Abstract
Background: In the inflammatory bowel diseases, chronic inflammation predisposes to dysplasia and colorectal carcinoma, leading to the need of surveillance colonoscopies. The most-used marker of colonic inflammation is faecal calprotectin. Its correlation with endoscopic and histological findings is well-documented. In this study, we evaluated the role of sequential faecal calprotectin measurements in predicting colorectal dysplasia, to identify patients with increased risk of dysplasia or colonic malignancy in ulcerative colitis.
Methods: We collected the faecal calprotectin measurements and colorectal histology reports of patients with ulcerative colitis treated in Helsinki University Hospital (Helsinki, Finland) between 2007 and 2017, with a focus on IBD-associated neoplasia, inflammatory activity, and sporadic adenomas. Using the time-weighted AUC of faecal calprotectin as a marker of inflammatory burden, we tested the performance of faecal calprotectin to predict the risk for colorectal neoplasia.
Results: In total, 982 patients with ulcerative colitis were included. Of them, 845 had pancolitis and 127 concomitant primary sclerosing cholangitis. Forty-one patients (4%) had IBD-associated colorectal dysplasia and seven (0.7%) developed adenocarcinoma. In patients with constantly elevated faecal calprotectin level (>500 µg/g), colorectal neoplasia was more frequent compared to those with low (<200 µg/g) calprotectin (13% and 4%, p < 0.05). Histological inflammatory activity was also related to more frequent dysplastic changes.
Conclusions: Colon dysplasia and adenocarcinoma are more common among ulcerative colitis patients with constantly elevated faecal calprotectin than in patients in remission. The role of inflammatory activity in inducing neoplastic changes in colon is further supported by histology, as histological inflammatory activity correlates with dysplasia.
Introduction
In the inflammatory bowel diseases (IBD), long-lasting active inflammation of the colonic mucosa predisposes to increased cell proliferation promoting carcinogenesis. Inflammation induces mutations in tumor suppressor genes and leads to dysplastic lesions of the mucosa and to colitis-related colorectal cancer (CRC) [Citation1–6]. There is some evidence of the chemopreventive effect of 5-ASA against colitis-related CRC [Citation1,Citation4,Citation6,Citation7], but the results are controversial [Citation8,Citation9]. The increased CRC-risk is associated with the male sex, early onset of IBD, duration and extent of the disease, severity of inflammation, and concomitant primary sclerosing cholangitis (PSC) [Citation1,Citation2,Citation5]. The cumulative risk of CRC in IBD varies from less than 1% to 2% by 10 years, 8% by 20 years, and to 8–18% by 30 years in different studies, the risk being higher in earlier studies [Citation1,Citation2,Citation7,Citation5,Citation10]. Regular surveillance colonoscopies are recommended for all patients with colonic IBD, excluding those with isolated proctitis [Citation11]. Due to improved therapies as well as dysplasia surveillance programs, the rate of CRC in IBD patients has been declining over the past years. In some recent studies, the risk of CRC has not exceeded that of the healthy persons [Citation1,Citation4,Citation12–15].
In IBD, faecal calprotectin (FC) levels correlate closely with endoscopic and histological inflammation [Citation16,Citation17]. In our clinic, the FC test was introduced to the daily clinical routine in 2007, replacing most colonoscopies in the evaluation of inflammatory activity in colonic IBD. FC test is performed on the regular basis at 3 to 12 months interval, at patient-reported flares, and before routine colonoscopies. After the introduction of FC tests into clinical practice, most colonoscopies in IBD patients are performed for dysplasia screening. The incidence and prevalence of IBD is rising in all countries, and as the patients get older, the prevalence is also growing. The peak incidence of IBD is at young adulthood [Citation14,Citation15,Citation18–20]. Thus, the need for repeated colonoscopies for the dysplasia surveillance lasts for decades and is strenuous for the patients as well as health-care providers. The extensive dysplasia surveillance program is allocated based on the known risk factors for CRC in IBD, and the findings of the latest colonoscopy [Citation11]. With the increasing number of patients, decreasing health-care resources, and declining incidence of IBD-associated CRC, however, we should be able to target both the screening and the surveillance even more concisely.
