Abstract
Objectives
The presence of autoimmune metaplastic atrophic gastritis (AMAG) may lead to an increased risk of associated gastric neoplastic lesions. This study aims to investigate the prevalence of gastric neoplasia in AMAG patients and to explore the possibility of PGI/II ratio as a predictor for AMAG diagnosis.
Patients and methods
Retrospective audit of 135 patients diagnosed with AMAG on endoscopic gastric biopsy between January 2017 and December 2020 at Beijing Friendship Hospital. The study was registered in Chinese Clinical Trial Registry (ChiCTR2000041163).
Results
A total of 135 patients (the mean age 61.9 ± 10.9 years,109 female) had histologically confirmed AMAG. 31.1% (42/135) had AMAG without neoplasia on the initial biopsy; 37% (50/135) had multiple type 1 gastric neuroendocrine tumors (g-NETs), 36 grade 1 and 14 grade 2, the median diameter was 5 mm (range 1–25); 31.9% (43/135) had multiple gastric hyperplastic polyps (GHPs), including 15 cases of GHPs with neoplastic transformation, the median diameter was 14.5 mm (range 3–50). 3.7% (5/135) had single gastric low-grade dysplasia/adenoma, the median diameter was 5 mm (range 3–15). 5.9% (8/135) had single or double gastric high-grade dysplasia or adenocarcinoma, the median diameter was 15 mm (range 8–43). 40.7% (55/135) had pepsinogen (PG) I< 10 ng/ml, 45.9% (62/135) had PG I/II ratio ≤1 and each group had a median of PG I/II ratio <1.
Conclusions
Lower serum PG I level and PGI/II ratio may be a predictor to indicate the diagnosis of AMAG. It’s necessary to perform regular endoscopic surveillance for AMAG patients to recognize associated gastric neoplasia timely.
Introduction
Autoimmune metaplastic atrophic gastritis (AMAG) is an immune-mediated chronic inflammatory disease characterized by immune-mediated destruction of gastric parietal cells in the fundus and corpus of the stomach, thereby slowly decreasing the ability to absorb adequate quantities of iron and vitamin B12. The histological findings of AMAG include full-thickness inflammation, destruction of oxyntic glands, metaplasia (intestinal, pseudopyloric, or pancreatic acinar) and parietal cell pseudohypertrophy [Citation1]. Destruction of gastric parietal cells leads to hypergastrinemia, stimulation of endocrine cells and development of type 1 gastric neuroendocrine tumors (g-NETs) and other associated lesions, such as gastric hyperplastic polyps (GHPs) [Citation2] or gastric carcinomas. But, in the early stage, it is lack of typical endoscopic features. As the severity of atrophy progression, chief cells in the corpus are replaced by pyloric glands, leading the concentration of pepsinogen (PG) I decreased, consequently, the PGI/II ratio is greatly reduced. Low level of PGI and PGI/II ratio are associated with severe gastric atrophy and may predict the diagnose of AMAG [Citation3]. Although the prevalence of AMAG is on the rise gradually, the incidence rate is still underestimated. As we know, the subsequently type 1 g-NET is strongly associated with AMAG, however, GHPs and gastric carcinoma should also be noticed. There is limited literature [Citation4] about the incidence of AMAG associated lesions. Therefore, the aims of this study were: (i) to report the prevalence of gastric neoplasia in AMAG patients in our hospital and (ii) to explore the possibility of PGI level and PGI/II ratio as a predictor for AMAG.
Material and methods
This is a retrospective audit of AMAG patients managed at Beijing Friendship Hospital, Capital Medical University, China during January 2017 and December 2020. The study was approved by Beijing Friendship Hospital Ethics Committee (Certificate number is 2020-P2-277-01) and was registered in Chinese Clinical Trial Registry (Registration number is ChiCTR2000041163). Written informed consent was obtained from all participants. All participants signed a written informed consent giving permission for publication.
