northern- and Central Finland population. Study inclusion criteria were HGD or EAC diagnosis with endoscopic surveillance for at least six months before the first HGD or EAC diagnosis. We identified 60 patients with HGD or EAC who met the inclusion criteria according to Oulu University Hospital and Jyväskylä Central Hospital records between the years 1987–2013 and 1995–2014, respectively. Control patients with non-progressing BE (n = 56) and LGD (n = 54) were identified from Oulu University Hospital and they were matched for sex, age, BMI and follow-up time. We defined BE as the presence of columnar epithelium in the esophagus, including patients with either gastric or intestinal metaplasia or both, and macroscopically visible lesion confirmed by the endoscopist.
Data collection
The study patients were identified from the archives of the Departments of Pathology, Oulu University Hospital, and Jyväskylä Central Hospital, Finland. In Finland, endoscopic surveillance for patients with BE has been implemented also in primary healthcare. In Northern Finland, endoscopic samples taken in primary health care centers have been centralized in the archives of the Oulu Pathology Laboratory of the Cancer Society of Finland, and in Central Finland samples are centralized in the archives of the Department of Pathology at the Jyväskylä Central Hospital since 1995. All previous endoscopy samples and diagnostic hematoxylin-eosin (HE) -stained slides were retrieved from the pathology archives mentioned earlier and from the archives of the Departments of Pathology at Central Ostrobothnia Central Hospital, Kokkola; Länsi-Pohja Central Hospital, Kemi; Kainuu Central Hospital, Kajaani, and Lapland Central Hospital, Rovaniemi. Clinical data for each patient was obtained from the patient records, including pathology reports.
Re-assessment of original pathological samples
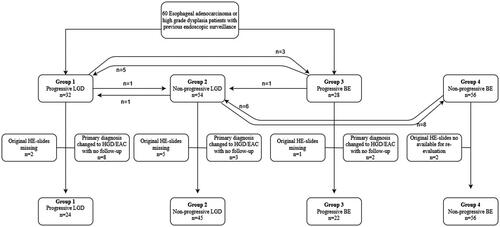
Of the original 170 patients, a total of 339 samples were identified from archives. Of these, 292 samples from 147 patients were available included in the study. Samples were independently re-assessed by two experienced GI pathologists (T.J.K and J.M) strictly blinded for clinical data, original diagnoses, possible duplicate samples from the same patients and each other’s diagnoses. Changes to the original diagnosis required agreement between both expert GI pathologists. If there was a disagreement between the expert GI pathologists in the re-evaluation, a consensus diagnosis was made after discussion. After re-evaluation, patients were divided into four groups according to findings during the follow-up: Group 1, progressive LGD, contained patients who had at least one endoscopy performed with a diagnosis of LGD at least six months before the diagnosis of HGD or EAC (= endpoint); Group 2, non-progressive LGD, included patients with LGD not developing to HGD or EAC; Group 3, progressive BE, included patients who initially showed non-dysplastic BE at least six months before progression to HGD or EAC; Group 4, non-progressive BE, included patients who did not progress to LGD or to more advanced lesions during the follow-up. The minimum length of follow-up in years in groups 1, 2, 3 and 4, was 1.8, 3.51, 2.4 and 5.5 years, respectively.
Outcomes
The main aims were to compare diagnoses of BE with and without dysplasia between general and specialized GI pathologists and to compare the prognostic value of diagnoses made by general pathologists and specialized GI pathologists, in terms of progression to HGD or EAC.
Statistical analysis
Statistical analysis was performed with IBM SPSS statistics 24.0 (IBM Corp., Armonk, NY, USA). For baseline information, we used frequency analyses and the Mann-Whitney U-test was used to compare differences between two independent groups with continuous variables. For categorical data analysis, we used the χ2-test and Fisher-test. The threshold for significance was set at p < .05. In all continuous variables, the median and interquartile range is presented. Follow-up times were calculated based on the time between the first and last endoscopy. Cohen’s kappa was calculated to analyze the interobserver agreement.
Results
Patients
All histological samples (n = 292) from 147 patients were re-evaluated by two expert GI pathologists (T.J.K and J.M) and classified as EAC, HGD, LGD, ID or BE. The distribution of patients according to re-evaluation into four groups is described in . Patient characteristics in each group are presented in . After re-evaluation, diagnosis in 13 patients with the initial diagnosis of LGD or BE were revised to HGD or EAC. Since this change led to the loss of the prior six-month follow-up criteria, and due to that, a total of 25 samples from these patients were excluded from further analysis. Twelve samples were non-diagnostic and alternative samples were not available resulting in exclusion.
