Abstract
Background and aims
The effect of colonoscopy withdrawal time (WT) beyond 6 min on colorectal adenoma detection rate (ADR) is unclear. We focused on the relationship between WT and ADR.
Materials and methods
This study was a prospective observational study involving 437 patients who underwent colonoscopy at Tongren Hospital in Shanghai from 1 July 2020 to 31 August 2020. Patients were divided into two groups according to whether the WT was >6 min. Age, sex, body mass index (BMI), defoaming rate score, Boston bowel preparation scale (BBPS), primary colonoscopy, hypertension, diabetes mellitus, dietary preparation 1 day before the examination, and abdominal surgery history factors were analysed by univariate and multivariable logistic regression to explore the odds ratios (ORs) of ADR in two WT groups. Restricted cubic spline regression was used to further analyze the relationship between WT and the ORs of adenoma detection.
Results
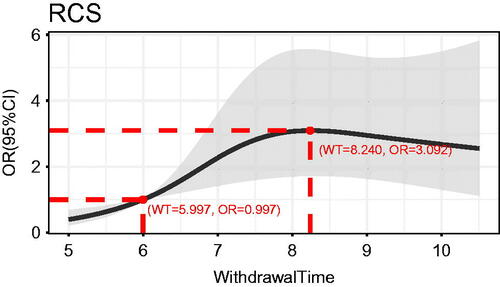
The ADR among 437 patients was 17.16% (75/437). Multivariable regression analysis showed that in the group with WT >6 min, patients aged ≥50 years old and male could have an increased risk of adenoma detection (OR 5.80, 95% CI 2.32–14.47; p < .001; OR 2.30, 95% CI 1.19–4.43; p = .013). The cubic spline curve showed that the ADR increased with time for WT of 6–8 min, and the highest ADR was achieved when the WT was controlled at 8 min (WT = 5.997, OR = 0.997; WT = 8.240 min, OR = 3.092).
Conclusion
The highest ADR was achieved when the WT of colonoscopy was controlled at 8 min.
Introduction
Colorectal cancer (CRC) is one of the major malignant tumours that seriously endanger human health, with an incidence rate of 10.2% and a mortality rate of 9.2%, ranking 3rd worldwide in terms of incidence and 2nd in terms of mortality. In recent years, with changes in people’s lifestyles and diet structures, colorectal cancer has been ranked 2nd in incidence and 5th mortality of malignant tumours in China [Citation1,Citation2], seriously threatening people’s lives and health. Studies have shown that nearly 90% of colorectal cancers develop adenomas through a series of genetic changes called the adenoma-carcinoma sequence [Citation3]. Thus, the detection and removal of colorectal adenomas by colonoscopy can reduce colorectal cancer mortality [Citation4,Citation5].
The quality metrics for colonoscopy published by the American College of Gastroenterology (ACG) state that the adenoma detection rate (ADR) should be ≥25% in the asymptomatic average risk population older than 50 years of age [Citation6]. Colonoscopy does not detect all colonic lesions, with a missed adenoma detection rate of 26% [Citation7]. There is evidence that the adenoma detection rate can be significantly improved by prolonging the colonoscopy withdrawal time (WT) [Citation8]. In the study conducted by Barclay et al. in 2053 colonoscopies performed by 12 endoscopists, a 16.5% increase in ADR was found at WT ≥6 min compared to WT <6 min [Citation9].
The ACG guidelines recommend that endoscopists spend at least 6 min examining the colorectal mucosa during exit colonoscopy to avoid missing colorectal lesions and to improve ADR [Citation10]. A multicentre, randomized, controlled trial in China showed that 1027 patients were randomized to the 9-min and 6-min groups, and the ADR was significantly higher with greater WT length (36.6% vs. 27.1%) [Citation11]. One study at the University of Illinois Rockford School of Medicine showed that endoscopic patients with WT ≥8 min had a significantly higher ADR than patients with WT <8 min (37.8% vs. 23.3%) [Citation12]. This finding suggests that a WT of 8 min or longer is more clinically meaningful. Based on the findings and problems of previous studies, we conducted a prospective cohort study for this purpose, focusing on the association between colonoscopy WT and ADR and inferring the optimal WT for ADR.
