Abstract
Objective
To determine the risk factors for emergency endoscopic variceal ligation (EVL) failure in acute variceal bleeding (AVB).
Methods
Data from 161 cirrhosis patients with oesophageal variceal bleeding who underwent emergency EVL treatment at the Second Hospital of Hebei Medical University from January 2018 to May 2021 were retrospectively analysed. Enrolled patients were divided into a successful treatment group and a failed treatment group. The variables studied were demographic, clinical, imaging, laboratory, and endoscopic data from the enrolled patients.
Results
Of the enrolled patients, 19 patients experienced emergency EVL failure. Of these patients, nine underwent emergency endoscopic treatment again, six patients were treated with a Sengstaken–Blakemore tube for haemostasis and endoscopic treatment, four patients received drug therapy. The presence of portal vein thrombosis (PVT) in the failure group was higher than that in the success group (p < .05). Active bleeding on endoscopy was associated with emergency EVL failure for patients with Child–Pugh class C (p < .05).
Conclusions
Child–Pugh class C with active bleeding on endoscopy or the presence of PVT could increase the risk of emergency EVL failure. The patient’s condition should be fully evaluated before emergency endoscopic treatment to reduce the risk of failure.
Introduction
Liver cirrhosis is the terminal stage of chronic liver disease caused by different factors, and it is characterized by the accumulation of fibrotic tissue and abnormal liver regenerative nodules. Decompensated cirrhosis results in impaired liver function and portal hypertension. Acute variceal bleeding (AVB) is the most life-threatening complication of portal hypertension, with mortality at 6 weeks in 20% [Citation1].
Endoscopic treatment of AVB includes endoscopic variceal ligation (EVL), sclerotherapy, and cyanoacrylate injection. The Baveno VII consensus recommended EVL as the first-line treatment for oesophageal variceal bleeding [Citation2]. The European Society of Gastrointestinal Endoscopy (ESGE) recommended that, following haemodynamic resuscitation, patients with AVB should undergo endoscopy within 12 h of presentation, with an on-call trained emergency team with necessary technical expertise available [Citation3]. An international consensus group recommended that endoscopy examination should be performed within 24 h after presentation for patients with acute upper gastrointestinal bleeding [Citation4]. Emergency endoscopy can identify the source of bleeding early and control it effectively. However, the haemostatic failure rate with emergency endoscopy is 10–20% [Citation5], and patients with haemostatic failure have a poor prognosis. It is important to analyse the causes of emergency endoscopic treatment failure, explore the relevant influencing factors, evaluate the risk of treatment, and actively implement effective prevention and interventions. Currently, there are only a few studies of the risk factors for emergency endoscopic treatment failure, and related factors are insufficiently comprehensive. This study was designed to explore the failure rate of emergency EVL and analyse the risk factors for emergency EVL failure.
Materials and methods
Patient selection
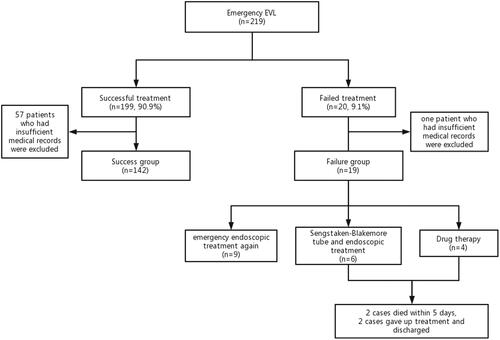
We conducted an observational study at the Second Hospital of Hebei Medical University from January 2018 to May 2021. A total of 219 patients who underwent emergency EVL treatment to control oesophageal variceal bleeding were collected. Among them, 58 patients who had insufficient medical records were excluded. One hundred sixty-one patients were analysed in our study. The case screening process is shown in . Most patients received fluid resuscitation and vasoactive drugs immediately on admission. According to the standard of Baveno consensus [Citation2], patients were divided into a successful treatment group and a failed treatment group.