We know from several previous studies that FC correlates well with the inflammatory activity in colonic IBD, and it is also elevated in colorectal carcinoma in both IBD and non-IBD patients. It could therefore be hypothesized that FC might have a role in predicting dysplasia in IBD. If true, FC would be an ideal non-invasive marker of increased risk of dysplasia or CRC, for better targeting resources for dysplasia screening and surveillance colonoscopies. The role of FC in evaluating disease activity in IBD is well-known, but there are no studies on the use of FC in predicting dysplasia or CRC.
In present study, we evaluated the role of inflammation measured by sequential FC in predicting neoplastic changes in colon, namely dysplasia and adenocarcinoma, in patients with ulcerative colitis (UC). We also assessed whether FC measurements enables us to modify surveillance colonoscopy protocol of UC patients, allowing us to identify patients with an increased risk of dysplasia or colonic malignancy.
Materials and methods
Patients and data collection
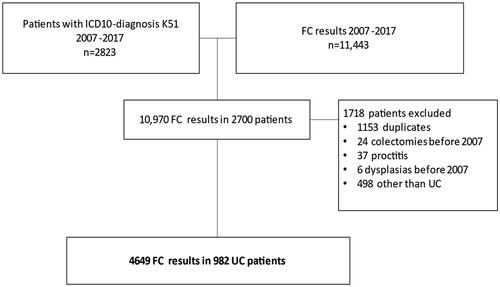
The flow chart of patient inclusion is presented in . In this retrospective study, we collected all FC test results from the laboratory database of the outpatient gastroenterology clinic of Helsinki University Hospital (Helsinki, Finland) from 1st January 2007 to 31st December 2017. The data comprised 11,443 FC test results. From the clinical database, we collected all patients (n = 2823) with an ICD-10 diagnosis of K51 (UC), who were treated at our clinic during this period. By combining these, we got 10,970 FC test results from 2700 patients. After exclusion of duplicates (n = 1153), patients with isolated proctitis only (n = 37), colon resection, total colectomy, colon neoplasia (dysplasia or CRC) before 2007 (n = 30), and patients with CD or diagnosis other than IBD (n = 498), we ended up with 4649 FC measurements from 982 UC patients. This constituted the final patient-cohort. Patients were divided into three groups: 1. patients with no dysplasia, 2. patients with IBD-associated neoplasias (dysplasia or adenocarcinoma), and 3. patients with sporadic adenomas. The distinction between sporadic adenomas and colitis-induced dysplasia was made endoscopically. Sporadic adenomas are defined as focal lesions identified by endoscopist as a polyp, whereas UC induced dysplasia is so called flat dysplasia, not noticed in endoscopy.
We collected demographic data from all participants, including the age at the time of UC diagnosis, duration and extent of UC, presence of associated PSC, and the use of 5-ASA. Biopsy and colectomy data of UC-associated dysplasias or carcinomas (diagnostic agreement of two experienced pathologists required) and sporadic adenomas were collected from the databases. Histological grade of colonic inflammation was collected using standard text mining methods on colonoscopy pathology reports recorded in Finnish. First, all sentences containing relevant keywords, such as ‘inflammation’, ‘active’, ‘inactive’ and ‘normal’, were collected. Then, based on the word content of sentences, they were labeled into mild (grade 1), moderate (grade 2), severe (grade 3) or no (grade 0) inflammation. In cases where two severity values were recorded in the same report, such as ‘mild-moderate’, higher grade value was selected. The criteria for the inflammatory activity of IBD of the pathology department of Helsinki University Hospital were as follows: 0 (no inflammation): none of the following features, 1 (mild inflammation): presence of cryptitis, but none of the following, 3 (moderate inflammation): presence of crypt abscesses, but no ulceration, 4 (severe inflammation): presence of ulcerations of the mucosa.
