Abstract
Background
The selection of endoscopic treatments for small rectal neuroendocrine tumors is controversial.
Objective
To retrospectively compare the effectiveness and safety of precut endoscopic mucosal resection (EMR-P) and endoscopic submucosal dissection (ESD) for small rectal neuroendocrine tumors (NETs).
Methods
Data from 98 patients with small rectal NETs who were hospitalized at Shenzhen Second People's Hospital between August 2014 and November 2021 were collected. The en bloc resection rate, pathological complete resection rate, radical resection rate, operation time, adverse event rate and hospital stay were compared between the two groups.
Results
The operation time in the EMR-P group was significantly shorter than that in the ESD group. The median hospital stay in the EMR-P group was also significantly shorter than that in the ESD group. There were no significant differences between the two groups in terms of the en bloc resection, complete resection or radical resection rates. There was also no significant difference in the incidence of adverse events between the two groups. The delayed bleeding and delayed perforation rates of the two groups were improved after conservative treatment without surgery. There was no significant difference in the rate of positive vertical margins and horizontal margins between the EMR-P group and the ESD group. No local recurrence or metastasis was found during follow-up.
Conclusion
EMR-P is an effective and safe endoscopic treatment for rectal NETs with a diameter of less than 10 mm. EMR-P is a significantly shorter procedure and requires a shorter hospital stay than ESD. EMR-P does not increase the cut margin positivity rate.
Introduction
Neuroendocrine neoplasm (NEN) generally refers to a family of heterogeneous tumors originating from peptidergic neurons and neuroendocrine cells. Clinically, this family of neoplasms consists of low-grade malignant tumors with inert and slow growth as well as high-grade malignant tumors with metastatic characteristics [Citation1]. The human digestive tract is the preferred site of neuroendocrine tumors. The endoscopic detection rate of rectal neuroendocrine tumors is approximately 0.17%, accounting for 12%∼27% of all neuroendocrine tumors and 20% of gastrointestinal neuroendocrine tumors [Citation2]. Rectal NETs with a diameter less than 10 mm have a low risk of distant metastasis (<3%). Most patients can undergo radical resection, and the long-term prognosis is good, with a five-year survival rate of approximately 98%∼100% [Citation2]. At present, it is reported that rectal NETs less than 10 mm can be completely removed endoscopically [Citation3,Citation4].
The conventional endoscopic resection methods for rectal NETs include polypectomy, EMR, ESD, modified EMR (including cap-EMR, ligation-EMR, EMR-P and so on), and endoscopic full-thickness resection. Because endoscopic polypectomy and EMR have low complete resection rates [Citation5,Citation6], they are not routinely used for treating rectal NETs. The use of endoscopic full-thickness resection [Citation7] is currently in the clinical exploration stage due to its high risk and difficulty, so it is not widely use as a treatment for rectal NETs at present. The use of ESD and mEMR for the treatment of rectal NETs is still controversial; some studies have shown that ESD is better than mEMR with regard to the whole en block resection rate and pathological complete resection rate [Citation8]. However, other studies have suggested that there is no significant difference between ESD and mEMR [Citation9]. Accordingly, we conducted a retrospective study to investigate the treatment outcomes of patients who underwent EMR-P and ESD and to compare the rates of adverse events between these two approaches.
Materials and methods
Study design and ethics
The was a single-center retrospective study conducted at Shenzhen Second People’s Hospital. The study was carried out in accordance with the Declaration of Helsinki (revised in 2008) and approved by the Ethics Committee of Shenzhen Second People's Hospital. The keywords ‘rectal neuroendocrine tumor’, ‘rectal neuroendocrine cancer’, ‘rectal NEN’, ‘rectal NET’ and ‘rectal NEC’ were used to search medical records in the Pathology Department of Shenzhen Second People's Hospital from August 2014 to November 2021. We sorted the retrieved patient medical data, including patient characteristics and clinical results. Informed consent was obtained from each patient for endoscopic resection. However, informed consent was waived for this study due to its retrospective nature.