Research object and method
Study population
This prospective cohort study was conducted at the Digestive Endoscopy Center of Tongren Hospital, Shanghai Jiao Tong University, starting on 1 July 2020, and ending on 31 August 2020. The study was recorded in the Chinese Clinical Trials Registry as ChiCTR1900025549; the trial was completed, and this report represents the final analysis of this subcentre only. All patients provided written informed consent to participate in the study. The research project was approved by the Ethics Committee of Tongren Hospital with ethical approval number Tongren Ethics Audit 2020-029-02.
Inclusion and exclusion criteria
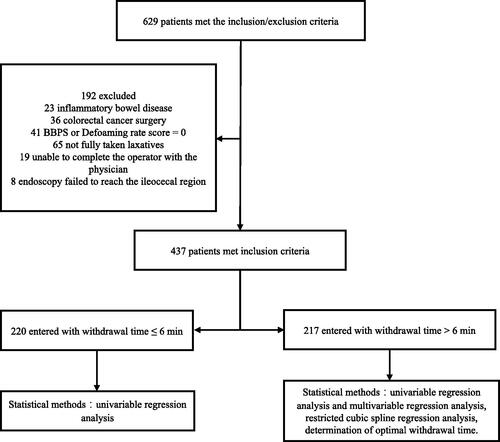
Patients aged ≥18 years old who were able to complete a full colon examination (including colonoscopy screening, surveillance colonoscopy and colonoscopy with indications) at the endoscopy centre were included in the study. The exclusion criteria were as follows: (a) inflammatory bowel disease; (b) postoperative colorectal cancer; (c) poor bowel preparation with one bowel segment defoaming rate score or Boston bowel preparation scale (BBPS) of 0; (d) patients who did not take laxatives completely or who did not take laxatives for emergency colonoscopy due to gastrointestinal bleeding; (e) those whose mental health conditions prevented them from cooperating with the physician to complete the procedure; (f) and the endoscope not reaching the ileocecal region. Those with one or more of the above conditions were excluded from inclusion ().
Data collection
Routine colonoscopy was performed using Olympus CF-260 and 290 series e-colonoscopies. There were 7 physicians participated in this research, who were all senior gastrointestinal endoscopists with >5000 cases of colonoscopy experience. The colonoscopies were performed following the relevant Chinese endoscopic procedure guidelines [Citation13]. A trained nurse performed data collection, including age, sex, body mass index (BMI, kg/m2), defoaming rate score, BBPS, whether it was the primary colonoscopy, whether they had hypertension, whether they had diabetes, whether they had dieted preparation the day before the examination, and whether they had a history of abdominal surgery.
The WT of colonoscopy was defined as the time used to observe the colorectal mucosa during the withdrawal process after reaching the ileocecal region, excluding the time for aspiration of residual faecal water, cleaning of the intestinal lumen, biopsy of the lesion, and resection [Citation14]. The WT was recorded on the spot by the operator using a timer. The number of adenomas detected was based on histology and pathologically confirmed adenomas. At the time of polyp removal, the endoscopist recorded the site, size and number of polyps. Polyp size was measured using biopsy forceps as a standard. The diameter of the largest polyp was recorded, and the pathology grade was the highest. The adenoma detection rate (ADR) was calculated as the number of patients with at least one adenoma divided by the total number of patients examined by colonoscopy.
Statistical analyses
After performing a normality test with continuous values of measurement data, nonnormality was expressed using medians and interquartile range, and normality was expressed as mean ± SD,and compared by independent samples t test,count data were expressed as percentages and analysed using the chi-square test or Fisher’s exact test. For potential risk factors for ADR, including age, sex, BMI, defoaming rate score, BBPS, primary colonoscopy, hypertension, diabetes mellitus, dietary preparation the day before the examination, and history of abdominal surgery, multivariable logistic regression was performed to determine the relevant factors associated with adenoma detection. A restricted cubic spline regression analysis was performed to directly fit the nonlinear relationship between the independent and dependent variables using the predominance ratio of detecting adenomas as the dependent variable and WT, gender, and age as the independent variables. All of the statistical analyses were statistically significant using R Statistics software, version 4.0.1.
