Abstract
Background & aims
Non-alcoholic fatty liver disease (NAFLD) and alcohol-related liver disease frequently coexist. While several blood-based indices exist for the detection of NAFLD, few studies have examined how alcohol use possibly impacts their diagnostic performance. We analysed the effects of alcohol use on the performance of indices for detecting fatty liver disease (FLD).
Methods
We included participants from the Cardiovascular Risk in Young Finns Study (Finnish sample) and KORA study (German sample) who underwent abdominal ultrasound or magnetic resonance imaging, respectively, for detection of FLD and had serum analyses available for calculation of Fatty Liver Index (FLI), Hepatic Steatosis Index (HSI), Lipid Accumulation Product (LAP), and Dallas Steatosis Index (DSI). Alcohol use was estimated by questionnaires as mean daily consumption and binge drinking (Finnish sample only). Predictive performance for FLD was assessed according to alcohol consumption.
Results
The study included 1426 (Finnish sample) and 385 (German sample) individuals, of which 234 (16%) and 168 (44%) had FLD by imaging. When alcohol consumption was <50 g/day, all indices discriminated FLD with area under the receiver operating characteristics (AUROC) of 0.82–0.88. AUROCs were 0.61–0.66 among heavy drinkers (>50 g/day). AUROCs decreased to 0.74–0.80 in the highest binge-drinking category (>2 times/week). Alcohol use correlated with FLI and LAP (r-range 0.09–0.16, p-range <.001–.02) in both samples and with DSI (r = 0.13, p < .001) in the Finnish sample.
Conclusions
Indices perform well and comparably for detection of FLD with alcohol consumption <50 g/day and with different binge-drinking behaviour.
Introduction
Fatty liver disease (FLD) is an increasing healthcare concern and is linked to increased mortality, severe comorbidity, decreased quality of life, and a significant economic burden on society [Citation1,Citation2]. Although some of the pathogenesis and risk factors are well known, the increasing prevalence of FLD suggests a need for improved prevention and interventions [Citation3]. Alcohol, obesity, and type 2 diabetes (T2DM) are known significant etiological factors for FLD. Traditionally, FLD has been dichotomized as either non-alcoholic fatty liver disease (NAFLD) or alcohol-related liver disease (ArLD) [Citation4,Citation5]. However, the two conditions often coexist [Citation6,Citation7], and the European Association for the Study of the Liver (EASL) states a need for studies to reveal the impact of alcohol use on diagnostic tests for FLD [Citation8].
The diagnosis of FLD can be made by imaging studies. Ultrasound (US) is the recommended first-line tool in clinical practice and magnetic resonance proton density fat fraction (MRI-PDFF) is the most accurate non-invasive method [Citation9]. However, population screening using imaging methods is time- and resource consuming. Hence, several different index scores based on routine blood tests and biometric characteristics have been developed to detect NAFLD [Citation4,Citation10]. Fatty Liver Index (FLI), based on waist circumference (WC), body mass index (BMI), and serum levels of gamma-glutamyl transferase (γ-GT) and triglycerides (TG), is one of the most validated indices and exhibits an area under the receiver operating characteristic (AUROC) from 0.72–0.97 for detecting NAFLD [Citation11–21]. The Hepatic Steatosis Index (HSI; based on sex, BMI, alanine transaminase (ALT) and aspartate transaminase (AST), and T2DM), Lipid Accumulation Product (LAP; based on WC, TG, and sex) and Dallas Steatosis Index (DSI; based on age, sex, diabetes status, hypertension, fasting glucose, ALT, BMI, and ethnicity) are other indices that also have been used in this context [Citation13,Citation16,Citation17,Citation20–24]. To date, no study has assessed the performance of FLD indices for detection of steatosis in ArLD [Citation9]. Accordingly, we sought to investigate whether various levels of alcohol use impact the diagnostic performance of several fatty liver indices.
In the present cross-sectional study, we aimed to assess the impact of alcohol consumption on the diagnostic performance of FLI, HSI, LAP, and DSI in the detection of FLD in two population-based cohorts from Finland and Germany.