Patients
A total of 145 patients were enrolled, and 19 patients undergoing endoscopic biopsy, one patient undergoing polypectomy, five patients undergoing EMR, 13 patients undergoing transabdominal sacral rectal resection, six patients without hospital consultation, one patient with thoracic metastasis, one patient with uterine metastasis and one patient with liver metastasis were excluded. The remaining 98 patients had rectal NETs and were included in the current study. Either EMR-P or ESD was performed.
Indications for endoscopic resection
The indications for endoscopic resection were as follows: (1) the diameter of rectal NETs was less than 10 mm; (2) no lymphatic metastasis or distant metastasis, which was confirmed by CT or MRI; and (3) patients in generally stable condition without severe cardiopulmonary insufficiency who can tolerate the operation.
Instruments
The following instruments and equipment were used: image processing device (Olympus, Japan, CLV-290sl); electronic colonoscopy (Olympus, Japan, HQ290i); high-frequency electric knife (ERBE, Germany, VIO300D); snare (Boston Scientific Corporation, America, M00561231); disposable endoscope injection needle (Jiangsu Antel Medical, China, ATE-ZSZ-23x2000x25x4); rotatable and repeated opening and closing soft tissue clip (Nanwei Medical Technology Co, Ltd, China, POCC-D-26-195); and disposable mucosal incision knife (Olympus, Japan, KD-650Q).
EMR-P procedure
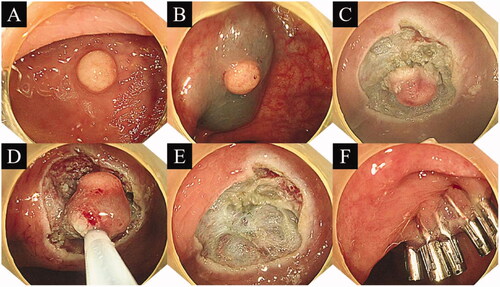
Normal saline and indigo carmine dye were mixed and then injected into the submucosal layer. The lesion was then lifted, and the metal front of the snare was extended approximately 1.5 mm. Circumferential incision was performed approximately 3 ∼ 5 mm along the periphery of the lesion. The resection depth reached the middle and lower parts of the submucosa. After circumferential resection, the snare was used to grasp the lesion. During this process, the snare was placed as deep as possible to ensure that the appropriate depth was reached. Then, the electric cutting device was applied. If bleeding of the wound occurred, electrocoagulation was administered to stop the bleeding. Finally, clip closure of the mucosal defect was performed ().
Figure 1. Procedure for precut endoscopic mucosal resection. (A) Yellow hemispherical mass in the rectum; (B) Injection into the submucosal layer; (C) Circumferential resection; (D) Snaring of the lesion; (E) Mucosal defect after endoscopic submucosal dissection; (F) Clip closure of the mucosal defect.
ESD procedure
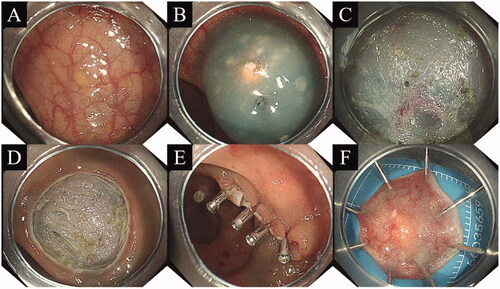
A dual knife was used to mark around the lesion as needed. Submucosal injection was the same as that used for EMR-P. A circumferential mucosal incision was made around the lesion, and the submucosal layer was dissected using a dual knife. Intraoperative bleeding was stopped by electrocoagulation, and the wound was sutured with a tissue clip ().
Figure 2. Procedure for endoscopic submucosal dissection. (A) Yellow hemispherical mass in the rectum; (B) Injection into the submucosal layer; (C) Entry into the submucosa for dissection; (D) Mucosal defect observed after endoscopic submucosal dissection; (E) Clip closure of mucosal defect; (F) The excised specimen was treated and analyzed in a standardized manner.