Results
A total of 629 patients were enrolled from 1 July 2020, to 31 August 2020, of whom 192 were excluded due to inflammatory bowel disease, postoperative colorectal cancer, poor bowel preparation, and refusal of consent. A total of 437 patients were included in the study, of whom 177 (40.5%) were male, and 260 (59.5%) were female, divided into two groups by time to exit >6 min. The baseline characteristics of patients in both groups were similar throughout the analysis set (). Age, sex, BMI, defoaming rate score, BBPS, primary colonoscopy, hypertension, diabetes, dietary preparation one day before the examination, and history of abdominal surgery were not significantly different in the distribution between the withdrawal groups. According to the age <50 years and ≥50 years, we divided the enrolled patients into two groups and calculated the difference of the mean WT and ADR between the two groups. As a result, there was no significant statistical difference in mean WT (p = .095) and significant statistical difference in ADR between the two groups (p < .001) (Supplementary Table 1).
Table 1. Baseline characteristics of the patients and bowel preparation.
At WT ≤6 min, there was no significant difference in the distribution of ADR between patients grouped according to age, sex, BMI, defoaming rate score, BBPS, primary colonoscopy, hypertension, diabetes, dietary preparation one day before the examination, or history of abdominal surgery (). There was a significant difference in the distribution of ADR between age and gender groups when the WT was >6 min. It was indicated that age ≥50 years old and male sex could increase the risk of adenoma detection (OR, odds ratio 5.80, 95% CI 2.32–14.47; p < .001; OR, odds ratio 2.30, 95% CI 1.19-4.43; p = .013; ).
Table 2. Odds ratios for ADR in Univariate and Multivariate logistic regression in WT ≤6 min.
Table 3. Odds ratios for ADR in univariate and multivariate logistic regression in WT >6 min.
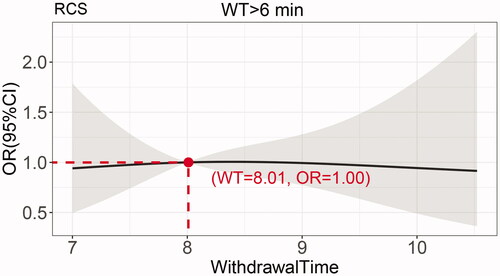
The cubic spline plot of WT versus OR of ADR is shown in . The cut-off value for the total dataset was 6 min (WT = 5.997, OR = 0.997), and the adenoma detection was significantly increased when the WT was >6 min compared to WT ≤ 6 min. When the WT was 6–8 min, the OR of adenoma detection increased significantly with the WT, and the OR of adenoma detection did not increase significantly after a WT >8.240 min (OR of adenoma detection = 3.092, WT = 8.240 min). To verify this finding, we set a sub dataset at WT >6 min and performed sub dataset analysis as shown in . The result showed adenoma detection OR at WT of 8 min was close to 1.0, and when the WT >8 min, the adenoma detection OR no longer changed significantly with the WT. This finding suggests that a balance between risk and benefit can be achieved by controlling the WT at 6–8 min, and the optimal WT for achieving the highest ADR is 8 min.
Discussion
Several studies presently show that WT with colonoscopy after it reaches the ileocecal region is an important factor affecting ADR, with substantial evidence from retrospective studies supporting the benefit of prolonged WT for ADR. Nevertheless, the available literature on the WT has been inconsistent. Some studies have reported positive results [Citation11,Citation12,Citation15], while others have shown no benefit of prolonged WT [Citation16,Citation17]. This inconsistency might be due to a retrospective analysis of the relationship between ADR and WT, rather than elucidation by prospective trials. To date, clinicians remain inconclusive about the WT. The Chinese domestic consensus recommends a WT of ≥6 min, but does a longer WT result in a higher ADR? It is generally accepted that the longer that the WT of colonoscopy is, the higher that the polyp detection rate is, but prolonged operations will cause increased patient discomfort, time and costs and reduce the efficiency of the endoscopist, so it is necessary to choose an appropriate WT, which was also a focus of this study.