Faecal calprotectin samples were quantitated in the major clinical laboratory in southern Finland, HUSLAB, with an ELISA kit from Calpro AS (Calpro/Calprolab, Oslo, Norway). The values quoted as normal and indicating remission were <100 μg/g [Citation21].
Clinical endpoints of the study are development of CRC, colectomy, or the end of the follow-up at the end of 2017, whichever occurred first.
Statistical methods
The demographics and clinical information of the patients in each group were summarized with descriptive statistics (mean, median, min, max, interquartile range (IQR)) for continuous variables. Categorical variables were summarized with frequencies and percentages. Faecal calprotectin time-weighted area under the curve (twAUC) was calculated for each patient using trapezoidal rule.
The receiver operator characteristics (ROC) curve analysis was performed to assess the prediction ability of detecting dysplasia or carcinoma using twAUC for FC (twAUC-FC) and twAUC for histological inflammation grade (twAUC-grade). Sensitivities and specificities were calculated for different thresholds of twAUC-FC. The maximum summation of sensitivity and specificity was selected as the optimal cut-off value for the classification.
The association between the presence of dysplasia or carcinoma and several risk factors was determined using odds ratios (OR) with 95% confidence intervals (CI). Stepwise logistic regression was performed with the following independent variables: age, age at the time of IBD diagnosis, sex, duration of IBD, extent of IBD, PSC, duration of PSC at the end of the follow-up, use of 5-ASA, twAUC-FC, and twAUC-grade. The model with the lowest Akaike Information Criteria (AIC) was selected as the final model. In the multiple logistic regression models, two-sided hypotheses were tested and p values <0.05 were considered significant. R version 3.6.0 and SPSS version 25 were used for all statistical analyses in the study.
Ethical consideration
No permission of local ethics committee or informed consent was required in accordance with Finnish regulations for register-based studies without contact with the study subjects. The study protocol was approved by Helsinki University Hospital Ethical Committee (HUS 115/2020).
Results
UC patient-cohort comprised 982 patients and 9803 patient-years. shows the demographic data. In total, 845 patients (86%) had pancolitis and 127 (12%) concomitant PSC. Almost all patients (99%) were on 5-ASA medication. The median disease duration at the end of the study was 8 years (range 0–48 years, 0 indicating the diagnosis and the end of follow-up at the same year).
Table 1. Demographic data of the included 982 patients with ulcerative colitis.
Colorectal dysplasia and adenocarcinoma (CRC)
Of all patients, 41 (4%) had IBD-associated dysplasia and 7 (0.7%) IBD-associated CRC. In the dysplasia group, 71% of patients were men, 16 patients (39%) had repeated dysplasia, and five patients (12%) had high-grade dysplasia. There was no difference in the use of 5-ASA between patients with or without dysplasia (96% and 99%, p = 0.178), neither in the prevalence of dysplasia between the patients with left-sided or total colitis (4% and 5%, p = 0.707). Of the patients with IBD-associated neoplasia, 26% had concomitant PSC with a median duration of 13 years (range 2–27). In the non-neoplasia group only 12% of the patients had PSC (p = 0.006), and the duration of PSC was shorter than in the neoplasia group (9 years, range 0–29, p = 0.03). Of the UC patients with PSC, 9% had dysplasia, as compared to 4% in the non-PSC group (p = 0.009).
Four out of seven CRC patients had previous dysplasia before the CRC diagnosis. Patients with IBD-associated neoplasias were older (median age 44 and 38 years, p = 0.002) at the end of follow-up, and the disease duration was longer (median 15 and 10 years, p < 0.001) than in patients without neoplasia.