Subjects and methods
Study samples
The present study is a cross-sectional study based on two samples from The Cardiovascular Risk in Young Finns Study [Citation25] (Finnish sample) and The Cooperative Health Research in the Region Augsburg study (KORA) [Citation26,Citation27] (German sample). In both samples, study design and procedures were approved by local ethics committees or institutional review boards. All participants gave written informed consent.
The cardiovascular risk in Young Finns Study
The Cardiovascular Risk in Young Finns Study is a follow-up study that started in 1980 [Citation28]. Children and adolescents aged 3–18 years from five different centres in Finland were enrolled by random selection from the population register. The original sample included 4320 participants. Participants were followed up every 3–9 years with surveys, clinical examination, imaging studies, blood samples, or combinations thereof. In 2010–2012, abdominal US was performed on participants [Citation29]. We included participants with available data on US, mean alcohol consumption, and γ-GT in our study, which consisted of 1426 participants. US was used to define FLD, as either mild or definitive, using a validated protocol based on liver-to-kidney contrast, parenchymal brightness, deep beam attenuation and bright vessel walls, with the operator blinded for participants’ characteristics [Citation25,Citation29,Citation30]. Clinical data and blood samples collected at the same time were used for calculation of FLD indices.
Cooperative health research in the region Augsburg study (KORA)
The KORA-MRI study is a sub-sample of the second follow up (KORA FF4 study, 2013–2014, N = 2279) of the population-based KORA S4 baseline survey, sampled in the region of Augsburg, Germany (KORA S4 study, 1999–2001, N = 4261) [Citation27]. The KORA-MRI study consisted of 400 participants who underwent whole-body MRI at 3 Tesla. Exclusion criteria were age >72 years, pregnancy/breastfeeding, history of stroke/myocardial infarction, or contraindication for gadolinium-enhanced MRI (e.g., implanted device, renal failure, allergy, claustrophobia) [Citation26]. Hepatic fat content was measured as proton density fat fraction (MRI-PDFF) by a multi-echo Dixon technique on a volumetric interpolated breath-hold examination (VIBE) sequence. A cut-off point of mean 5.56% fat content at the level of the portal vein was used to define the presence of FLD [Citation31]. Details of other covariates are described in Supplementary methods. Of the 400 participants, 14 were excluded due to missing MRI-PDFF-data and 1 was excluded because of revocation of participation. The final study sample consisted of 385 participants.
Fatty liver disease indices and alcohol consumption
We calculated FLI, HSI, LAP, and DSI for each participant based on variables obtained at the time of the imaging study according to the equations previously described (Supplementary Table 1) [Citation11,Citation22,Citation24,Citation32]. Based on established cut-offs, FLI was sub-grouped as <30, 30–60, and >60, HSI was sub-grouped as <30, 30–36, and >36, LAP was sub-grouped as <23 or >23 for females and <30.5 or >30.5 for males, and DSI was sub-grouped as < −1.4, −1.4–0, and >0. Ethnicity was not documented, but we can safely assume that most participants were white in both Finnish and German study groups.
For the KORA study, alcohol intake was assessed by self-report using during a standardized interview by trained staff. Alcohol consumption was divided into the following groups: Group 1, <10 g/day; Group 2, females: 10–20 g/day, males: 10–30 g/day; Group 3, females 20–50 g/day, males 30–50 g/day; Group 4, > 50 g/day.
Binge drinking was recorded in the Finnish sample as the frequency of drinking at least six standard alcohol units per occasion and was divided into the following six sub-groups according to this frequency: Group 1, >2 times/week; Group 2, 1 time/week; Group 3, 2–3 times/month; Group 4, 1 time/month; Group 5, 2–6 times/year; Group 6, More seldom. Binge drinking data were available for 1385 participants.