Specimen processing
All resected specimens were treated according to the following specifications: the specimen was unfolded and fixed at the cutting edge with a long needle. The cells were fixed in 10% formalin solution for 24 ∼ 48 h. Sections were cut at 2-mm interval and stained with hematoxylin and eosin. The pathological diagnoses and evaluation of curability were made by expert gastrointestinal pathologists at our hospital. The tumor size, differentiation, grade, positive margin and vascular infiltration were evaluated.
Clinical outcomes
We analyzed the endoscopic en bloc resection rate, pathological complete resection rate, radical resection rate, operation time, hospital stay, operation cost and incidence of adverse events, including delayed bleeding or delayed perforation. Follow-up was conducted to determine whether there was recurrence and metastasis. The operation time was defined as the time from submucosal injection to complete resection of the lesion. Hospital stay was defined as the time from the day of admission to the day of discharge. Operation cost was defined as all medical expenses incurred during the operation. En bloc resection was defined as complete resection of the specimen, not multiple specimens. Pathological complete resection was defined as en bloc resection with no tumor cells observed at the horizontal and vertical margins. Radical resection was defined as no vessel and lymph infiltration on the basis of pathological complete resection. Delayed perforation was defined as the presence of free gas in the abdominal cavity as detected by abdominal X-ray film or abdominal computed tomography (CT). Delayed bleeding was defined as postoperative hematochezia and decreased hemoglobin requiring blood transfusion or endoscopic hemostasis. Local recurrence was defined as the presence of a new lesion at the original resection site during follow-up that was confirmed as NET by biopsy. Metastatic recurrence was defined as lymphatic or organ metastasis identified by imaging examination during the follow-up period.
Statistical analysis
All data were statistically analyzed by SPSS v.28 statistical software. Since this was a retrospective study, the sample size could not be calculated. In addition, this was not a randomized controlled study. Logistic regression analysis was used to analyze the two endoscopic treatment methods (EMR-P vs. ESD). The following factors were analyzed based on the reliability score: age (year), sex (male/female), tumor location (distance from perianal skin), tumor size (mm), and tumor shape (flat/hemispherical). Continuous variables with abnormal distributions are expressed as the median and interquartile interval. Fisher's exact test was used to compare the treatment results of the two groups. The Mann–Whitney U-test was used to analyze continuous data with a non-normal distribution. p < .05 was used to indicate significance.
Results
Baseline characteristics of the EMR-P and ESD groups
shows the patient enrollment flow chart. Forty patients underwent EMR-P, and 58 patients underwent ESD. The EMR-P group included significantly fewer men than the ESD group. There were no significant differences in age, tumor location, tumor morphology or tumor size between the EMR-P group and the ESD group. Therefore, there was no need to match the two groups ().
Figure 3. Flowchart of patient enrollment. EMR: Endoscopic mucosal resection; EMR-P: Precut endoscopic mucosal resection; ESD: Endoscopic submucosal dissection; IQR: Interquartile range.
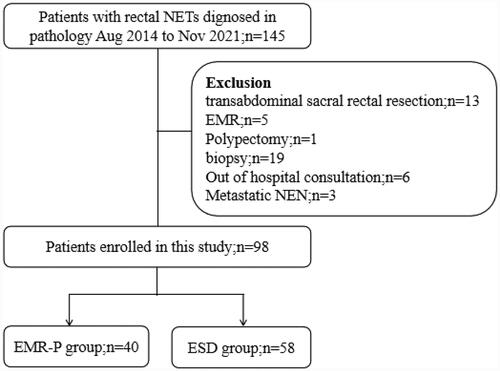
Table 1. Comparison of the baseline clinical characteristics of patients in the EMR-P and ESD groups.