Colectomy
In total, 189 (19%) patients underwent colectomy during the study period, 158 (83%) of them due to active IBD and 32 (17%) because of neoplasia. Of those with dysplasia, 60% underwent colectomy.
Adenomas
We analyzed colonic adenomas separately. Of all patients, 73 (6%) had one or more adenomas. Of these, 78% were tubular adenomas, 10% tubulovillous, and 12% serrated. The median age at diagnosis of the first adenoma was 51 (range 25–82) years.
Histological grade of inflammatory activity
In total, 4289 colonoscopies were performed. In 36% of colonoscopies (n = 1546), the histological grade of inflammatory activity was 0 indicating histological remission. Mild inflammatory activity (grade 1) was seen in 21% of colonoscopies (n = 880), moderate inflammatory activity (grade 2) in 28% (n = 1186), and severe inflammatory activity (grade 3) in 12% of colonoscopies (n = 533). In 144 colonoscopies (3%) the colonic inflammation was not graded. Of all patients, 36% (n = 357) had severe inflammatory activity at least in one colonoscopy. Of patients with severe histological inflammatory activity, 7% had dysplasia at least once, compared to 3% of patients with histological remission, or mild or moderate inflammatory activity (p < 0.05). On the other hand, 63% of patients with dysplasia (26/41) had severe histological inflammatory activity at least once.
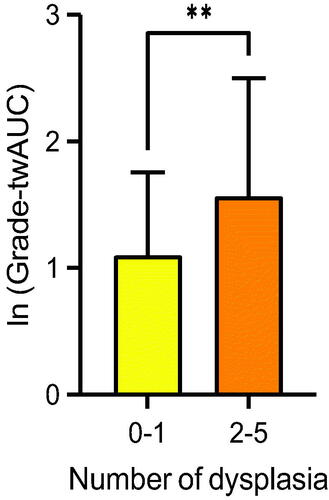
We calculated time-weighted area under the curve (AUC) for the histological inflammatory activity grade (twAUC-grade), which reflects the long-time burden of inflammatory activity. The prevalence of dysplasia in patients with moderate to severe histological inflammatory activity (grade 2–3) was equal to those with remission or mild inflammatory activity (grade 0–1). The twAUC-grade for patients with dysplasia detected more than once was higher than those with no dysplasia, or dysplasia detected only once (). However, the OR of twAUC-grade for neoplasia was 1.11 (95% CI 0.73–1.66) in all patients, and 1.09 (95% CI 0.39–2.78) in PSC-patients, meaning the long-time histological inflammatory activity burden did not predict dysplasia in these patient-cohorts.
Faecal calprotectin
The results of FC values are presented in . Of all patients, 79% had provided more than one stool sample for FC, and the median number of FC measurements was 3 (range 1–31, IQR 2–6). The median time interval between 2 consecutive measurements was 12 months (range 0–119 months, 0 indicating that the patient had only one FC sample). The median time between the first and last FC measurements was 12 months (range 1–119, IQR 2–31). In patients with dysplasia/CRC, the median time between first FC measurement and the diagnosis of IBD associated neoplasias was 13 months (range 5–105, IQR 8.75–17).
Table 2. Faecal calprotectin values of the study patients (n = 982).
The median FC of all patients was 184 µg/g (range 5–13,887, IQR 568). In patients without IBD-associated neoplasias, the median FC was 181 µg/g (range 5–13,887, IQR 567), and of those with neoplasia 232 µg/g (range 6–10,316, IQR 633) (p = 0.271). No statistically significant differences in FC values were found between patients with one or more dysplasia findings or low-grade or high-grade dysplasia. Of all 4649 FC values, 1890 (41%) were below 100 µg/g indicating remission. There was no difference between the dysplasia and non-dysplasia groups (40% and 41%, p = 0.785).
The median FC in patients with CRC was 485 (range 7–2215), which did not differ significantly from that of non-CRC patients (p = 0.161) (). Patients who ended up having a colectomy had a significantly higher median FC than patients with an intact colon (147 µg/g and 441 µg/g, p < 0.001).