All patients had blood taken to measure their fasting serum gastrin-17 concentration (35–100 pg/ml, ELISA), serum PGI/II ratio (immunoassay), hemoglobin (male 120–160 g/l, female 110–150 g/l), serum vitamin B12 (abnormal < 190 pg/ml), serum iron (7.8–32.2 umol/l), serum ferritin (11.0–306.0 ng/ml), serum anti-parietal cell antibody (PCA, abnormal >1:80, IIFA), serum anti-intrinsic factor antibody (IF, abnormal >1:20, ELISA), serum thyroid function tests (TFTs) and Helicobacter pylori serology. H. pylori status was determined by serum H. pylori IgG and 13C-urea breath test (UBT) or by histological evaluation of gastric biopsies. H. pylori negative patients did not receive any eradication therapy or proton pump inhibitor/H2 receptor antagonist before the test. Hypergastrinemia was diagnosed when the fasting serum gastrin concentration was two times higher than the upper limit of the normal range.
All the AMAG patients had undergone esophagogastroduodenoscopy (EGD). The typical endoscopic finding of AMAG is advanced corpus dominant mucosal atrophy, which is distinguished from the pattern of H. pylori-induced atrophic gastritis. The atrophic mucosa manifests with varying degrees of mucosa thinning, flattened or diminished gastric rugal folds. And submucosal grid vessels may be visible in cases of moderate to severe atrophy [Citation5]. Other endoscopic appearances include remnant oxyntic mucosa [Citation6], sticky adherent dense mucous and scattered minute whitish protrusions [Citation7]. According to the manifestation of EGD, we classified the degree of mucosal atrophy into mild, moderate and severe (). Background mucosa (fundus, corpus and antrum) and associated lesions, such as g-NETs, GHPs, gastric dysplasia, or gastric cancer were taken for biopsy in all the cases (). The endoscopic classification of gastric early cancer or superficial lesions was assessed according to the Paris endoscopic classification [Citation8]. The majority of lesions were treated by endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD) or surgery.
Figure 1. Mild atrophic gastritis, rough mucosa in the corpus, flattened rugal folds and absence of submucosal vessel.
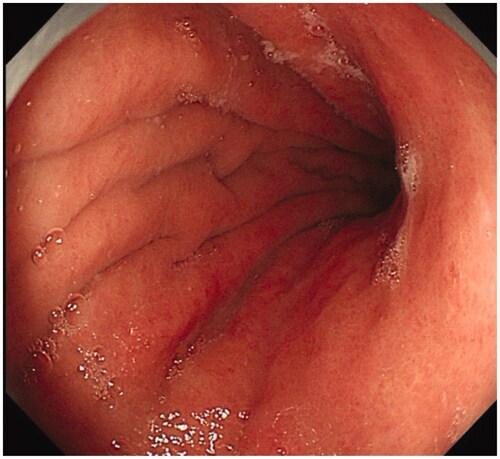
Figure 2. Moderate atrophic gastritis, thinning mucosa, flattened rugal folds and submucosal micro-vessel in the corpus.
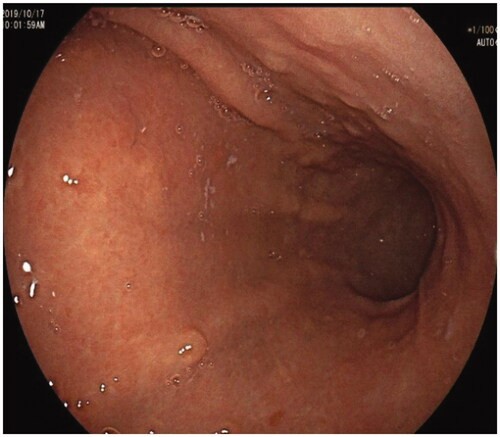
Figure 3. Severe atrophic gastritis, diminished rugal fold and submucosal grid vessel in the corpus.