We included patients who met the following criteria: oesophageal variceal haemorrhage in liver cirrhosis diagnosed according to Baveno V definitions [Citation6]; patients or their authorized family members agreeing to the undergoing of emergency EVL treatment and a signed informed consent form; and EVL treatment performed within 24 h of presentation.
Patients were excluded if they met any of the following criteria: noncirrhotic variceal bleeding, such as due to peptic ulcer; endoscopic treatment of variceal haemorrhage performed after 24 h of presentation; endoscopic contraindications, such as gastrointestinal perforation; poor physical condition or heart failure, renal failure and respiratory failure.
Emergency EVL treatment
Preparation before EVL
Volume resuscitation was started to ensure tissue perfusion and maintain haemodynamic stability. Vasoactive drugs (somatostatin 250–500 µg/h or octreotide 25–50 µg/h with vein drip) were initiated as soon as possible before endoscopy treatment and continued for 2–5 days. Transfusion of red blood cells was performed conservatively with a haemoglobin level between 7 and 8 g/dL. General anaesthesia with trachea intubation was performed for patients with altered consciousness to protect the airway. Prophylactic antibiotic therapy was instituted at admission.
EVL treatment
The endoscope (Olympus-260 endoscope, Olympus, Tokyo, Japan) was moved from the oesophagogastric junction to the mouth, and the oesophageal varices were ligated (6 Shooter Multi-Band Ligator, Cook Endoscopy, Inc., Winston-Salem, NC) in a spiral shape. Multiple ligation rings could be set for each vein as needed, with an interval of approximately 1.5 cm between the two rings, for a total of 6–12 rings.
Postoperative care
Vital signs were monitored closely, and patients were asked to avoid strenuous exercise and remain in bed as much as possible. Patients were required to refrain from eating or drinking for 24 h after the endoscopic operation. After the endoscopic operation, patients were given somatostatin to reduce portal pressure, prophylactic antibiotics, proton pump inhibitors to inhibit acid [Citation7], and gastric mucosal protective agents. Postoperative attention was paid to whether the patient had bleeding, perforation, stenosis, fever, nausea, vomiting, retrosternal pain, or other complications.
Definitions
In our study, emergency EVL treatment failure was defined either of failure to control bleeding by EVL or of rebleeding within 120 h after EVL [Citation2]. Failure to control bleeding was defined as one of the following criteria: fresh haematemesis or nasogastric tube aspiration of ≥100 mL of fresh blood ≥2 h after therapeutic endoscopy; development of hypovolaemic shock; and a 3-g/dL drop in haemoglobin (9% drop of haematocrit) within any 24-h period if no transfusion was administered [Citation6]. The size of oesophageal varices was classified into three forms: small and straight as form 1 (F1); enlarged and tortuous (F2); or large and spiral-shaped, occupying more than one-third of the lumen (F3) [Citation8].
Data collection
The variables studied were demographic, clinical, laboratory, imaging, and endoscopic data at the time of variceal bleeding. We collected the patients' sex, age and aetiology; whether it was the first haemorrhage; whether orotracheal intubation was performed under intravenous anaesthetic induction during treatment; whether the case was one with or without hepatocellular carcinoma (HCC); and previous endoscopic treatment history. Some data were provided by abdominal ultrasound with Doppler examination and contrast-enhanced computed tomography scans, such as whether the case was complicated with portal vein thrombosis (PVT), whether there was a spontaneous gastrorenal shunt (SGRS) or spontaneous splenorenal shunt (SSRS), the degree of ascites and the patient's portal vein trunk width. Some laboratory data (haemogram and coagulation indices, hepatic function index), Child–Pugh class and some endoscopic data (time of endoscopic treatment, grade of varicose vein and bleeding manifestations under endoscopy) were also collected.