Statistical analyses
Groups were compared using the t-test or Mann–Whitney U-test for continuous variables (for normally and non-normally distributed variables, respectively), or Chi-Squared or Fisher’s exact for categorical variables (Fisher’s exact test was used when samples in at least one of the subgroups ≤5). Correlations were calculated using Spearman’s correlation. Performance of indices was analysed by area under the receiver operating characteristic (AUROC) and sensitivities, specificities, positive predictive values (PPV) and negative predictive values (NPV). Binary logistic regression analysis was performed to calculate the modifying effect of alcohol use on the association between FLD indices and imaging-based FLD by including both the FLD index, alcohol use, and their interaction term in the regression model, with imaging-based FLD as the dependent variable. Statistical analyses were performed with SPSS for Windows 27.0 (SPSS, Chicago, IL). A p-value of <.05 was considered statistically significant.
Results
Of the 1426 participants (men 42.6%) in the Finnish sample, FLD was found in 234 (16%) individuals (male predominance, 67.1%); FLD was either mild (n = 186) or definitive (n = 48). Amongst 385 participants in the German sample, FLD was found in 168 (44%) participants. Characteristics for the study groups and by FLD status are shown in . Of 1385 participants with available binge drinking data, 309 (22.3%) reported binge drinking more frequently than once monthly. In this binge drinking group, prevalence of FLD was 27.5% (n = 85), while prevalence of FLD was 12.7% (n = 137) in those with less frequent binge drinking (p < .001).
Table 1. Characteristics of the study populations.
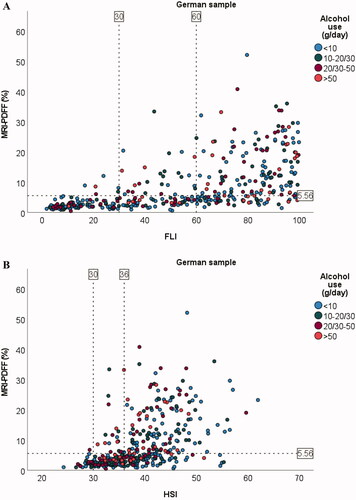
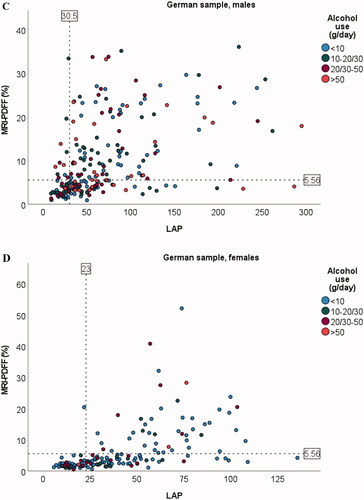
Correlations between anthropometric variables and amount of hepatic steatosis as determined by MRI-PDFF in the German sample were r = 0.59 (BMI), r = 0.70 (WC), and r = 0.67 (WHR) (all p < .001). The corresponding correlations between amount of hepatic steatosis and other metabolic characteristics were r = 0.51 (triglycerides), r = 0.52 (fasting glucose), and r = 0.20 (alcohol/day) (all p < .001) (Supplementary Figure 1). In comparison, the correlation between FLD indices and amount of hepatic steatosis were r = 0.72 (FLI), r = 0.61 (HSI), r = 0.66 (LAP), and r = 0.72 (DSI) (all p < .001) ().
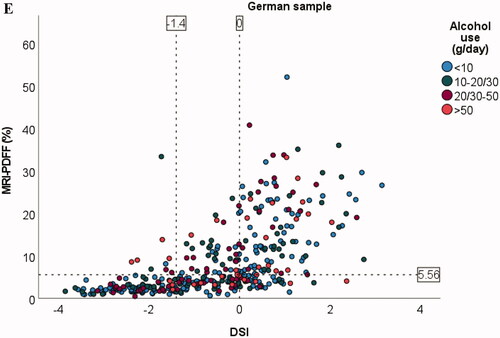
Figure 1. Scatter plots for association between amount of liver fat and FLI (A), HSI (B), LAP (C, D), and DSI (E) in the German sample. Dotted line on y-axis for MRI-PDFF = 5.56% (cut-off value used for fatty liver disease). Dotted line/lines on x-axis indicate cut-off values for the indices. For LAP, males and females are shown separately because of different cut-off values. Alcohol consumption cut-offs (g/day) for males were <10, 10–30, 30–50, and >50. Corresponding cut-offs for females were <10, 10–20, 20–50, and >50.