Figure 2. Demonstrating original HE microscopic slides in which primary diagnosis was changed from non-dysplastic metaplasia to low-grade dysplasia (a) after expert GI pathologist re-evaluation. (b) original LGD was downgraded to BE, (c) original LGD did not change and (d) original LGD diagnosis was upgraded to HGD. All images are taken with 20x magnification and 100 µm scalebar is found in image D.
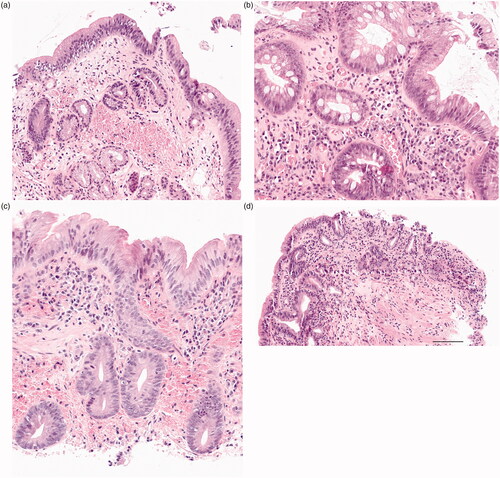
Table 1. Characteristics of patients grouped into different progression patterns according to re-assessed diagnoses.
Primary outcomes
After re-evaluation, the original diagnosis was changed in 34% of the original histological samples. More specifically, 25% of original BE (34/136), 59% of LGD (46/78) and 33% of HGD (9/27) diagnoses were changed after re-evaluation. Of original LGD diagnoses, 53% (41/78) were downgraded to BE or ID (). Re-evaluation results and changes in diagnosis are presented in detail in .
Table 2. Comparison of original and expert GI pathologist diagnoses for each sample. Groups were stratified into progressors (progression to HGD or LGD) and non-progressors accordingly based on expert GI pathologist diagnoses.
Expert GI pathologists disagreed in 30% of samples providing a kappa value of 0.62, and these cases were evaluated jointly in an attempt to reach a consensus. Consensus evaluation was needed in 35%, 48% and 49% of samples for final HGD, LGD and ID, respectively. Only 8.5% of the final BE diagnoses needed a consensus. The need for consensus evaluation in different categories is presented in .
When expert GI pathologists evaluated LGD lesions, they downgraded the original diagnosis in the progressors group in only 31% of samples, whereas in the non-progressors group downgrading occurred in 70% (p = .001, and ). Of the initial LGD diagnoses made by an expert GI pathologist, 61% were in the progressive LGD group, whereas only 42% of general pathologists’ LGD diagnoses were in the progressive LGD group ( and ). Of the initial BE diagnoses made by an expert GI pathologist 35% were in the progressor group and 37% of general pathologists’ BE diagnoses were in the progressor group ( and ).
Expert GI pathologists diagnosed HGD or EAC 4.6 months (median, IQR 1.3–13.0) earlier compared to general pathologists. In three cases expert GI pathologists diagnosed HGD or CA 5, 4 and 2 years earlier compared to general pathologists.
Secondary outcomes
In the progressive LGD group (Group 1), macroscopically long Barrett’s segments (over 10 cm) were more frequent (37% of cases) compared to other groups (7–11 cm; p < .001, ). In the comparison of progressive and non-progressive LGD (Groups 1 and 2), the length of follow-up time, age, sex, reflux symptoms and prior PPI use were similar (). Patients with progressive BE (Group 3) suffered more from reflux symptoms (91%) than non-progressive BE (Group 4) patients (66%) (p < .001 (). Otherwise, there were no differences between the groups ().
Discussion
This study shows that LGD is over-diagnosed among general pathologists. Initial LGD diagnosis as re-evaluated by two expert GI pathologists was associated with a high risk of progression to HGD or EAC. Moreover, the agreement between two expert GI pathologists was substantial, suggesting that two independent expert GI pathologists are needed to diagnose LGD as current guidelines also state. Near perfect agreement in the diagnosis of non-dysplastic BE was found among the expert GI pathologists, suggesting there is no need for two separate expert evaluations in cases without features of dysplasia.