Data analysis
All statistical analyses were performed using SPSS software, version 25.0 (SPSS Inc., Chicago, IL). The continuous data were first tested for normality. If they were normally distributed, they were represented by the mean ± standard deviation, and the statistical method was the t-test. If they were not normally distributed, they were represented by the median (interquartile range), and the Mann–Whitney U test was used for analysis. The categorical data are shown as absolute and relative frequencies and were analysed using the χ2 test or Fisher's exact test. In the multivariable analysis, binary logistic regression models were used to investigate the risk factors associated with emergency endoscopic treatment failure. Variables with a p-value < .05 in the univariate analysis were selected for possible inclusion in the multivariable analysis. Data that were included in the regression analysis are presented as the odds ratio (OR) with 95% confidence interval (CI).
Results
Patient characteristics
A total of 219 patients who underwent emergency EVL treatment to control oesophageal variceal bleeding were collected. One hundred ninety-nine patients (90.9%) were successfully treated. Among them, 57 patients who had insufficient medical records were excluded. One hundred forty-two patients were classified into the ‘success group’. Twenty patients (9.1%) who experienced emergency EVL failure. Among them, one patient who had insufficient medical records were excluded. 19 patients were classified into the “failure group”. One hundred sixty-one patients were analysed in our study. Of the 19 failed patients, 9 patients underwent emergency endoscopic treatment again. Six patients were treated with a Sengstaken–Blakemore tube for haemostasis and endoscopic treatment again. Four patients received drug therapy. Among them, 2 failed patients died within five days and 2 failed patients gave up treatment and discharged (). The two patients died of haemorrhagic shock and circulatory failure. The baseline characteristics of 161 patients are shown in . The cohort comprised 102 men (63.4%) and 59 women (36.6%), with a mean age of 55.0 years old (range: 26–80). The main aetiology of cirrhosis was hepatitis B virus (HBV) (92 cases, 57.1%). PVT was present in 43 patients (26.7%). There were 15 patients (9.3%) with SGRS/SSRS and 51 patients (31.7%) with moderate/abundant ascites. A total of 99 patients (61.5%) had first bleeding, and 34 cases (21.1%) had a previous EVL history. Of the 161 enrolled patients, 32 patients (19.9%) were given orotracheal intubation under intravenous anaesthetic induction during treatment. The number of patients with concomitant HCC was 30 (18.6%). During the endoscopic operations, 118 cases (73.3%) were observed as oesophageal varices F3, and 73 cases (45.3%) had active bleeding.
Table 1. Baseline characteristics of the enrolled patients and comparison of characteristics between the failure and success groups.
Comparison of the characteristics between the success group and failure group
The analysis of the two groups is shown in . There were more patients with PVT in the failure group than that in the success group (57.9% versus 22.5%, p = .001). The proportion with Child–Pugh class C in the failure group was significantly greater than that in the success group, and the difference between the groups was statistically significant (47.4% versus 19.0%, p = .010). In the study, patients showed a higher rate of active bleeding on endoscopy in the failure group than that in the success group (78.9% versus 40.8%, p = .002). The duration of emergency EVL treatment was divided into two periods: less than 12 h and 12–24 h. There were more patients treated within 12 h and fewer patients treated within 12–24 h in the failure group, but without statistical significance (42.1% versus 33.1%, 57.9% versus 66.9%, p = .437). Other baseline characteristics did not show significant difference between the two groups (p>.05) ().
Analysis of potential risk factors for emergency EVL failure
We explored potential risk factors for emergency EVL treatment failure. In the univariable analysis, Child–Pugh class C (p = .008), the presence of PVT (p = .002) and active bleeding on endoscopy (p = .004) were associated with emergency EVL failure (). Baseline variables that were thought to be clinically relevant to EVL failure or showed a univariable relationships with emergency EVL treatment failure were included in the multivariable analysis. Variables included were carefully selected to guarantee parsimony. In the multivariable analysis, the severity of hepatic disease as measured by the presence of PVT and active bleeding on endoscopy were identified as risk factors for emergency EVL treatment failure ().
Table 2. Univariable analyses of potential risk factors for emergency EVL failure.
Table 3. Multivariable analyses of potential risk factors for emergency EVL failure.
We further compared the relationship between active bleeding on endoscopy and Child–Pugh class in . Active bleeding on endoscopy was associated with emergency EVL failure only for patients with Child–Pugh class C (p < .05).