Comparison of treatment results between the EMR-P and ESD groups
A comparison of the treatment results in the EMR-P and ESD groups is shown in . The operation time in the EMR-P group was significantly shorter than that in the ESD group [5.5 (4–9.5) min vs. 32 (25–94.5) min, p < .001]. The median hospital stay in the EMR-P group was also significantly shorter than that in the ESD group [5(3–7) d vs. 7(5–11) d, p = .01]. There were no significant differences between the two groups in terms of the en bloc resection, complete resection or radical resection rates [en bloc resection rate: 97.5% vs. 98.28%, p = .29; complete resection rate: 85.0% vs. 82.76%, p = .18; radical resection rate: 85.0% vs. 82.76%, p = .18]. There was also no significant difference in the incidence of adverse events between the two groups [2.50% vs. 3.45, p = .21]. The delayed bleeding and delayed perforation rates of the two groups were successfully improved after conservative treatment without surgery. No serious deaths occurred in either group. There was no significant difference in the rate of positive vertical margins or horizontal margins between the EMR-P group and the ESD group [12.50% vs. 12.01%, p = .36; 5.00% vs. 6.90%, p = .28]. No local recurrence or metastasis was noted during follow-up.
Table 2. Comparison of the treatment results between EMR-P and ESD.
Discussion
Rectal NETs show light yellow polypoid micro-uplift under endoscopy, and the surface is covered with normal epithelium. Approximately 80% of rectal NETs are less than 10 mm in diameter and are usually located in the middle of the rectum [Citation10,Citation11]. Rectal NETs need to be distinguished from rectal polyps. Rectal polyps are hyperplasia of intestinal epithelial glands. Rectal polyps can be observed with the naked eye, and the coated epithelium is different from the normal intestinal mucosa. In contrast, rectal NETs are covered with normal intestinal epithelium. Therefore, it is easy to identify them theoretically. However, in practical endoscopic examinations, it is still difficult for inexperienced endoscopists to identify rectal NETs and rectal polyps. Through the retrospective analysis of cases in Shenzhen Second People's Hospital, we found that 19 cases of rectal NETs were biopsied. This indicates that the operating surgeon did not master the key points of distinguishing rectal polyps from rectal NETs. Rectal NETs also need to be differentiated from rectal lipoma. The surface of lipomas are also covered with normal intestinal epithelium, but the color is more yellow, and lipomas are softer than NETs. Ultrasonic enteroscopy can distinguish them well. Rectal NETs appear as a mixed echo mass, while lipoma appear as a hyperechoic mass.
Some studies have confirmed that for rectal NETs less than 10 mm (small rectal NETs), the risk of distant metastasis is less than 3%. Most rectal NETs can be cured by local resection, and the long-term prognosis is good, with a 5-year survival rate of approximately 98% ∼100% [Citation2]. For rectal NETs larger than 20 mm, the rate of lymphatic metastasis and distant metastasis is high, and the 5-year survival rate is approximately 15%∼37% [Citation2]. In the selection of treatment methods, many current guidelines note that rectal NETs with a diameter less than 10 mm have a low risk of lymphatic metastasis and myometrial infiltration and rarely show malignant potential, so they can be completely removed by endoscopy [Citation3,Citation4]. Our data also support these views. Vascular infiltration, myometrial infiltration and distant metastasis were not found in the 98 patients enrolled in this work. This proves that endoscopic treatment is reliable for small rectal NETs.