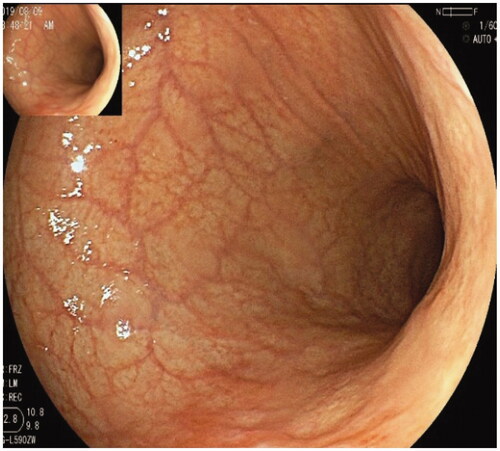
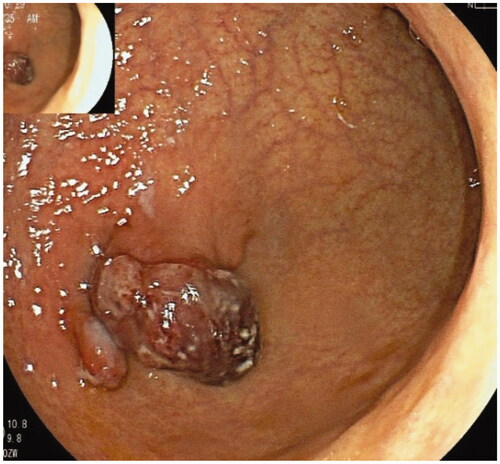
Figure 6. Two well-differentiated carcinomas in the corpus and antrum in the same patient were arisen from AMAG.
Histological slides were evaluated by the same experienced pathologist according to the WHO Classification of Tumors of the Digestive System, the updated Sydney system for the classification of gastritis [Citation9], and related literatures for AMAG [Citation10–13].
A diagnosis of AMAG had been based on a combination of (i) histologically confirmed atrophy and gastric enterochromaffin-like (ECL) cell hyperplasia in the fundus or corpus. Pseudo pyloric metaplasia or pancreatic acinar metaplasia may be visible, (ii) hypergastrinemia, (iii) the presence of anti-PCA or anti-intrinsic factor antibodies.
Inclusion criterion of AMAG patients were: pathological diagnosed with AMAG, with (i) negative H. pylori test, or (ii) positive H. pylori test, but meanwhile 13C UBT value <6; endoscopic manifestation was without current H. pylori infection appearances according to endoscopic Kyoto classification of H. pylori infection [Citation14]; positive serum PCA, PGI/II ratio < 1; coexist with anemia or Hashimoto’s disease.
Statistical analysis was performed using SPSS version 22 (SPSS Inc., Chicago, IL). Quantitative variables were described using the mean ± SD, whereas categorical variables were described using frequency and proportions. The Chi-square test was used to test for differences between groups. Fisher’s exact test was used for statistical comparisons as appropriate. Continuous variables were compared using t test. The measurement data of non-normal distribution were described using median, and the Kruskal–Wallis non-parametric test was used for pairwise comparison among the three subgroups. p Values <.05 were considered statistically significant. All p values were two-sided.
Results
Demographics features
A total of 135 patients diagnosed with AMAG were included in our study, comprised 105 women and 30 men, with a female to male ratio of 3.5:1. The mean age of all patients at diagnosis was 61.9 ± 10.9 years old (range 30–82), the median body mass index (BMI) was 23.3 kg/m2 (range 19.0–35.4). Absence of H. pylori was found in 94.8% (128/135). The initial indications for performing EGD were: bloating or abdominal pain (67/135), anemia (32/135), screening for early gastric cancer (29/135), hypergastrinemia (2/135), neurological symptoms (2/135), weight loss (2/135) and low level PGI(1/135). Of these patients, 45 patients had a history of anemia, 25 had Hashimoto’s disease, 4 had subacute spinal cord degeneration (SCD) and 10 had diabetes mellitus type 2. Only five patients used to take proton pump inhibitor.
According to EGD and pathological findings, 135 patients were divided into 6 groups: 42 cases of AMAG without neoplasia, 38 cases of type 1 g-NETs, 33 cases of GHPs, 9 cases of g-NETs and GHPs, 5 cases of low-grade dysplasia or adenoma, 8 cases of high-grade dysplasia or well-differentiated adenocarcinoma(coexist 2 g-NETs) ().
Table 1. Demographics features of AMAG patients and AMAG-associated neoplasia.
Endoscopic characteristics of AMAG and associated neoplasia
All 135 patients underwent EGD. EGD showed a varying degree of mucosal thinness, flattened or diminished rugal folds, and submucosal grid vessels in the fundus or corpus while the antral mucosa was relatively normal. There were 27 cases of mild atrophic gastritis, 64 cases of moderate and 44 severe atrophic gastritis. Gastric xanthoma was found in 10 cases, sticky adherent dense mucous was rigidly attached to the mucosa of fundus in 18 cases, which was different from the whitish clouded mucous produced by H. pylori infection.