Table 4. Comparison of bleeding on endoscopy in Child–Pugh class A/B/C patients between the failure and success groups.
An additional comparison of the characteristics of PVT between the failure and success groups is shown in . There was no significant difference between the two groups regarding the characteristics of PVT, such as location, degree, extent or duration (p>.05) ().
Table 5. Comparison of characteristics in PVT patients between the failure and success groups.
Discussion
AVB due to endoscopy treatment failure is associated with poor prognosis in patients. Thus, it is important to evaluate the relevant risk factors. However, there are limited data with respect to the prognostic factors of emergency endoscopic failure. Therefore, we analysed the risk factors for emergency EVL treatment failure, providing evidence for the early clinical assessment of treatment risk and effect. The incidence of emergency EVL failure in our study was 9.1%, and the study yielded findings similar to those of previous research (10–20%) [Citation5]. In our study, we found that Child–Pugh class C with active bleeding on endoscopy and the presence of PVT increased the risk for emergency EVL treatment failure.
Kim et al. [Citation9] pointed out that the failure rate of emergency EVL was 10.4%, which is close to that in our study. They also reported that active bleeding on endoscopy was an independent risk factor for emergency EVL failure. Evidence supports that patients with active bleeding and indications of recent bleeding have a poor prognosis, while patients without the above symptoms have a good prognosis, even with low haemoglobin levels [Citation10]. Magaz et al. [Citation1] pointed out that Child–Pugh class B patients with active bleeding on endoscopy have a worse prognosis than Child–Pugh B without active bleeding. In our study, active bleeding on endoscopy had a high risk of emergency EVL failure, which might be related to haemodynamic instability and a poor endoscopic visual field during active bleeding. These patients should be given volume resusciation to maintain haemodynamic stability and adequate evaluation. It was reported that Child–Pugh class B patients with active bleeding should be performed preemptive TIPS as soon as possible [Citation1]. However, active bleeding on endoscopy in Child–Pugh B class patients was not risk factor in our study. We need to expand the sample size for further research.
It is well known that the Child–Pugh class is commonly used to estimate prognosis for AVB, with scores based on five parameters: the presence and severity of hepatic encephalopathy and ascites, serum bilirubin concentrations, serum albumin concentrations and prothrombin time. The liver synthesis and metabolic function of decompensated cirrhosis patients are poor. Study has confirmed that serum albumin was associated with portal hypertension and could be used to assess the risk of bleeding in patients with cirrhosis [Citation11]. Serum total bilirubin levels were associated with rebleeding after prophylactic ligation [Citation12]. These two parameters are not risk factors for failure in our study. However, our research reported that Child–Pugh class C with active bleeding was a prognostic factor for failure of emergency EVL. There is also evidence that patients with Child–Pugh class C is related to an increased risk of variceal bleeding [Citation3]. Child–Pugh assessment during the acute bleeding episode can predict outcome. Survival rate is near 100% for patients with Child–Pugh class A while death is common in patients with Child–Pugh class C (>13 points) [Citation1]. Two studies supported that 1-year mortality rate of patients with Child–Pugh class C that treated with the standard therapy was 39–40% and the 1-year risk of failure or rebleeding was 50–66% [Citation13, Citation14]. Cho et al. [Citation15] also found that Child–Pugh class C was a risk factor for emergency endoscopic treatment failure, consistent with the results of our study.