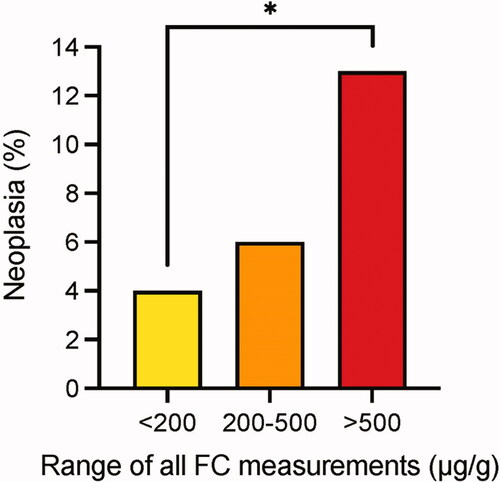
To evaluate the role of inflammatory activity more closely, we divided patients in 3 groups according to the FC results: 1. those with FC <200 µg/g constantly in every measurement (n = 285), 2. those with FC constantly between 200 and 500 µg/g (mild/moderate inflammation, n = 38), and 3. those with FC constantly >500 µg/g (severe inflammation, n = 89). Patients whose FC varied between classes, were not included in this analysis (n = 570). In the group of excluded patients there were 10 patients with dysplasia but none with CRC. The histological inflammatory burden was significantly higher in patients with FC constantly >500 µg/g than those with FC <500 µg/g (). In patients with severe inflammation (FC >500 µg/g), IBD-associated neoplasia was significantly more common compared to patients with FC <200µg/g (13% and 4%, p = 0.015). In the group of mild/moderate inflammation, the difference in the number of IBD-associated neoplasias (6%) compared to those with remission or severe inflammation was not significant ().
Figure 3. Long-term grade of histological inflammation (twAUC-grade) according to FC values. FC values are presented as µg/g; ****p < 0.05. twAUC-grade: time-weighted area under the curve for the grade of histological inflammation.
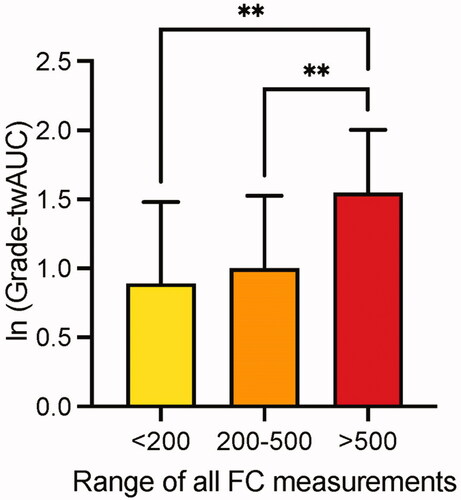
Figure 4. Correlation of IBD associated neoplasia and the severity of colonic inflammation measured with FC. *p = 0.015; FC: faecal calprotectin.
The median FC in patients with sporadic adenomas was 159 µg/g (range 5–6170), with no significant difference compared to the non-adenoma group (p = 0.561). There was also no difference in FC values between the different types of adenomas.
We also calculated time-weighted area under the curve values of FC (twAUC-FC), reflecting the long-term inflammatory burden of the mucosa, and compared them between different patient-groups (). The mean twAUC-FC in the non-neoplasia group was 554 µg/g and in the neoplasia group 648 µg/g (p = 0.303). There was no significant difference in twAUC-FC between patients with once or more often detected dysplasia. ORs of twAUC-FC for neoplasia did not differ between all patients and PSC-patients (1.00 for each group) ( and ). The optimal time-weighted FC cut-off for neoplasia was 705 µg/g with a specificity of 75% and a sensitivity of 34% (AUC 0.519) ( and ).