In the Finnish sample, mean alcohol consumption correlated with FLI (r = 0.16), LAP (r = 0.09), and DSI (r = 0.13) (all p < .001). The correlation between alcohol consumption and HSI was non-significant (r = 0.02, p = .54). In the German sample, alcohol consumption correlated with FLI (r = 0.14, p = .007) and LAP (r = 0.12, p = .02), while correlations with HSI and DSI were non-significant (r= −0.06, p = .25 and r = 0.10, p = .06, respectively).
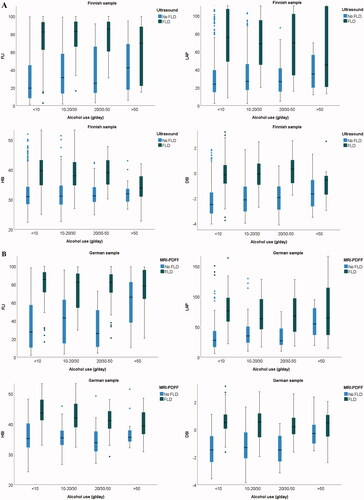
All index scores were higher in the presence of FLD (). This same finding was consistent in all the alcohol subgroups (). The distributions of participants with low, intermediate, or high index score were also consistent between alcohol groups when alcohol consumption was <50 g/day ().
Figure 2. Boxplots of differences in index distribution between no FLD or FLD shown for different alcohol consumption groups; Finnish sample (A), German sample (B). Boxes represent the middle two quartiles, the horizontal line inside box represents median. Upper and lower quartiles are expressed as vertical lines within the range. Outliers are marked by dots. Alcohol consumption cut-offs (g/day) for males were <10, 10–30, 30–50, and >50. Corresponding cut-offs for females were <10, 10–20, 20–50, and >50.
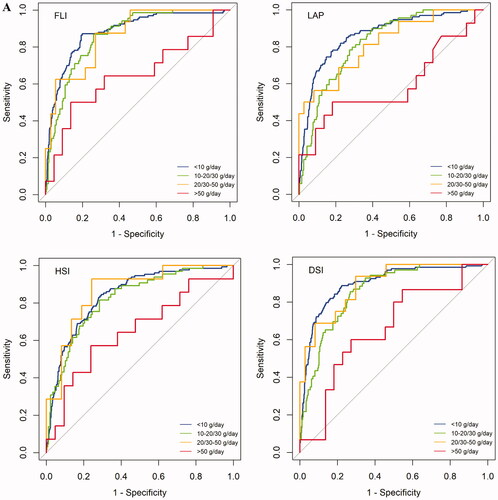
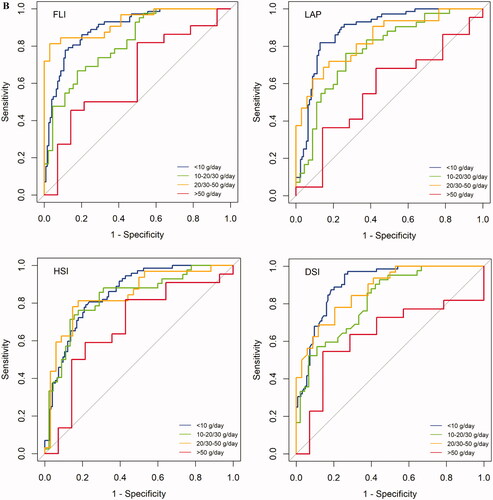
In the Finnish sample overall, AUROC values of FLI, HSI, LAP, and DSI for detection of FLD were 0.87, 0.83, 0.84, and 0.88, respectively (). With alcohol consumption <50 g/day, AUROC values ranged between 0.82–0.90 for FLI, HSI, LAP, and DSI (, ). AUROC ranged between 0.61–0.66 when alcohol consumption was >50 g/day. In the German sample, AUROC values for FLI, HSI, LAP, and DSI were 0.87, 0.82, 0.83, and 0.86, respectively (, ). AUROC values ranged between 0.78–0.93 with alcohol use <50 g/day. AUROC ranged between 0.57–0.68 when alcohol consumption was >50 g/day.