In previous studies, the definitions of WT were slightly different. In American College of Gastroenterology (ACG) colorectal cancer screening guidelines, withdrawal time was defined as the time measured from when the colonoscope reaches the cecum to the time the scope is withdrawn from the anus in the absence of polyp removal [Citation18]. In United Kingdom Bowel cancer screening programme, the definition of WT was defined as the average time (in minutes) taken to withdraw the colonoscope (from caecum to extubation) for all complete colonoscopies where the procedure outcome was normal (no abnormalities found) [Citation19]. From these, the definition of WT in the guidelines was often just the observation time which excluded the impact of polyp factors. In clinical studies, there were usually some polyp-related or lesion-related manipulations, such as excision or biopsy, etc., during the withdrawal phase of colonoscopy. To avoid the potential bias of those manipulations, researchers had used a variety of diverse approaches. In study of Robert et al. since WT included time taken for manoeuvres such as polypectomy performed during the withdrawal phase of colonoscopy, they separately calculated WT for procedures involving the removal of polyps and for those in which no polyps were manipulated [Citation9]. As the similar definition of WT, Lee et al. only calculated the WT of procedures with no therapeutic manoeuvres (including excision or biopsy) of polyps [Citation20]. Meanwhile, a considerable number of studies defined the WT as the time excluding the time of polyp-related or lesion-related manipulations, such as excision or biopsy, etc., during the withdrawal phase of colonoscopy to avoid the potential bias [Citation8,Citation14,Citation21]. Referring to previous study [Citation14], we measured the time of withdrawal phase of colonoscopy after reaching the ileocecal region and excluded the time for aspiration of residual faecal water, cleaning of the intestinal lumen, biopsy of the lesion and resection, which might erroneously increase the WT, to avoid the potential bias. Our statistical result showed that there was no difference in mean WT and a significant difference in ADR between the patients age <50 years and ≥50 years (Supplementary Table 1), which suggested that the WT was not affected by factors related to polyps, such as excision or biopsy, etc. Our study was a single-centre cohort study that prospectively demonstrated that extending the WT from 6 to 8 min significantly improved ADR on colonoscopy. The robustness of these findings was confirmed by restricted cubic spline regression analysis. This outcome also suggests that endoscopists can benefit most from an 8-minute WT. In addition, multivariable regression models adjusted for differences between possible confounding factors, including bowel preparation and history of previous abdominal surgery, identified advanced age and male sex as high-risk factors for ADR.
In this study, using restricted cubic spline regression analysis, the OR value of adenoma detection was cut off at 6 min, after which adenoma detection gradually increased with increasing WT from 6 to 8 min. After 8 min, the curve gradually became horizontal, and the subset of data proved that, after 8 min, adenoma detection no longer increased significantly with increasing time. Therefore, we concluded that the ADR increased with time when the WT was 6–8 min, and the ADR was highest when the WT was controlled at 8 min.
Recent studies of ADRs have shown that the ADR in the Chinese population is approximately 14–15% [Citation22,Citation23], and the total population ADR in this study was 17.2%, with an ADR of 23.2% in men and 13.1% in women. The ADR in this study was slightly higher than that reported in the literature, which could be related to the inclusion of patients who were all operated on by physicians with extensive experience in colonoscopy and to the geographical differences in the distribution of adenomas.