A recent study reported that the prevalence of PVT in patients with cirrhosis is positively correlated with the severity of liver disease: 10% in patients with compensated cirrhosis, 17% in patients with Child–Pugh class B/C, and 26% for liver transplant candidates [Citation16]. The decrease in portal vein flow played the most important role in the pathogenesis of PVT. PVT could increase the degree of portal hypertension, further increasing the risk of portal hypertensive bleeding [Citation17]. The presence of PVT caused patients of AVB to be ones more susceptible to failure to control bleeding and early rebleeding, which was independent predictors of 5-day failure [Citation18]. Our study also showed that PVT was an independent risk factor for emergency EVL failure. Gao et al. [Citation19] reported that PVT was associated with high 14-day and 6-week rebleeding rates in patients after EVL. However, their study excluded patients with HCC. HCC invasion of the portal vein can form portal vein tumour embolus, increasing the degree of portal hypertension. Lee et al. [Citation20] reported that AVB patients with HCC had higher rates of 5-day treatment failure and 6-week mortality than those without HCC. It should be noted that many studies have excluded critically ill patients, such as patients with advanced liver cirrhosis and multifocal HCC. To identify patients whose prognosis was negative, Amitrano et al. [Citation18] analysed a series of unselected cirrhotic patients admitted for AVB. However, they showed that HCC was not a predictor of 5-day failure in AVB. Our study also indicated that HCC was not a risk factor for emergency EVL failure.
Previous studies have pointed out that the degree of portal hypertension is predictive of bleeding risk, and variceal size was associated with portal pressure. The grade of oesophageal varices was positively correlated with the size of the varices in their classification [Citation8]. Simonetto et al. [Citation21] pointed out that variceal diameter greater than 10 mm was the most important predictor of variceal bleeding. In addition, Drolz et al. [Citation12] reported that a high grade of varicose vein was a risk factor for bleeding in patients after prophylactic endoscopic variceal ligation. They also pointed out that the larger that the oesophageal varices were, the higher that the incidence of procedure-associated bleeding events was. Cho et al. [Citation22] also reported that concomitant gastric varices F3 was a risk factor for EVL-induced ulcer bleeding. However, our study showed that the grade of oesophageal varices was not a risk factor for emergency EVL failure, which might be related to the change in varicose degree when vein rupture and bleeding occurred. Furthermore, our study did not measure the hepatic venous pressure gradient (HVPG), and further studies related to HVPG are needed. For patients with a history of ligation, a scar will be formed at the ligation site. There was also a finding that the history of previous EVL was a significant predictor of emergency EVL treatment [Citation9]. When the adjacent varicose vein ruptures and requires EVL again, it is not easy to completely ligate it, often leading to treatment failure. With the increase in ligation times, the failure rate of ligation increases gradually [Citation9]. However, our study did not support this point. Cho et al. [Citation22] also reported that a previous treatment history was not a risk factor in their study, consistent with our findings.
For AVB patients with altered consciousness, endoscopy should be performed with protection of the airway, such as under general anaesthesia with trachea intubation, and extubation should be performed as quickly as possible after endoscopy [Citation2,Citation3]. Prophylactic intubation prior to endoscopy is of critical importance for patients with greater illness severity, planned upper endoscopy, and haematemesis 24 h previously [Citation23]. However, regarding the influence of sedation on emergency endoscopy, some endoscopy physicians have suggested that patients with cirrhosis should not be sedated during endoscopic treatment, considering the potential risks with sedation [Citation24]. The use of sedatives was considered a relative contraindication in emergency endoscopic treatment [Citation25]. However, when under nonsedation, patients are prone to anxiety, hiccups and involuntary movement, which can lead to additional variceal bleeding and endoscopic treatment failure [Citation26]. In contrast, patients with sedation cooperated well, and the process of endoscopic therapy was relatively smooth [Citation27]. In our study, orotracheal intubation under intravenous anaesthetic was not a risk factor for failure of emergency EVL. Park et al. [Citation28] concluded that there were no significant differences in adverse reactions or mortality after emergency endoscopy between the sedated and nonsedated groups, consistent with the results of our study. However, their study also pointed out that sedation could reduce the operation time of emergency endoscopy, and it was safe to give sedation to patients in emergency endoscopy for the treatment of variceal haemorrhage.