There are many endoscopic methods for the treatment of rectal NETs, including polypectomy, EMR, modified EMR (m-EMR), ESD, and endoscopic full-thickness resection. Because polypectomy cannot completely remove the lesion, secondary surgical intervention is often required after operation, so it is not recommended as a routine endoscopic treatment for rectal NET resection [Citation5]. EMR is a simple and minimally invasive treatment, but it can only cut to the superficial submucosa, so the complete resection rate with this approach is approximately 30%∼70%. Therefore, it is not a routine endoscopic treatment for rectal NET resection [Citation6]. m-EMR and ESD have advantages in terms of the complete resection rate, and both can often achieve negative margins and satisfactory treatment effects [Citation6]. Therefore, ESD and m-EMR are recommended as the first choice for the treatment of rectal NETs. m-EMR consists of many procedure types: cap-assisted EMR (EMR-C), ligation-assisted EMR (EMR-L), precutting EMR (EMR-P), and dual-channel endoscopic EMR [Citation6,Citation9,Citation12,Citation13]. Lee. [Citation6] found that EMR-L was higher than EMR-C in terms of the complete resection rate, so they asserted that EMR-L is a better treatment method for rectal NETs. So et al. [Citation13] noted that due to the high rate of en bloc resection and complete resection, EMR-P is an effective treatment for cutting rectal NETs. Meanwhile, the operation time is short, and no extra costs are incurred.
Compared with other m-EMR methods, EMR-P has certain advantages because it has few limitations related to the size of the tumor [Citation13]. Some studies have found that EMR-P has fewer requirements for operators than ESD. At the same time, there was no significant difference between them in terms of the en bloc resection rate, complete resection rate and complication rate [Citation14]. This conclusion is the same as that reported in our study. Although our study identified one patient with ESD who suffered perforation, there was no significant difference in the adverse events between the two groups.
Many studies have shown that the operation time of m-EMR is significantly shorter than that of ESD [Citation15,Citation16,Citation17]. Our study found the same results. In addition, the length of hospital stay in the EMR-P group was also significantly shorter than that in the ESD group. ESD is a level IV surgery, and EMR-P is a level III surgery; often, clinicians often believe that level IV surgeries have more postoperative complications than level III surgeries. To ensure good postoperative recovery, changes in the patient’s condition can be evaluated, and the hospital stay can be prolonged.
Moon et al. [Citation18] conducted a multicenter study across 16 university hospitals in South Korea and found that 407 patients with positive or unclear pathological margins did not have a significantly increased recurrence rate after the endoscopic resection of rectal nets after 45 months of follow-up. Our results revealed that 16 patients had positive margins. These patients were followed up for 5 ∼ 102 months. During the follow-up period, no local recurrence, lymphatic metastasis or distant metastasis was found in any of our patients. At the same time, our results showed that there was no significant difference between EMR-P and ESD in terms of the cut margin positivity rate. This further shows that for rectal NETs, ESD is not superior to EMR-P in terms of the pathological complete resection rate.
This study has several limitation. First, because this was a retrospective study, so the number of cases was limited and randomized grouping was unsuitable. Second, the median follow-up time of this study was short, which cannot better evaluate the postoperative recurrence and metastasis, It is necessary to extend the follow-up time in the further study. Third, five endoscopists performed endoscopic resection in this study. We cannot avoid the difference of our staff ability in endoscopic evaluation and treatment, but all endoscopists had more than 5 years endoscopic working experience, and they all had completed more than 1000 cases of EMR and more than 200 cases of ESD. Fourth, in selecting endoscopic treatment strategies (ESD or P-EMR), endoscopists chose according to the requirements of the guidelines [Citation4] and personal experience, this may lead to some biased results. However, due to they owned rich endoscopic experience, this deviation would be minimized.
In conclusion, EMR-P and ESD are safe and effective endoscopic treatment methods for rectal NETs with diameters ≤10 mm. EMR-P is superior to ESD in terms of the operation time and hospital stay. At the same time, EMR-P is less challenging for endoscopists and is suitable for wide-ranging promotion and application, especially for grassroots hospitals or hospitals that have not yet performed ESD.
Acknowledgements
The authors extend special acknowledgements to the study population for their generous participation.
References
- Digestive diseases group, pathology branch, chinese medical association, consensus expert group on pathological diagnosis of gastrointestinal and pancreatic neuroendocrine tumors in China in 2020 consensus on pathological diagnosis of gastrointestinal and pancreatic neuroendocrine tumors in China (2020 edition). Chinese J Pathol. 2021;50(1):14–20.