The major strength of the current study is that it is a geographically representative population-derived case-control study showing results from a routine clinical follow-up and diagnostic chain of BE patients. The collection of biopsy material for the re-evaluation was also as systematic as possible and only isolated original diagnostic HE slides were unavailable. Furthermore, strict blinding of clinical and inter-observer data was maintained during the re-evaluation. The major weakness of the study is the limited number of progressive LGD and BE patients. However, according to Finland’s cancer register and previous publications, EAC annual incidence in Finland is approximately 9.23/100.000 in males and 3.08/100.000 in females (https://syoparekisteri.fi/tilastot/tautitilastot/ accessed 15 April 2021). With retrospective evaluation only 60 patients with prior esophageal biopsies and Barrett’s diagnosis were identified, suggesting that selection bias is inevitable in this sort of study design, and only a minority of EAC patients undergo prior endoscopies resulting in surveillance. Another weakness is the retrospective study design where previous endoscopic samples were obtained based on identifying patients treated for HGD or EAC. This population-based approach resulted in the identification of patients with previous BE or LGD progressing to HGD or EAC, but we were not able to detect patient follow-ups due to non-progressing BE or LGD. Instead, the control patients were identified in the pathology database and matched with the population samples for sex and age. Accordingly, although the identification of studied patients was population-based, the final study is a case-control study. Therefore, our study does not provide data on real population-based progression risks of BE and LGD, but on odds related to non-selected and non-progressing lesions. However, the main aim of the current study was to compare BE-associated diagnoses between expert GI and general pathologists in Finland, to observe whether expert GI pathologists could distinguish between progressive and non-progressive disease, and to investigate realized endoscopic surveillance, which has not been previously published.
Patients with BE have a significantly increased risk of developing EAC over that of the general population (∼30 ×) [Citation9], however annual malignant progression rate is only 0.33–0.5% [Citation2]. The annual progression rate of LGD to EAC in the literature is 0.4–13.4% [Citation4,Citation8,Citation10]. This highly variable rate is due to challenges with the histological diagnosis of LGD, which could be microscopically indistinguishable from, for example, reactive abnormalities related to active inflammation [Citation5]. Concordance among pathologists regarding the criteria for LGD is also low [Citation11]. However, the grade of dysplasia is currently still the best available prognostic factor for evaluating progression, and it guides management and treatment options for dysplasia [Citation2,Citation11,Citation12]. The Vienna classification is a globally accepted standard for determining dysplasia in gastrointestinal mucosal specimens, but it still harbors an inherent degree of subjectivity [Citation13]. Our study shows good interobserver agreement between two expert GI pathologists, with a kappa value of 0.62. In previous studies with the re-evaluation of Barrett’s LGD, kappa values were 0.32–0.69 and re-evaluations were done by at least two expert GI-pathologists [Citation14–16]. In the presented study, 53% of the original routine LGD diagnoses were downgraded to ID or BE after expert re-evaluations, which is in a line with previous studies [Citation4,Citation14]. In our material, in 6.4% of cases with HGD or EAC at re-evaluation, expert GI pathologists diagnosed HGD or EAC at least two years earlier compared to general pathologists. However, whether earlier diagnosis in these patients could have improved prognosis remains speculative.
Current guidelines for endoscopic surveillance of BE patients vary from no surveillance to surveillance every three years for metaplasia with no dysplasia depending on the length of BE area, 6–12 months for LGD, or intervention with possible endoscopic ablation procedures [Citation7,Citation8]. Our findings support the role of length of BE mucosa in progression risk assessment since progressive LGD patients’ Barrett segments were significantly longer. In our material, patients with progressive LGD and with non-progressive LGD had endoscopic surveillance intervals of 14 and 18 months, indicating that in Central and Northern Finland LGD endoscopic follow-up occurs too seldom when compared to common guidelines [Citation7]. However, in both groups, there were also non-dysplastic biopsies taken between LGD samples which might have influenced the executed surveillance. In addition, in patients with progressive BE, the median endoscopy surveillance rate was 0.34 endoscopies/year, and with non-progressive BE the rate was 0.25 endoscopies/year. None of the patients underwent endoscopic procedures during follow-up for LGD.