There have been a number of previous studies of the timing of endoscopy. The Baveno VII consensus pointed out that endoscopic examination should be performed immediately after haemodynamic resuscitation, preferably within 12 h of presentation [Citation2]. Nevertheless, some comments have pointed out that the endoscopic field of vision is poor and that endoscopic operation is difficult when active bleeding has occurred. Wysockie et al. [Citation29] found that, if AVB patients did not receive emergency endoscopic treatment (defined as within 24 h after admission), the mortality rate increased from 8.25% to 15.3%. It was also reported that delayed endoscopy more than 15 h after admission is an independent risk factor for patient death [Citation30]. Chen et al. [Citation31] pointed out that endoscopy performed within 12 h of admission for cirrhotic patients with haematemesis had a low correlation with rebleeding rate and mortality at 6 weeks. However, Jung et al. [Citation32] pointed out that the timing of endoscopic examination did not affect the mortality or rebleeding rate of AVB patients. And Huh et al. [Citation33] conducted a stratified study of the timing of endoscopy according to the severity of liver disease and found that endoscopic treatment within 12 h after admission had a poor prognosis in low-risk patients (MELD score ≤17), while it had no effect on the mortality of high-risk patients. In our study, the timing of emergency endoscopy was divided into two periods of less than 12 h and within 12–24 h, and there was no effect on emergency EVL.
Our study had several limitations. First, this study was a retrospective study with some limitations, but we conducted a systematic analysis of the valid case data collected. Second, we collected a limited number of samples. However, we included variables that were believed to be clinically relevant to EVL failure or showed a univariable relationship with emergency EVL failure in the multivariable analysis. The variables included were carefully selected to guarantee parsimony. The results of the index characteristics are similar to those of previous similar studies. Considering that the sample quantity of our study was a limitation, we will expand the sample size in future studies.
Conclusion
In conclusion, emergency EVL treatment should be conducted at an appropriate time within 24 h after presentation according to the condition of each patient with AVB. The successful implementation of emergency EVL could be hindered by active bleeding on endoscopy, especially for Child–Pugh class C patients, and the presence of PVT. Adequate fluid resuscitation and condition assessment before endoscopy are more important. The patient’s condition should be fully evaluated before emergency endoscopic treatment. Salvage treatments should be administered for failed patients to improve their prognosis.
Disclosure statement
No potential conflicts of interest were reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Magaz M, Baiges A, Hernández-Gea V. Precision medicine in variceal bleeding: are we there yet? J Hepatol. 2020;72(4):774–784.
- de Franchis R, Bosch J, Garcia-Tsao G, Baveno VII Faculty, et al. Baveno VII – renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–974.
- Karstensen JG, Ebigbo A, Bhat P, et al. Endoscopic treatment of variceal upper gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Cascade guideline. Endosc Int Open. 2020;8(7):E990–E997.
- Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the international consensus group. Ann Intern Med. 2019;171(11):805–822.
- Habib A, Sanyal AJ. Acute variceal hemorrhage. Gastrointest Endosc Clin N Am. 2007;17(2):223–252.
- de Franchis R, Baveno V, Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53(4):762–768.
- Xu XY, Ding HG, Jia JD, et al. Guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding in cirrhotic portal hypertension. J Clin Hepatol. 2016;32(2):203–219.
- North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319(15):983–989.
- Kim DH, Cho E, Jun CH, et al. Risk factors and on-site rescue treatments for endoscopic variceal ligation failure. Korean J Gastroenterol. 2018;72(4):188–196.
- Horibe M, Ogura Y, Matsuzaki J, et al. Absence of high-risk stigmata predicts good prognosis even in severely anemic patients with suspected acute upper gastrointestinal bleeding. United European Gastroenterol J. 2018;6(5):684–690.
- Qi X, Dai J, Yang M, et al. Association between portal vein thrombosis and survival in non-liver-transplant patients with liver cirrhosis: a systematic review of the literature. Gastroenterol Res Pract. 2015;2015:480842.
- Drolz A, Schramm C, Seiz O, et al. Risk factors associated with bleeding after prophylactic endoscopic variceal ligation in cirrhosis. Endoscopy. 2021;53(03):226–234.
- Lv Y, Yang Z, Liu L, AVB-TIPS Study Group, et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4(8):587–598.
- García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–2379.