Figure 3. AUROC for FLI, HSI, LAP, and DSI in detection of FLD. Samples are grouped according to mean alcohol consumption. AUROC charts for Finnish sample (A) and German sample (B). Color-coded based on alcohol consumption; blue: <10 g/day, green: 10-20/10-30 g/day, yellow: 20–50/30–50 g/day, red: >50 g/day. Alcohol consumption cut-offs (g/day) for males were <10, 10–30, 30–50, and >50. Corresponding cut-offs for females were <10, 10–20, 20–50, and >50.
Table 2. AUROCs and 95% CIs for FLD indices to detect FLD according to alcohol consumption.
Binge drinking modified the performance of indices in detection of FLD. AUROCs decreased with increasing binge drinking frequency. AUROCs ranged between 0.87–0.93 among non-binge drinkers, while AUROCs ranged between 0.70–0.80 among weekly binge drinkers (Supplementary Table 2). For comparison, AUROCs for WC to detect FLD ranged between 0.80–0.87 when alcohol use was <50 g/day; AUROCs ranged from 0.64–0.71 with alcohol use >50 g/day (Supplementary Table 3).
Performance measures for the various indices using established cut-offs are shown in . For FLI, HSI and DSI, which are divided into three-step scales, values are given for lower and higher cut-off points. When observing the low-risk groups in the Finnish sample, NPV was 97.5% (FLI), 97.6% (HSI), 96.1% (LAP), and 96.6% (DSI). Corresponding NPV results in the German sample were 94.6% (FLI), 96.0% (HSI), 88.7% (LAP), and 92.2% (DSI). In high-risk groups, PPVs in the Finnish sample were 45.6% (FLI), 41.5% (HSI), 26.6% (LAP), and 62.7% (DSI). The corresponding PPVs in the German sample were 72.0% (FLI), 61.3% (HSI), 55.9% (LAP), and 75.5% (DSI). When grouped according to alcohol use, NPV in particular weakened for all indices among heavy drinkers (>50 g/day).
Table 3. Performance measures for FLD indices.
In separate logistic regression analyses with FLD as the dependent variable, and the indices, alcohol use, and their interaction term as independent variables, we observed a significant interaction effect between alcohol use and FLI (p < .001), LAP (p = .03), and DSI (p < .001) in the Finnish sample. In the German sample, a significant interaction effect was found for FLI (p = .005), LAP (p < .001), and DSI (p < .001). For HSI, p-values were .39 and .32 (Finnish and German samples, respectively).
Discussion
FLD is highly prevalent worldwide and has serious life-threatening consequences, which emphasize the importance of increasing global awareness of the disease and in developing valid tools for clinical practitioners in early detection of persons at risk [Citation33]. Early identification of FLD is essential, as steatosis can progress to NASH and fibrosis [Citation34,Citation35]. Alcohol use and binge drinking are associated with hepatic steatosis and disease progression, suggesting that significant overlap exists between NAFLD and ArLD [Citation36,Citation37]. The findings of the present cross-sectional study support the use of FLI, HSI, LAP, and DSI for detection of FLD when alcohol use is limited to <50 g/day. AUROC values ranged 0.82–0.90 for all indices when alcohol use was within these limits (i.e. excellent discrimination for FLD). Regarding sensitivity, specificity, and NPV, a dip could be seen among heavy drinkers; in particular, the NPV of a low index score weakened in the >50 g/day alcohol group. Only 2.6% of the Finnish sample and up to 9.4% of the German sample reported such alcohol use.
Despite its limitations, US is recommended as a first-line tool for diagnosis of FLD in clinical practice [Citation9]. MRI-PDFF is considered the most accurate non-invasive method in this context, but its higher price and poorer availability limit its use [Citation9]. As a new finding, the results of the present study showed good performance of the four indices to detect both US- or MRI-PDFF-diagnosed FLD among non-drinkers and up to those who consume alcohol moderately. This is consistent with earlier findings of external validation for FLI and LAP using magnetic spectroscopy for detection of liver fat [Citation13]. Previous studies suggest that the parameters included in the FLD-index algorithms may be influenced by alcohol consumption and binge drinking [Citation38]. From this perspective, the present study provides novel and crucial data concerning the reliability of these indices in the context of alcohol consumption.