Previous studies have shown that colorectal adenomas are strongly associated with several important factors, such as age, sex, smoking, obesity, diabetes, hypertension, BMI, and bowel preparation [Citation24–30]. This study showed a significantly higher ADR in the older age group (age ≥50 years old) than in the younger age group (age <50 years old) (21.8% vs. 8.5%). The ADR was significantly higher in men than in women (23.2%% compared to 13.1%). This finding is in line with the previous association of older age groups and male sex with increased adenoma detection rates [Citation31–36]. It was found that the adenoma detection rate increased significantly with increasing BMI [Citation29]. Bowel cleanliness and the number of intestinal air bubbles significantly influenced ADR, with higher ADR with higher cleanliness and fewer air bubbles and significantly lower ADR with inadequate bowel preparation [Citation30,Citation37]. Differences between the results of the present study and previous studies were considered to be due to the small sample size of each group.
Regarding limitations, the WT in this study is a human factor. Although the operating physicians included in the study were all senior gastrointestinal endoscopists with >5000 cases of colonoscopy experience, there were still inevitable impacts of characteristics of the endoscopist, including examination techniques, experience, and individual differences. Second, this study was a single-centre study data, so its results cannot be applied to all populations. We subsequently plan to conduct a large, multicenter trial to expand the sample size of patients with WT >6 min and prospectively assess whether an 8-minute WT significantly improves ADR and can be used as the optimal WT for the highest ADR.
Conclusion
Analysis of a prospective cohort study of 437 patients concluded that advanced age and male sex are relevant factors for ADR. The highest detection rate of colonic adenoma was observed when the exit time of colonoscopy was controlled at 8 min.
Ethical approval
All patients provided written informed consent to participate in the study. The research project was approved by the Ethics Committee of Tongren Hospital with the ethical approval number: Tongren Ethics Audit 2020-029-02.
Author contributions
Study concept and design (Min Zhao; Ying Xu; Haixia Peng; Daming Yang); Acquisition of data (Min Zhao, Weijie Sun, Jiaying Chen, Haijing Zhu); Analysis and interpretation of data (Min Zhao; Ying Xu); Drafting of the manuscript (Min Zhao); Critical revision of the manuscript for important intellectual content (Min Zhao; Ying Xu; Haixia Peng); Administrative, technical, or material support (Haixia Peng;Daming Yang); Supervision of study (Haixia Peng); All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (16.7 KB)Acknowledgments
We thank Hong Yi, the nurse who collected the data, for her contribution; we also thank all the Endoscopists who participated in this study, Fengli Zhou, Weiyi Wang, Ji Li, Rong Kuai, and Yimin Chu, for their help; and we thank Fan Hu, a teacher of statistics at the School of Public Health, Shanghai Jiao Tong University School of Medicine, for his guidance in data analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The experimental data are non-public; if the industry needs data support, please contact the corresponding authors of this paper directly.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134(7):783–791.
- Cotton S, Sharp L, Little J. The adenoma-carcinoma sequence and prospects for the prevention of colorectal neoplasia. Crit Rev Oncog. 1996;7(5–6):293–342.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696.
- Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30.
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72–90.
- Zhao S, Wang S, Pan P, et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: a systematic review and meta-analysis. Gastroenterology. 2019;156(6):1661–1674.e11.
- Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest Endosc. 2000;51(1):33–36.
- Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355(24):2533–2541.
- Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101(4):873–885.
- Zhao S, Yang X, Wang S, et al. Impact of 9-minute withdrawal time on the adenoma detection rate: a multicenter randomized controlled trial. Clin Gastroenterol Hepatol. 2020;20(2):e168-e181.
- Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6(10):1091–1098.
- 中华医学会消化内镜学分会, 中国抗癌协会肿瘤内镜学专业委员会. 中国早期结直肠癌筛查及内镜诊治指南(2014, 北京). 中华医学杂志. 2015;95(28):2235–2252.
- Jung Y, Joo YE, Kim HG, et al. Relationship between the endoscopic withdrawal time and adenoma/polyp detection rate in individual colonic segments: a KASID multicenter study. Gastrointest Endosc. 2019;89(3):531–532.
- Shaukat A, Rector TS, Church TR, et al. Longer withdrawal time is associated with a reduced incidence of interval cancer after screening colonoscopy. Gastroenterology. 2015;149(4):952–957.