A total of 50 cases (37%) of multiple g-NETs were detected, of which 9 cases were found to coexist with GHPs, 1 case was found to coexist with gastric adenoma and 2 coexist with gastric early cancer. There were 43 cases (31.9%) of GHPs, of which only GHPs in 33 cases (15 cases contained of neoplastic transformation), 1 case had GHPs coexisting with early cancer and 9 cases had NET coexisting with GHPs. LGDs/adenoma were found in 5 cases (3.7%), of which 1 case coexisted with NETs. There were 8 cases (5.9%) of early cancer, 2 cases were double lesions, so there were 10 lesions. The distribution of gastric lesions listed in .
The median diameter of g-NETs was 5 mm (range 1–25 mm), the median number of lesions was 3 (range 1–20), 45 cases had multiple lesions. All the g-NETs located in the gastric fundus and corpus, morphological characteristic shows mainly protruding polypoid. Central depression of the lesion occurred in 6 cases, surface ulceration with lymph node metastasis was found in 1 case, and 40 cases had yellowish appearance. Magnifying endoscopy-narrow band imaging (ME-NBI) was applied to 18 cases, all of which showed submucosal blue-green ‘tree-like’ vessel.
The median diameter of GHPs was 14.5 mm (range 3–50 mm), the median number of lesions was 3 (range 1–17), 23 cases had multiple lesions. The polyps mainly distributed in the gastric fundus and corpus and were in sessile or protruding appearance. Polyps were often redness and hematose, and showed a ‘strawberry-like’ appearance with erosions or purulent surface at the head of polyps when GHPs contained with neoplastic transformation.
The median diameter of gastric low-grade dysplasia or adenoma was 5 mm (range 3–15 mm), all of them were single with clear margin, 4 cases distributed in the fundus and corpus, 1 was in antrum. The macroscopic morphology was type 0-Is in three cases and type 0-IIa in two cases.
The median diameter of gastric high-grade dysplasia or well-differentiated adenocarcinoma was 15 mm (range 8–43 mm), 2 cases were double lesions, 10 lesions in total distributed in the fundus, corpus or antrum. The morphology was type 0-Is in 1 case, type 0-IIa in 4, type 0-IIc in 2, type 0-IIa + IIc in 1 and lateral spreading tumor in two cases. ME-NBI was performed in 10 lesions and showed clear demarcation line, irregular microvascular and microsurface pattern. Endoscopic characteristics are listed in .
Table 2. Endoscopic characteristics of AMAG and AMAG associated gastric lesions.
Serum tests in patients of AMAG and associated neoplasia
About 92.6% (125/135) had hypergastrinemia, the positive rate of serum anti-gastric PCA was up to 85.9% (116/135), the positive rate of anti-intrinsic factor antibodies (IF) was 28.1% (38/135). 67/135 cases (49.6%) were diagnosed with anemia, including 63 cases (46.7%) of megaloblastic anemia with a mean age 63.9 ± 9.5 years old and 45 (33.3%) iron deficiency anemia with a mean age 58.9 ± 12.2 years old. All the patients had normal folic acid level. A total of 80 cases (59.3%) had decreased level of PGI and PGI/II ratio <3, 55 patients (40.7%) had PGI level <10 ng/ml. 62 (45.9%) of them had PGI/II ratio ≤1 ().
Table 3. The characteristics of AMAG patients.
The mean age of AMAG patients with early gastric cancer was higher than that of g-NETs patients (71.75 ± 8.12 versus 57.53 ± 11.06, p= .004), there was no statistically significant difference in age among other groups. Statistical analysis was conducted on the values of serum gastrin, hemoglobin, vitamin B12 and PGI/II ratio in each group, which showed that there was a statistical difference in serum gastrin between groups (p= .041), but there was no statistical difference between groups after pair comparison ().
Table 4. Serum tests in patients of AMAG and AMAG associated neoplasia.