Even low alcohol intake in FLD is associated with increased risks for advanced liver disease [Citation39,Citation40], and binge drinking is related to an increased risk of liver disease independent of average alcohol intake and confounders [Citation41,Citation42]. In this regard, the present study provides novel evidence that binge-drinking behaviour did not impair the performance of the four FLD indices, thus increasing their validity in this context. Despite a well-established association between alcohol and liver disease, the results of the present study suggest that the correlation between alcohol and liver fat is smaller than the respective correlation of metabolic factors, such as WC and BMI. This further supports the usage of FLD indices regardless of alcohol consumption and the abandoning of strict alcohol criteria in the nomenclature and diagnosis of FLD. In addition, ArLD and FLD might have supra-additive harmful effects on the liver [Citation43,Citation44].
The prevalence of FLD amongst study participants varied significantly (16% and 44%, p < .001) between the two study groups. The differences between study groups may in part be explained by known etiological factors for FLD being more prevalent in the German sample (e.g., degree of obesity, age, alcohol use, and diabetes). Furthermore, the modality used for FLD detection differed between the study groups (US vs. MRI-PDFF). The inclusion criteria in study enrolment also differed; although both original study samples included participants from the general population, further enrolment into imaging studies differed [Citation26]. Both observed prevalence values matched previously reported values in western countries [Citation4,Citation45]. Sensitivity and specificity values of the four indices were generally similar between the Finnish and the German samples except for HSI, which had low specificity at the low-risk cut-off in the German sample. Among heavy drinkers, the Finnish sample had almost consistently a more profound reduction in sensitivity, but higher specificity compared to the respective values in the German sample. Along with the higher overall prevalence of FLD in the German sample, the PPVs of the four indices were also correspondingly higher in each group of alcohol consumption in the German sample. Our findings suggest that the various FLD indices perform well and are suitable for screening FLD in both high- and low-prevalence populations and with alcohol use up to 50 g/day.
Comparison of correlation for commonly used obesity markers and degree of steatosis revealed strongest correlations for WC and WHR. It is noteworthy that these correlations and corresponding AUROC values were of comparable magnitude as the correlations between the indices and degree of steatosis, suggesting a possibility of using these simple body composition measures in screening for liver disease in low-resource settings. This is in accordance with a previous study that showed that WC and HC both show independent association with liver disease [Citation46].
Interestingly, FLI has recently been suggested to mirror some of the risk of cardiovascular diseases [Citation47,Citation48]. This suggested association between FLD and cardiovascular disease further emphasizes the need for screening the population with FLD indices; the performances of these indices are only minimally impacted by moderate alcohol consumption.
The strengths of this study were two geographically differing cohorts with prevalence of FLD matching previously reported data, well-documented parameters, and objective measurements. Validation of the indices was successfully achieved in two different cohorts using both US and MRI-PDFF as criteria for FLD.
One of the limitations of this study was alcohol consumption data that relied on questionnaires. A more objective way to assess this parameter in future studies could be through blood tests for metabolites of alcohol use (e.g., phospatidylethanol [Citation49]). Although the total number of participants was high compared to previous studies, both study groups were analysed individually, and sub-grouping according to alcohol use inevitably led to small counts in some of the sub-groups.
In conclusion, the four indices were valid in detecting FLD as measured by US and MRI-PDFF, suggesting that these indices may have a clinically relevant role in the primary diagnosis of this emerging health problem. Alcohol consumption up to 50 g/day and binge drinking had minimal impact on diagnostic performance of these indices. FLD indices offer an inexpensive and convenient tool to identify patients at risk when compared with imaging modalities such as US or MRI.