- Maione F, Chini A, Milone M, et al. Diagnosis and management of rectal neuroendocrine tumors (NETs).Diagnostics. 2021;11:771.
- Caplin M, Sundin A, Nillson O, Barcelona Consensus Conference participants, et al. ENETS consensus guidelines for the management of patients with digestive NeuroendocrineNeoplasms: Colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95(2):88–97.
- Ramage JK, De Herder WW, Delle Fave G, Vienna Consensus Conference participants, et al. Vienna consensus conference participants. ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology. 2016;103(2):139–143.
- Zhou P-H, Yao L-Q, Qin X-Y, et al. Advantages of endoscopic submucosal dissection with needle-knife over endoscopic mucosal resection for small rectal carcinoid tumors: a retrospective study. Surg Endosc. 2010;24(10):2607–2612.
- Lee J, Park YE, Choi JH. Comparison between cap-assisted and ligation-assisted endoscopic mucosal resection for rectal neuroendocrine tumors. Ann. Gastroenterol. 2020;33:385–390.
- Brand M, Reimer S, Reibetanz J, et al. Endoscopic full thickness resection vs. transanal endoscopic microsurgery for local treatment of rectal neuroendocrine tumors-A retrospective analysis. Int J Colorectal Dis. 2021;36(5):971–976.
- Lee DS, Jeon SW, Park SY, et al. The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: Comparison with endoscopic mucosal resection. Endoscopy. 2010;42(8):647–651.
- Lee W-H, Kim S-W, Lim C-H, et al. Efficacy of endoscopic mucosal resection using a dual-channel endoscope compared with endoscopic submucosal dissection in the treatment of rectal neuroendocrine tumors. Surg Endosc. 2013;27(11):4313–4318.
- Basuroy R, Haji A, Ramage JK, et al. Review article: the investigation and management of rectal neuroendocrine tumours. Aliment Pharmacol Ther. 2016;44(4):332–345.
- Bang BW, Park JS, Kim HK, et al. Endoscopic resection for small rectal neuroendocrine tumors: Comparison of endoscopic submucosal resection with band ligation and endoscopic submucosal dissection. Gastroenterol Res Pract. 2016;2016:6198927.
- Chen TH, Lin CJ, Wu RC, et al. The application of miniprobe ultrasonography in the diagnosis of colorectal subepithelial lesions. Chang Gung Med J. Jul-Aug. 2010;33(4):380–388.
- So H, Yoo SH, Han S, et al. Efficacy of precut endoscopic mucosal resection for treatment of rectal neuroendocrine tumors. Clin Endosc. 2017;50(6):585–591.
- Oh CK, Cho YW, Choi IH, et al. Comparison of precutting endoscopic mucosal resection and endoscopic submucosal dissection for large (20-30 mm) flat colorectal lesions. J Gastroenterol Hepatol. 2022; 37(3):568–575.
- Xiang-Yao W, Ning-Li C, En-Qiang L, et al. The outcomes of modified endoscopic mucosal resection and endoscopic submucosal dissection for the treatment of rectal neuroendocrine tumors and the value of endoscopic morphology classification in endoscopic resection. BMC Gastroenterol. 2020;20(1):200.
- Cheung DY, Choi SK, Kim H-K, et al. Circumferential submucosal incision prior to endoscopic mucosal resection provides comparable clinical outcomes to submucosal dissection for well-differentiated neuroendocrine tumors of the rectum. Surgical Endoscopy. 2015;29(6):1500–1505.
- Cha JH, Jung DH, Kim J-H, et al. Long-term outcomes according to additional treatments after endoscopic resection for rectal small neuroendocrine tumors. Sci Rep. 2019; 9(1):4911.
- Moon CM, Huh KC, Jung S-A, et al. Long-Term clinical outcomes of rectal neuroendocrine tumors according to the pathologic status after initial endoscopic resection: a KASID multicenter study. Am. J. Gastroenterol. 2016;111(9):1276–1285.