Pathological characteristics of AMAG and associated neoplasia
All patients demonstrated atrophic gastritis affecting the corpus. All cases showed different extent of atrophy or intestinal metaplasia of background mucosa, including 18 cases of mild atrophic gastritis, 63 moderate and 54 severe atrophic gastritis. Intestinal metaplasia in the corpus was diagnosed in 108 cases. Pathological findings indicated a varying degree of gastric ECL cell hyperplasia in the fundus or corpus, of which, 51 cases showed linear hyperplasia, 67 cases in micronodular, 4 cases in dysplasia, 9 cases in adenomatoid and 4 cases in micro carcinoid. Pseudo pyloric metaplasia was found in 30 cases and pancreatic acinar metaplasia in 10 cases. A totalo f 132 cases had the sparing of the antral mucosa. Three cases with H. pylori infection had antral intestinal metaplasia.
There were 50 cases of type 1 gastric NETs, including 36 cases of G1 grade, 14 of G2 grade, Ki-67 proliferation index was between 3% and15%. A varying degree of ECL cell hyperplasia and oxyntic gland atrophy occurred in the background mucosa.
A total of 43 cases of GHPs were detected, including 15 cases of GHPs with neoplastic transformation and 28 cases without neoplastic change. Among them, 3 GHPs with LGD, 3 GHPs with HGD,7 GHPs with well-differentiated carcinoma, 2 GHPs with moderate- differentiated carcinoma.
One case had low-grade tubular adenoma and four cases showed LGD. Background mucosal pathology showed different extends of ECL cell hyperplasia, mild atrophy of oxyntic gland with intestinal metaplasia in one case, and severe atrophy with intestinal metaplasia in four cases.
Eight patients had gastric early cancer, including two lesions of high-grade dysplasia and eight lesions of well-differentiated carcinomas. Background mucosal pathology showed ECL cell hyperplasia. The pathological characteristics are listed in .
Table 5. Pathological characteristics of AMAG and AMAG associated gastric lesions.
Treatments
A total of 95 patients underwent endoscopic resection, of them, 25 patients who had gastric NETs underwent EMR and 16 underwent ESD; 26 patients who had GHPs underwent EMR and 15 underwent ESD; 3 patients who had gastric LGD/adenoma underwent EMR, 2 underwent ESD; 1 patient who had gastric early cancer underwent EMR, 7 patients underwent ESD. One g-NET patient and 2 GHPs patients required surgery. Eight g-NETs patients had regular surveillance without additional treatment. Different treatments are listed in .
Table 6. The treatments of AMAG-associated gastric lesions.
AMAG patients who had megaloblastic anemia or iron deficiency anemia received vitamin B12 intramuscular injection or oral administration of ferrous sulfate. Patients who had hypothyroidism received thyroxine orally.
Discussion
AMAG is an immune-mediated chronic inflammatory disease characterized by the damage of oxyntic glands and destruction of gastric parietal cells, which leads to hypergastrinemia, stimulation of endocrine cells and most commonly development of type 1 g-NETs. In the early stage of AMAG, the endoscopic manifestation is not unique compared with the healthy situation, whereas flattened rugal folds and submucosal vessels may be visible in cases of extensive atrophy. And pseudo-polyps or polyps (hyperplastic or adenomatous) might be present. In our study, we found that the features of ‘thinning mucosa, flattened rugal folds and submocasal micro-vessel’ were very useful for the endoscopic diagnosis of AMAG. The typical endoscopic characteristic of AMAG is advanced corpus dominant mucosal atrophy, that is, discolored mucosa with marked submucosal vascular visibility and non-atrophic pattern in the antrum. It is distinguished from the pattern of H. pylori-induced atrophic gastritis as classified by Kimura-Takemoto [Citation15,Citation16]. Other endoscopic appearances include remnant oxyntic mucosa, sticky adherent dense mucous and scattered minute whitish protrusions [Citation7]. Progression of corpus atrophy over a few years of follow-up was found to have multiple pseudo-polyps presenting as reddish nodules at the initial endoscopic examination.