| Abbreviations | ||
| FLD | = | fatty liver disease |
| BMI | = | body mass index |
| WHR | = | waist-hip ratio |
| WC | = | waist circumference |
| HC | = | hip circumference |
| FLI | = | fatty liver index |
| HSI | = | hepatic steatosis index |
| LAP | = | lipid accumulation product |
| DSI | = | Dallas steatosis index |
| ArLD | = | alcohol-related liver disease |
| US | = | ultrasound |
| MRI | = | magnetic resonance imaging |
| MRI-PDFF | = | magnetic resonance proton density fat fraction |
| AUROC | = | area under the receiver operating characteristic |
| PPV | = | positive predictive value |
| NPV | = | negative predictive value |
| 95% CI | = | 95% confidence interval |
Supplemental Material
Download MS Word (201.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Kim D, Li AA, Gadiparthi C, et al. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155(4):1154–1163.e3.
- Younossi ZM. Non-alcoholic fatty liver disease – a global public health perspective. J Hepatol. 2019;70(3):531–544.
- Carr RM, Oranu A, Khungar V. Nonalcoholic fatty liver disease. Gastroenterol Clin North Am. 2016;45(4):639–652.
- EASL– E. Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402.
- Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209.
- Åberg F, Färkkilä M. Drinking and obesity: alcoholic liver disease/nonalcoholic fatty liver disease interactions. Semin Liver Dis. 2020;40(02):154–162.
- Younossi ZM, Stepanova M, Ong J, et al. Effects of alcohol consumption and metabolic syndrome on mortality in patients with nonalcoholic and alcohol-related fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(8):1625–1633.e1.
- Zelber-Sagi S, Sheron N, Hydes T. Policy Statement on the Coexistence of Non-Alcoholic Fatty Liver Disease (NAFLD) and Alcohol-Related Liver Disease (ARLD) 2020. Accessed January 18, 2022. https://easl.eu/wp-content/uploads/2020/08/Full-version-Policy-Statement-on-the-coexistence-of-NAFLD-and-ARLD_26Aug2020.pdf.
- Berzigotti A, Tsochatzis E, Boursier J, et al. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. Journal of Hepatology. 2021;75(3):659–689.
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American gastroenterological association, American association for the study of liver diseases, and American college of gastroenterology. Gastroenterology. 2012;142(7):1592–1609.
- Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6(1):33.
- Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States national health and nutrition examination survey. Aliment Pharmacol Ther. 2015;41(1):65–76.
- Cuthbertson DJ, Weickert MO, Lythgoe D, et al. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. 2014;171(5):561–569.
- Koehler EM, Schouten JNL, Hansen BE, et al. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11(9):1201–1204.
- Shen YN, Yu MX, Gao Q, et al. External validation of non-invasive prediction models for identifying ultrasonography-diagnosed fatty liver disease in a Chinese population. Medicine. 2017;96(30):e7610.
- Fedchuk L, Nascimbeni F, Pais R, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1209–1222.
- Meffert PJ, Baumeister SE, Lerch MM, et al. Development, external validation, and comparative assessment of a new diagnostic score for hepatic steatosis. Am J Gastroenterol. 2014;109(9):1404–1414.
- Zelber-Sagi S. Comparison of fatty liver index with noninvasive methods for steatosis detection and quantification. WJG. 2013;19(1):57.
- Yang BL, Wu WC, Fang KC, et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS ONE. 2015;10(3):e0120443.
- Kim JH, Kwon SY, Lee SW, et al. Validation of fatty liver index and lipid accumulation product for predicting fatty liver in Korean population. Liver Int. 2011;31(10):1600–1601.
- Xia MF, Yki-Järvinen H, Bian H, et al. Influence of ethnicity on the accuracy of non-invasive scores predicting non-alcoholic fatty liver disease. PLoS ONE. 2016;11(8):e0160526.
- Dai H, Wang W, Chen R, et al. Lipid accumulation product is a powerful tool to predict non-alcoholic fatty liver disease in Chinese adults. Nutr Metab (Lond). 2017;14(1):49.
- Almeida NS, Rocha R, Cotrim HP, et al. Anthropometric indicators of visceral adiposity as predictors of non-alcoholic fatty liver disease: a review. World J Hepatol. 2018;10(10):695–701.
- McHenry S, Park Y, Browning JD, et al. Dallas steatosis index identifies patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18(9):2073–2080.e7.