Figure 5. Time-weighted AUC for FC and detected dysplasias in different groups of patients; AUC: area under the curve; twAUC: time-weighted area under the curve; FC: faecal calprotectin; PSC: primary sclerosing cholangitis.
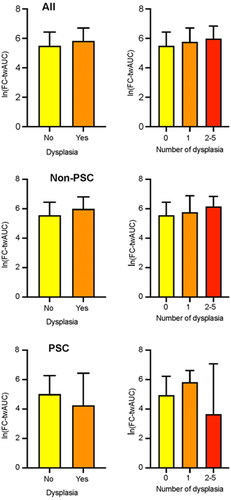
Figure 6. ROC curve of time-weighted FC for predicting occurrence of dysplasia or colorectal carcinoma. FC: faecal calprotectin; twAUC: time-weighted area under the curve; AUC: area under the curve; CRC: colorectal carcinoma; ROC: receiver operating characteristic.
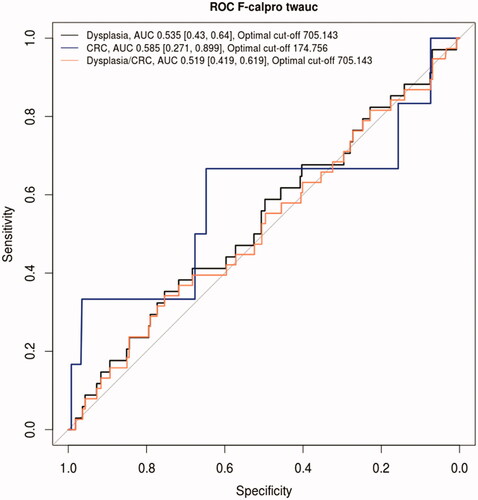
Table 3. Binary logistic regression to predict the composite of dysplasia or colorectal carcinoma in the whole cohort.
Table 4. Binary logistic regression to predict the composite of dysplasia or colorectal carcinoma in PSC patients.
Discussion
In the present study, FC did not predict dysplasia in the whole patient-cohort, and neither did the histological grade of inflammatory activity. However, in patients with constantly elevated FC (>500 µg/g) and active colonic inflammation, IBD-associated neoplasia was significantly more common than in patients with low FC (<200 µg/g).
In line with previous studies, the risk factors for IBD-associated dysplasia in our study were long disease duration, concomitant PSC, and male sex [Citation1,Citation2,Citation7,Citation5,Citation22]. In our data, we did not find any association of dysplasia with the extent of the disease. This can be due to the small number of patients with distal colitis (14%), being up to 50% in previous studies [Citation23,Citation24]. The reason for this difference is unclear, but it can be due to the patient enrolment from the tertiary referral centre where the most complicated cases of IBD are treated.
In our study, approximately half of the patients were in remission based on FC (FC <100 µg/g). This is in line with a Danish study showing that approximately 50% of UC patients are in remission during each year of follow-up [Citation24]. In a large European epidemiologic study, the colectomy rate was 6% after 1 year and 10% after 5 years of follow-up [Citation25]. The total colectomy rate in our study was 19%, the main indication for colectomy being refractory or fulminant colitis, confirming our previous findings on the indications for colectomy in UC [Citation26]. This explains that FC values in the colectomy group were higher than in the non-colectomy group.
In non-IBD patients with CRC, the median FC varies between 50 and 200 µg/g and is higher in CRC patients than in non-CRC patients [Citation27,Citation28]. The FC levels of non-IBD patients with a high risk of either colonic adenoma or colorectal carcinoma were evaluated in a large study, and the cutoff limit of 10 µg/g produced 74% sensitivity for carcinoma and 43% for adenoma [Citation29]. In 2000, a new stool sample preparation procedure for the measurement of FC was developed, yielding a fivefold increase in FC results compared to the earlier procedure [Citation30]. Still, this cutoff limit is too low for clinical practice in IBD patients. In our clinical practice remission is defined as FC <100 ug/g, although it has been suggested that in IBD patients FC values of up to 250 µg/g could be considered as normal [Citation31,Citation32]. In our study, the mean FC levels of the patients with colonic neoplasia or sporadic adenoma did not differ from the patients without neoplasia. However, conclusions should be drawn with caution, due to the limited number of patients with CRC.