As a chronic inflammatory disease, AMAG can also lead to GHPs [Citation1] and even neoplastic changes, such as gastric adenoma or adenocarcinoma [Citation13]. A limited literature reported that the risk of gastric cancer in patients with severe fundal atrophy was 5.76 times greater than that in healthy subjects [Citation17]. In addition, patients with intestinal metaplasia of the corpus have a higher risk of developing gastric cancer than patients without intestinal metaplasia [Citation18]. Epidemiological data showed that the risk of gastric cancer in H. pylori- negative AMAG patients was 10–20 times higher than that in H. pylori infection patients [Citation19,Citation20]. Japanese data [Citation7] showed that the gastric lesions associated with AMAG were: 11.4% were type 1 g-NETs, 21.1% GHPs, 0.8% adenoma and 9.8% adenocarcinoma. In our study, about 31.1% of patients had AMAG without neoplasia on initial biopsy; 37% had type 1 g-NETs, 31.9% had GHPs, 3.7% had gastric LGD/adenoma and 5.9% had early gastric cancer (high-grade dysplasia or adenocarcinoma). These data were similar to the above Japan’s study [Citation7]. Moreover, evaluation of background mucosa histological status (biopsy in the gastric fundus/corpus/antrum) is a necessary procedure for the diagnosis of AMAG [Citation2]. Comprehensive data showed that neoplastic lesions except type 1 g-NETs should be suspected of those patients presenting corpus dominant atrophic mucosa. Therefore, it is important to perform regular endoscopic surveillance for AMAG patients in order to recognize the associated gastric neoplasms early.
Serous PG concentration reflects the morphological and functional status of the gastric mucosa. Concentration of PG I and PG I/II ratio are noninvasive methods of assessing atrophic gastritis and the degree of atrophy. It has been found that the serum PGI < 70 ng/ml, PGI/II ratio <3 is considered to be the optimal cut-off value for the diagnosis of atrophic gastritis with high sensitivity and specificity [Citation21]. PGI/II ratio was negatively correlated with the degree of mucosal atrophy [Citation22]. As the severity of atrophy progression, chief cells are replaced by pyloric glands, leading the concentration of PGI decreased, consequently, the PGI/II ratio is greatly reduced. An Italian study suggested that a low PGI/II ratio may characterize patients with AMAG and g-NETs [Citation3]. In our study, we found AMAG patients may have lower PGI concentration and PGI/II ratio than that in H. pylori-induced atrophic gastritis. A total of 55 patients (40.7%) had PGI level lower than 10 ng/ml, 62 cases (45.9%) had PGI/II ratio lower than 1, and each group had a median of PG I/II ratio <1. So, we propose that lower PG I and PG I/II ratio may become a good diagnostic biomarker for AMAG prediction.
In this study, we included 7 AMAG patients with H. pylori infection. Endoscopic manifestation was without current H. pylori infection appearances. Intestinal metaplasia of the antrum was present in three cases. All cases displayed ECL cell hyperplasia in the corpus without multifocal atrophic gastritis. They all had positive serum anti-parietal antibody and PG I/II ratio <1. Meanwhile, they were also coexisting with vitamin B12 or iron deficiency anemia or Hashimoto’s disease. Although the cases were with H. pylori infection, it was still critically considered to be included in this AMAG cohort. The correlation between H. pylori infection and AMAG remains controversial. H. pylori infection may trigger the onset of autoimmune gastritis owing to homology between H. pylori and the proton pump H+/K+-ATPase on parietal cells [Citation23]. Achlorhydria in AMAG might allow urease positive bacteria other than H. pylori to colonize in the stomach, causing positive 13C-UBT results. Mean 13C-UBT value in AMAG patients was 5.4 versus 17.8 in non-AMAG patients [Citation24]. Further cases must be studied to determine its correlation.
This study is limited by its retrospective nature. A further clinical study is necessary to determine the optimal cut-off value for AMAG diagnose. In addition, we attempt to correlate histological observations with secretory activity and endoscopic manifestation to verify the accuracy of PG I/II ratio prediction.
Conclusions
Neoplastic lesions are more likely to occur on basis of AMAG. Lower serum PGI level and PGI/II ratio may be a predictor to indicate the diagnose of AMAG. It is necessary to perform regular endoscopic surveillance for AMAG patients to recognize associated gastric neoplasia timely.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Approval was granted by Beijing Friendship Hospital Ethics Committee (Certificate number is 2020-P2-277-01). All the patients gave consent to participating in this study. Written informed consent was obtained from all participants.