- Juonala M, Viikari JSA, Raitakari OT. Main findings from the prospective cardiovascular risk in young Finns study. Curr Opin Lipidol. 2013;24(1):57–64.
- Bamberg F, Hetterich H, Rospleszcz S, et al. Subclinical disease burden as assessed by whole-body MRI in subjects with prediabetes, subjects with diabetes, and normal control subjects from the general population: the KORA-MRI study. Diabetes. 2017;66(1):158–169.
- Holle R, Happich M, Löwel H, et al. KORA - a research platform for population based health research. Gesundheitswesen. 2005;67(S 01):19–25.
- Raitakari OT, Juonala M, Ronnemaa T, et al. Cohort profile: the cardiovascular risk in young Finns study. Int J Epidemiol. 2008;37(6):1220–1226.
- Suomela E, Oikonen M, Pitkänen N, et al. Childhood predictors of adult fatty liver. The cardiovascular risk in young Finns study. J Hepatol. 2016;65(4):784–790.
- Edens MA, van Ooijen PMA, Post WJ, et al. Ultrasonography to quantify hepatic fat content: validation by 1H magnetic resonance spectroscopy. Obesity. 2009;17(12):2239–2244.
- Caussy C, Reeder SB, Sirlin CB, et al. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018;68(2):763–772.
- Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–508.
- Lazarus J v, Mark HE, Villota-Rivas M, et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J Hepatol. Published Online. December 2021;76(4):771–780.
- McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148–1155.
- Perumpail BJ, Khan MA, Yoo ER, et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23(47):8263–8276.
- Niezen S, Trivedi HD, Mukamal KJ, et al. Associations between alcohol consumption and hepatic steatosis in the USA. Liver Int. 2021;41(9):2020–2023.
- Long MT, Massaro JM, Hoffmann U, et al. Alcohol use is associated with hepatic steatosis among persons with presumed nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18(8):1831–1841.e5.
- Traversy G, Chaput JP. Alcohol consumption and obesity: an update. Curr Obes Rep. 2015;4(1):122–130.
- Åberg F, Puukka P, Salomaa V, et al. Risks of light and moderate alcohol use in fatty liver disease: follow-up of population cohorts. Hepatology. 2020;71(3):835–848.
- Nivukoski U, Niemelä M, Bloigu A, et al. Combined effects of lifestyle risk factors on fatty liver index. BMC Gastroenterol. 2020;20(1):109.
- Åberg F, Helenius-Hietala J, Puukka P, et al. Binge drinking and the risk of liver events: a population-based cohort study. Liver Int. 2017;37(9):1373–1381.
- Sahlman P, Nissinen M, Puukka P, et al. Genetic and lifestyle risk factors for advanced liver disease among men and women. J Gastroenterol Hepatol. 2020;35(2):291–298.
- Hart CL, Morrison DS, Batty GD, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340(mar11 1):c1240–c1240.
- Åberg F, Puukka P, Salomaa V, et al. Combined effects of alcohol and metabolic disorders in patients with chronic liver disease. Clin Gastroenterol Hepatol. 2020;18(4):995–997.e2.
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285.
- Danielsson O, Nissinen MJ, Jula A, et al. Waist and hip circumference are independently associated with the risk of liver disease in population‐based studies. Liver Int. 2021;41(12):2903–2913.
- Iwasaki Y, Shiina K, Matsumoto C, et al. Correlation of the fatty liver index with the pathophysiological abnormalities associated with cardiovascular risk markers in Japanese men without any history of cardiovascular disease: comparison with the fibrosis-4 score. J Atheroscler Thromb. 2021;28(5):524–534.
- Cicero AFG, Gitto S, Fogacci F, et al. Fatty liver index is associated to pulse wave velocity in healthy subjects: data from the brisighella heart study. Eur J Intern Med. 2018;53:29–33.
- Röhricht M, Paschke K, Sack P, et al. Phosphatidylethanol reliably and objectively quantifies alcohol consumption in adolescents and young adults. Alcohol Clin Exp Res. 2020;44(11):2177–2186.