Presented results support previously published data showing that most EAC develops in patients with no previous endoscopic surveillance. Moreover, our results are in a line with the current recommendation that diagnosis of LGD should be done by at least two expert GI pathologists. Duits et al. demonstrated an increase in LGD progression risk when the diagnosis was done by expert GI pathologists [Citation5,Citation17]. When three pathologists agreed on LGD diagnosis, the odds of progression increased 47-fold (95% CI 13.10–169.70). The annual progression to HGD/EAC increased to 2.4%, 6.3%, and 20% when one, two, and three pathologists confirmed the diagnosis of LGD, respectively [Citation17]. However, among the 3 pathologists, an overall agreement was poor with the highest disagreement noted for differentiating the LGD (k = 0.21) [Citation12]. Accordingly, predictive immunohistochemical markers for BE and LGD malignant progression are desperately needed for routine clinical work. For instance, p53 immunostaining has shown promising results but it is still not in routine clinical use [Citation18–20].
According to our results, a high proportion of LGD diagnoses are downgraded after expert GI pathologists’ re-evaluation. This finding supports current recommendations for LGD diagnostics, which should be done by at least two expert GI pathologists. Agreement on the diagnosis of columnar metaplasia without dysplasia between expert GI pathologists was high, suggesting no need for multiple evaluations. Furthermore, LGD consensus diagnosis by two expert GI pathologists results in a significant risk for malignant transformation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Rubenstein JH, Shaheen NJ. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology. 2015;149(2):302–317.e301.
- Schlottmann F, Patti MG. Current concepts in treatment of Barrett's esophagus with and without dysplasia. J Gastrointest Surg. 2017;21(8):1354–1360.
- Fitzgerald RC. Molecular basis of Barrett's oesophagus and oesophageal adenocarcinoma. Gut. 2006;55(12):1810–1820.
- Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett's esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105(7):1523–1530.
- Duits LC, Phoa KN, Curvers WL, et al. Barrett's oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2015;64(5):700–706.
- Krishnamoorthi R, Lewis JT, Krishna M, et al. Predictors of progression in Barrett's esophagus with low-grade dysplasia: results from a multicenter prospective BE registry. Am J Gastroenterol. 2017;112(6):867–873.
- Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy. 2017;49(02):191–198.
- Qumseya BJ, Wani S, Gendy S, et al. Disease progression in Barrett's low-grade dysplasia with radiofrequency ablation compared with surveillance: systematic review and meta-analysis. Am J Gastroenterol. 2017;112(6):849–865.
- Hage M, Siersema PD, van Dekken H, et al. Oesophageal cancer incidence and mortality in patients with long-segment Barrett's oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol. 2004;39(12):1175–1179.
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365(15):1375–1383.
- Naini BV, Souza RF, Odze RD. Barrett's esophagus: a comprehensive and contemporary review for pathologists. Am J Surg Pathol. 2016;40(5):e45–e66.
- Kinra P, Gahlot GPS, Yadav R, et al. Histological assessment & use of immunohistochemical markers for detection of dysplasia in Barrett's esophageal mucosa. Pathol Res Pract. 2018;214(7):993–999.
- Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47(2):251–255.
- Pech O, Vieth M, Schmitz D, et al. Conclusions from the histological diagnosis of low-grade intraepithelial neoplasia in Barrett's oesophagus. Scand J Gastroenterol. 2007;42(6):682–688.
- Kerkhof M, van Dekken H, Steyerberg EW, et al. Grading of dysplasia in Barrett's oesophagus: substantial interobserver variation between general and gastrointestinal pathologists. Histopathology. 2007;50(7):920–927.
- Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32(4):368–378.
- Duits LC, van der Wel MJ, Cotton CC, et al. Patients with Barrett's esophagus and confirmed persistent low-grade dysplasia are at increased risk for progression to Neoplasia. Gastroenterology. 2017;152(5):993–1001.e1001.
- Skacel M, Petras RE, Rybicki LA, et al. p53 expression in low grade dysplasia in Barrett's esophagus: correlation with interobserver agreement and disease progression. Am J Gastroenterol. 2002;97(10):2508–2513.
- Kastelein F, Biermann K, Steyerberg EW, et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett's oesophagus. Gut. 2013;62(12):1676–1683.
- Janmaat VT, van Olphen SH, Biermann KE, et al. Use of immunohistochemical biomarkers as independent predictor of neoplastic progression in Barrett's oesophagus surveillance: a systematic review and Meta-analysis. PLOS One. 2017;12(10):e0186305.