Author contributions
Dr Haiyi Hu designed and performed the study, wrote the manuscript and gave final approval of the version to be published. Dr Rongxue Li and Dr Linlin Shao took charge of data collection. Dr Qian Zhang who is a member of clinical epidemiology and EBM unit performed statistical analysis of data. Dr Rui Xu evaluated the histological slides as a pathologist. Professor Shutian Zhang revised the manuscript critically. All authors read and approved the final manuscript.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Rustgi SD, Bijlani P, Shah SC. Autoimmune gastritis, with or without pernicious anemia: epidemiology, risk factors, and clinical management. Therap Adv Gastroenterol. 2021;14:17562848211038771.
- Hu HY, Zhang Q, Chen GY, et al. Risk factors and clinical correlates of neoplastic transformation in gastric hyperplastic polyps in chinese patients. Sci Rep. 2020;10(1):2582.
- Magris R, Re VD, Maiero S, et al. Low pepsinogen I/II ratio and high gastrin-17 levels typify chronic atrophic autoimmune gastritis patients with gastric neuroendocrine tumors. Clin Transl Gastroenterol. 2020;11(9):e00238.
- Mahmud N, Stashek K, Katona BW, et al. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: a renewed call for surveillance. Ann Gastroenterol. 2019;32(1):67–72.
- Kamada T, Maruyama Y, Monobe Y, et al. Endoscopic features and clinical importance of autoimmune gastritis. Dig Endosc. 2022;34(4):700–713.
- Kato M. Endoscopic features of early-stage autoimmune gastritis. Intern Med. 2020;59(23):2969–2970.
- Terao S, Suzuki S, Yaita H, et al. Multicenter study of autoimmune gastritis in Japan: clinical and endoscopic characteristics. Dig Endosc. 2020;32(3):364–372.
- The paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach and Colon: November 30 to december 1,2002. Gastrointest Endosc. 2003;58:S3–S43.
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated sydney system. International workshop on the histopathology of gastritis, houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181.
- Neumann WL, Coss E, Rugge M, et al. Autoimmune atrophic gastritis-pathogenesis, pathology and management. Nat Rev Gastroenterol Hepatol. 2013;10(9):529–541.
- Massironi S, Zilli A, Elvevi A, et al. The changing face of chronic autoimmune atrophic gastritis: an updated comprehensive perspective. Autoimmun Rev. 2019;18(3):215–222.
- Pittman ME, Voltaggio L, Bhaijee F, et al. Autoimmune metaplastic atrophic gastritis: recognizing precursor lesions for appropriate patient evaluation. Am J Surg Pathol. 2015;39(12):1611–1620.
- Arai J, Niikura R, Hayakawa Y, et al. Clinicopathological features of gastric cancer with autoimmune gastritis. Biomedicines. 2022;10(4):884.
- Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020;26(5):466–477.
- Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1(03):87–97.
- Kotelevets SM, Chekh SA, Chukov SZ. Updated Kimura-Takemoto classification of atrophic gastritis. World J Clin Cases. 2021;9(13):3014–3023.
- Tatsuta M, Iishi H, Nakaizumi A, et al. Fundal atrophic gastritis as a risk factor for gastric cancer. Int J Cancer. 1993;53(1):70–74.
- Shichijo S, Hirata Y, Sakitani K, et al. Distribution of intestinal metaplasia as a predictor of gastric cancer development. J Gastroenterol Hepatol. 2015;30(8):1260–1264.
- Brenner H, Arndt V, Stegmaier C, et al. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am J Epidemiol. 2004;159(3):252–258.
- Ekström AM, Held M, Hansson LE, et al. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121(4):784–791.
- Kitahara F, Kobayashi K, Sato T, et al. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44(5):693–697.
- Tong YL, Wang HG, Zhao Y, et al. Diagnostic value of serum pepsinogen levels for screening gastric cancer and atrophic gastritis in asymptomatic individuals: a cross-sectional study. Front Oncol. 2021;11:652574.
- Choudhuri J, Hall S, Castrodad-Rodriguez CA, et al. Features that aid identification of autoimmune gastritis in a background of active helicobacter pylori infection. Arch Pathol Lab Med. 2021;145(12):1536–1543.
- Furuta T, Baba S, Yamade M, et al. High incidence of autoimmune gastritis in patients misdiagnosed with two or more failures of H. pylori eradication. Aliment Pharmacol Ther. 2018;48(3):370–377.