We are aware of the strengths and limitations of this study. To our knowledge, this study is among the first studies to evaluate the role of FC in predicting dysplasia in patients with UC. We had a representative patient cohort and a long follow-up of 9803 patient-years. The prevalence of dysplasia and CRC in our cohort were like in a large Finnish register-based study evaluating the prevalence and risk factors of dysplasia and CRC in IBD patients between 1996 and 2008 [Citation1]. Due to efficient therapy, most patients were in remission, and were therefore at a lower risk of colitis-associated neoplasia compared to patients with active disease. Even if FC was not useful in predicting dysplasia in unselected IBD patients or in patients in remission, it did, however, predict neoplasia in patients with active colonic inflammation. Of note, patients with PSC-associated IBD usually have a quiescent disease course with a low grade of inflammatory activity, but still a lifetime risk for CRC of 30% [Citation33]. The patients for this study were enrolled from a tertiary referral center where the most severe cases of IBD are treated. Majority of the colectomies were performed due to chronic active UC refractory to medical therapy, and the colectomy rate was higher than in IBD patients in general. This most likely decreased the number of dysplasia and especially CRC. The number or the frequency of FC samples per patient was not standardized, but only one fifth of the patients had only one FC measurement.
As a conclusion, in the present study we demonstrated that the level of FC in repeated measures could not predict dysplasia in 10 years of follow-up in unselected patients with UC. Also, the grade of histological inflammation did not correlate with risk of colonic neoplasia in this study cohort. However, in patients with constantly markedly elevated FC level, dysplasia and CRC were significantly more common than in patients with low or moderately elevated FC level. This is an important finding. The finding that dysplasia or CRC in patients with FC constantly <200 µg/g is significantly less common compared to patients with prolonged active inflammation, can help to target the surveillance colonoscopies even more optimally, and reduce the need for tight surveillance especially in patients with long-term remission and low FC level.
| Abbreviations | ||
| 5-ASA | = | 5-aminosalisylic acid |
| AUC | = | area under the curve |
| CRC | = | colorectal carcinoma |
| FC | = | faecal calprotectin |
| IBD | = | inflammatory bowel disease |
| IQR | = | interquartile range |
| OR | = | odds ratio |
| PSC | = | primary sclerosing cholangitis |
| ROC | = | receiver operator characteristics |
| twAUC | = | time-weighted area under the curve |
| UC | = | ulcerative colitis. |
Acknowledgements
No additional writing assistance was used for this manuscript. AMP and MF contributed to the design of the study. AMP, TV, JA, and AM collected data. AMP, SQ, SH, AM and SK analysed the data. AMP, SQ, KLK and MF drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors approved the final version of this manuscript.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data underlying this article are available in the article.
Additional information
Funding
References
- Nieminen U, Jussila A, Nordling S, et al. Inflammation and disease duration have a cumulative effect on the risk of dysplasia and carcinoma in IBD: a case–control observational study based on registry data. Int J Cancer. 2014;134(1):189–196.
- Jess T, Rungoe C, Peyrin–Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10(6):639–645.
- Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20(29):9872–9881.
- Lopez A, Pouillon L, Beaugerie L, et al. Colorectal cancer prevention in patients with ulcerative colitis. Best Pract Res Clin Gastroenterol. 2018;32–33:103–109.
- Manninen P, Karvonen A, Laukkarinen J, et al. Colorectal cancer and cholangiocarcinoma in patients with primary sclerosing cholangitis and inflammatory bowel disease. Scand J Gastroenterol. 2015;50(4):423–428.