- Cho H, Nagata N, Shimbo T, et al. Recurrence and prognosis of patients emergently hospitalized for acute esophageal variceal bleeding: a long-term cohort study. Hepatol Res. 2016;46(13):1338–1346.
- Senzolo M, Garcia-Tsao G, García-Pagán JC. Current knowledge and management of portal vein thrombosis in cirrhosis. J Hepatol. 2021;75(2):442–453.
- Noronha Ferreira C, Seijo S, Plessier A, et al. Natural history and management of esophagogastric varices in chronic noncirrhotic, nontumoral portal vein thrombosis. Hepatology. 2016;63(5):1640–1650.
- Amitrano L, Guardascione MA, Manguso F, et al. The effectiveness of current acute variceal bleed treatments in unselected cirrhotic patients: refining short-term prognosis and risk factors. Am J Gastroenterol. 2012;107(12):1872–1878.
- Gao Z, Zhao J, Liu X, et al. Portal vein thrombosis associated with high 14-day and 6-week rebleeding in patients after oesophageal variceal band ligation: a retrospective, multicentre, nested case-control study. Hepatol Int. 2021;15(5):1183–1195.
- Lee YR, Park SY, Tak WY. Treatment outcomes and prognostic factors of acute variceal bleeding in patients with hepatocellular carcinoma. Gut Liver. 2020;14(4):500–508.
- Simonetto DA, Liu M, Kamath PS. Portal hypertension and related complications: diagnosis and management. Mayo Clin Proc. 2019;94(4):714–726.
- Cho E, Jun CH, Cho SB, et al. Endoscopic variceal ligation-induced ulcer bleeding: what are the risk factors and treatment strategies? Medicine. 2017;96(24):e7157.
- Smischney NJ, Seisa MO, Kumar M, et al. Determinants of endotracheal intubation in critically ill patients undergoing gastrointestinal endoscopy under conscious sedation. J Intensive Care Med. 2019;34(6):480–485.
- Saeian K, Staff D, Knox J, et al. Unsedated transnasal endoscopy: a new technique for accurately detecting and grading esophageal varices in cirrhotic patients. Am J Gastroenterol. 2002;97(9):2246–2249.
- Martins NB, Wassef W. Upper gastrointestinal bleeding. Curr Opin Gastroenterol. 2006;22(6):612–619.
- Carey EJ, Sorbi D. Unsedated endoscopy. Gastrointest Endosc Clin N Am. 2004;14(2):369–383.
- Kim SI, Jin YJ, Lee SH, et al. Conscious sedation using midazolam and sequential flumazenil in cirrhotic patients for prophylactic endoscopic variceal ligation. Digestion. 2015;92(4):220–226.
- Park CH, Park SW, Jung JH, et al. Clinical outcomes of sedation during emergency endoscopic band ligation for variceal bleeding: multicenter cohort study. Dig Endosc. 2020;32(6):894–903.
- Wysocki JD, Srivastav S, Winstead NS. A nationwide analysis of risk factors for mortality and time to endoscopy in upper gastrointestinal haemorrhage. Aliment Pharmacol Ther. 2012;36(1):30–36.
- Hsu YC, Chung CS, Tseng CH, et al. Delayed endoscopy as a risk factor for in-hospital mortality in cirrhotic patients with acute variceal hemorrhage. J Gastroenterol Hepatol. 2009;24(7):1294–1299.
- Chen PH, Chen WC, Hou MC, et al. Delayed endoscopy increases re-bleeding and mortality in patients with hematemesis and active esophageal variceal bleeding: a cohort study. J Hepatol. 2012;57(6):1207–1213.
- Jung DH, Huh CW, Kim NJ, et al. Optimal endoscopy timing in patients with acute variceal bleeding: a systematic review and meta-analysis. Sci Rep. 2020;10(1):4046.
- Huh CW, Kim JS, Jung DH, et al. Optimal endoscopy timing according to the severity of underlying liver disease in patients with acute variceal bleeding. Dig Liver Dis. 2019;51(7):993–998.