- Adler A, Wegscheider K, Lieberman D, et al. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12,134 examinations (Berlin colonoscopy project 3, BECOP-3). Gut. 2013;62(2):236–241.
- Gellad ZF, Weiss DG, Ahnen DJ, et al. Colonoscopy withdrawal time and risk of neoplasia at 5 years: results from VA Cooperative Studies Program 380. Am J Gastroenterol. 2010;105(8):1746–1752.
- Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021;116(3):458–479.
- Bowel cancer screening programme standards: valid for data collected from 1 April 2018. Available from: https://www.gov.uk/government/publications/bowel-cancer-screening-programme-standards/bowel-cancer-screening-programme-standards-valid-for-data-collected-from-1-april-2018#bcsp-s14-interventiontreatment-scope-withdrawal-time
- Lee TJW, Blanks RG, Rees CJ, et al. Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: evidence from the Bowel Cancer Screening Programme in England. Endoscopy. 2013;45(1):20–26.
- Lee RH, Tang RS, Muthusamy VR, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos). Gastrointest Endosc. 2011;74(1):128–134.
- Bai Y, Fang J, Zhao SB, et al. Impact of preprocedure simethicone on adenoma detection rate during colonoscopy: a multicenter, endoscopist-blinded randomized controlled trial. Endoscopy. 2018;50(2):128–136.
- Zhang S, Zheng D, Wang J, et al. Simethicone improves bowel cleansing with low-volume polyethylene glycol: a multicenter randomized trial. Endoscopy. 2018;50(04):412–422.
- Shin A, Hong CW, Sohn DK, et al. Associations of cigarette smoking and alcohol consumption with advanced or multiple colorectal adenoma risks: a colonoscopy-based case-control study in Korea. Am J Epidemiol. 2011;174(5):552–562.
- Wu H, Zhang J, Zhou B. Metabolic syndrome and colorectal adenoma risk: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2021;45(5):101749.
- Zhou H, Shen Z, Zhao J, et al. [Distribution characteristics and risk factors of colorectal adenomas]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21(6):678–684.
- Ortiz AP, Thompson CL, Chak A, et al. Insulin resistance, central obesity, and risk of colorectal adenomas. Cancer. 2012;118(7):1774–1781.
- Huang KW, Leu HB, Wang YJ, et al. Patients with nonalcoholic fatty liver disease have higher risk of colorectal adenoma after negative baseline colonoscopy. Colorectal Dis. 2013;15(7):830–835.
- Ben Q, An W, Jiang Y, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology. 2012;142(4):762–772.
- Sulz MC, Kröger A, Prakash M, et al. Meta-analysis of the effect of bowel preparation on adenoma detection: early adenomas affected stronger than advanced adenomas. PLoS ONE. 2016;11(6):e0154149.
- Wernly S, Wernly B, Semmler G, et al. A sex-specific propensity-adjusted analysis of colonic adenoma detection rates in a screening cohort. Sci Rep. 2021;11(1):17785.
- Ferlitsch M, Reinhart K, Pramhas S, et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. Jama. 2011;306(12):1352–1358.
- Roy HK, Bianchi LK. Differences in Colon adenomas and carcinomas among women and men: potential clinical implications. JAMA. 2009;302(15):1696–1697.
- Brenner H, Altenhofen L, Hoffmeister M. Sex, age, and birth cohort effects in colorectal neoplasms: a cohort analysis. Ann Intern Med. 2010;152(11):697–703.
- Cheng WC, Chen PJ, Kang JW, et al. Age, male sex, smoking and metabolic syndrome as risk factors of advanced colorectal neoplasia for fecal immunochemical test negative patients. J Formos Med Assoc. 2021;121:402–408.
- Jung YS, Park JH, Park CH. Comparison of risk of metachronous advanced colorectal neoplasia in patients with sporadic adenomas aged <50 versus ≥50 years: a systematic review and meta-analysis. J Pers Med. 2021;11(2):120.
- Guo R, Wang YJ, Liu M, et al. The effect of quality of segmental bowel preparation on adenoma detection rate. BMC Gastroenterol. 2019;19(1):119.