- Keller D, Windsor A, Cohen R, et al. Colorectal cancer in inflammatory bowel disease: review of the evidence. Tech Coloproctol. 2019;23(1):3–13.
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–535.
- Bernstein C, Nugent Z, Blanchard J. 5-aminosalicylate is not chemoprophylactic for colorectal cancer in IBD: a population based study. Am J Gastroenterol. 2011;106(4):731–736.
- Jess T, Loftus EJ, Velayos F, et al. Risk factors for colorectal neoplasia in inflammatory bowel disease: a nested case–control study from Copenhagen county, Denmark and Olmsted county, Minnesota. Am J Gastroenterol. 2007;102(4):829–836.
- Rutter M, Saunders B, Wilkinson K, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130(4):1030–1038.
- Magro F, Gionchetti P, Eliakim R, European Crohn’s and Colitis Organisation [ECCO], et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670.
- Manninen P, Karvonen A, Huhtala H, et al. The risk of colorectal cancer in patients with inflammatory bowel diseases in Finland: a follow-up of 20 years. J Crohn's Colitis. 2013;7:551–557.
- Jess T, Simonsen J, Jørgensen K, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143(2):375–381.
- Jussila A, Virta LJ, Kautiainen H, et al. Increasing incidence of inflammatory bowel diseases between 2000 and 2007: a nationwide register study in Finland. Inflamm Bowel Dis. 2012;18(3):555–561.
- Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol. 2015;50(8):942–951.
- Tibble JA, Sigthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119(1):15–22.
- Puolanne A, Kolho K, Alfthan H, et al. Rapid faecal tests for detecting disease activity in colonic inflammatory bowel disease. Eur J Clin Invest. 2016;46(10):825–832.
- Molodecky N, Soon I, Rabi D, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.
- Jussila A, Virta L, Salomaa V, et al. High and increasing prevalence of inflammatory bowel disease in Finland with a clear North–South difference. J Crohn's Colitis. 2013;7:256–262.
- Manninen P, Karvonen A, Huhtala H, et al. The epidemiology of inflammatory bowel diseases in Finland. Scand J Gastroenterol. 2010;45(9):1063–1067.
- Kolho K, Alfthan H. Concentration of fecal calprotectin in 11,255 children aged 0–18 years. Scand J Gastroenterol. 2020;55(9):1024–1027.
- Gupta R, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133(4):1099–1105.
- Langholz E. Current trends in inflammatory bowel disease: the natural history. Ther Adv Gastroenterol. 2010;3(2):77–86.
- Langholz E, Munkholm P, Davidsen M, et al. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107(1):3–11.
- Burisch J, Pedersen N, Čuković-Čavka S, EpiCom-group, et al. East–west gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63(4):588–597.
- Kolehmainen S, Lepistö A, Färkkilä M. Impact of anti-TNF-alpha therapy on colectomy rate and indications for colectomy in ulcerative colitis: comparison of two patient cohorts from 2005 to 2007 and from 2014 to 2016. Scand J Gastroenterol. 2019;54(6):707–711.
- Kristinsson J, Røseth A, Fagerhol M, et al. Fecal calprotectin concentration in patients with colorectal carcinoma. Dis Colon Rectum. 1998;41(3):316–321.
- Lehmann F, Trapani F, Fueglistaler I, et al. Clinical and histopathological correlations of fecal calprotectin release in colorectal carcinoma. WJG 2014;20(17):4994–4999.
- Kronborg O. Colon polyps and cancer. Endoscopy. 2000;32(2):124–130.
- Tøn H, Brandsnes Ø, Dale S, et al. Improved assay for fecal calprotectin. Clin Chim Acta. 2000;292(1–2):41–54.
- Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28(10):1221–1229.
- D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224.
- Broomé U, Löfberg R, Veress B, et al. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22(5):1404–1408.