Abstract
Objectives
In 2010, 27,000 inhabitants (45% of the population) of Östersund, Sweden, contracted clinical cryptosporidiosis after drinking water contaminated with Cryptosporidium hominis. After the outbreak, local physicians perceived that the incidence of inflammatory bowel disease (IBD), including ulcerative colitis (UC), Crohn’s disease (CD), and IBD-unclassified, and microscopic colitis (MC) increased. This study assessed whether this perception was correct.
Materials and methods
This observational study included adult patients (≥18 years old) from the local health care region who were diagnosed with pathology-confirmed IBD or MC during 2006–2019. We collected and validated the diagnosis, date of diagnosis, age at diagnosis, and sex from the Swedish quality register SWIBREG and electronic patient records. Population data were collected from Statistics Sweden. The incidences for 2006–2010 (pre-outbreak) and 2011–2019 (post-outbreak) were evaluated by negative binomial regression analysis and presented as incidence rate ratios (IRRs). Data were analyzed for IBD, for UC and CD separately, and MC.
Results
During the study period, we identified 410 patients with new onset IBD and 155 new cases of MC. Overall, we found a trend toward an increased incidence of IBD post-outbreak (IRR 1.39, confidence interval (CI) 0.99–1.94). In individuals ≥40 years old, the post-outbreak incidence significantly increased for IBD (IRR 1.69, CI 1.13–2.51) and CD (IRR 2.23, CI 1.08–4.62). Post-outbreak incidence of MC increased 6-fold in all age groups (IRR 6.43, CI 2.78–14.87).
Conclusions
The incidence of late-onset IBD and MC increased after the Cryptosporidium outbreak. Cryptosporidiosis may be an environmental risk factor for IBD and MC.
Introduction
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis (UC) as well as IBD-unclassified (IBD-U) when differentiation between CD and UC is not possible. In Sweden, the prevalence of IBD is approximately 0.65% [Citation1]. Onset usually occurs at a young age, with peak incidence in the third decade, although IBD can present at any time throughout one’s life [Citation2,Citation3].
The worldwide incidence of IBD is increasing, especially in developing countries where the population has started to adopt a Western lifestyle [Citation4]. According to the prevailing hypothesis, inflammation occurs due to an interaction between different environmental factors in genetically predisposed individuals, with the interaction between the intestinal flora, local immune system in the intestinal mucosa, and dysfunction in the mucosal barrier seeming to play a crucial role [Citation5–7]. Several studies have linked gastrointestinal infections to an increased risk of developing IBD later in life [Citation8–10].
Microscopic colitis (MC) is another chronic inflammatory bowel disease but usually not included when speaking of IBD because of different disease presentation and treatment. MC can be further divided into lymphocytic and collagenous colitis, affects more women than men and is mainly a disease of the elderly, with a peak incidence in the seventh decade [Citation11]. Global incidences of MC are increasing, but incidence rates vary widely between countries. In Sweden, incidence rates have been stable for the last two decades [Citation11,Citation12]. The pathophysiology of MC is largely unknown, but it is thought to be caused by an interaction between the immune system, environmental factors and an aging microbiota [Citation13]. Some recent studies have shown an elevated risk of developing MC after gastrointestinal infections [Citation14,Citation15].
Cryptosporidium is the most common protozoa to cause diarrhea among humans [Citation16]. There are more than 40 different subspecies, but Cryptosporidium hominis and Cryptosporidium parvum cause >90% of human cases [Citation17,Citation18]. In immunocompetent individuals, the infection is self-limiting. The acute symptoms (diarrhea, abdominal pain, vomiting and fever) usually last a few days but sometimes up to 3 weeks [Citation19]. Persistent sequelae, such as chronic diarrhea, fatigue, joint pain and eye problems, have been reported up to 2 years after the acute infection has subsided [Citation20–23]. Cryptosporidium infections are associated with the development of IBD-like symptoms in animal models with defective T-cell receptor-α, but a connection between human cryptosporidiosis and increased risk of IBD or MC has not yet been established [Citation8].
A few major Cryptosporidium outbreaks have been described in the literature. The largest outbreak affected Milwaukee, WI, in 1993 with more than 400,000 symptomatic individuals [Citation24]. In the autumn of 2010, Östersund, Sweden, was hit by a major outbreak of C. hominis subtype IbA10G2. An estimated 27,000 people, approximately 45% of the city's population, reported symptoms of clinical cryptosporidiosis [Citation25,Citation26]. Östersund is the only city in Region Jämtland Härjedalen (RJH), a sparsely populated area of almost 50,000 km2 with roughly 130,000 inhabitants. The region’s only hospital is located in Östersund, and all healthcare professionals work in the same patient record system.
After the outbreak in Östersund, local physicians perceived that the incidence of IBD and MC in RJH increased. In the present study, we investigated whether an increase in the incidence of IBD and MC was chronologically associated with the C. hominis outbreak.
Materials and methods
Study design and data collection
We conducted an observational study to compare the incidences of IBD and MC before and after the C. hominis outbreak in RJH. For this purpose, we collected data from the national quality register SWIBREG, the electronic patient records in Cosmic® and its predecessor VAS®, and population data from Statistics Sweden [Citation27]. SWIBREG is the Swedish quality register for IBD and has been in use since 2005 [Citation28]. In RJH, adult individuals (≥18 years old) with a pathology-confirmed diagnosis of IBD or MC are registered by the Gastrointestinal clinic at Östersund Hospital after providing informed consent. Data are entered by the patient’s physician and/or a dedicated nurse upon diagnosis and during subsequent visits at the clinic. The collected data include information on diagnosis, treatment, endoscopies, laboratory results, and patient-reported symptoms. Currently, a little more than 800 of the region’s 130,000 inhabitants (0.6%) are registered in SWIBREG.
For the present study, we included all patients from RJH with a new, pathology-confirmed diagnosis of IBD or MC between 1 January 2006 and 31 December 2019, who was aged ≥18 years. Data were collected from SWIBREG and a search of the patient records in Cosmic® and VAS®, based on International Classification of Diseases (ICD)-codes for IBD and MC (K50.0–52.9). Collected variables were diagnosis, date of diagnosis, age at diagnosis, and sex. The year 2006 was used as a starting point because electronic patient records were then in place at the medical and surgical departments, both of which are involved in the process of diagnosing IBD. After validation, included patients were divided into pre- and post-outbreak groups, with the post-outbreak period starting 1 January 2011. Patients were also divided into age categories (18–39 years or ≥40 years) based on the Montreal classification system used to define subgroups of IBD depending on age at diagnosis and disease distribution [Citation29].
Statistical analysis
The cohort was described in its entirety. The data were analyzed for IBD overall, UC and CD separately, and MC. Independent sample T-test and Mann–Whitney U test were used to assess differences in demographic variables. The yearly incidence of IBD was calculated by dividing the number of new patients by the number of adult individuals residing in RJH on the last day of the actual year and is presented per 100,000 adult inhabitants. IBD incidence was evaluated by a negative binomial regression analysis and results presented as incidence rate ratios (IRRs) with 95% confidence intervals (CIs). The number of inhabitants ≥18 years old was used as the offset variable. Independent variables were period of diagnosis (pre- versus post-outbreak), sex, and age category. IBM Statistics SPSS version 26 (SPSS Inc., Chicago, IL) was used for all statistical calculations. The significance level was set at 0.05.
Ethical considerations
This study was reviewed and approved by the regional research ethics review. Board in Umeå (reg. no. 2018/168-31) and the Swedish Ethical Review Authority (2019-05841).
Results
Cohort description
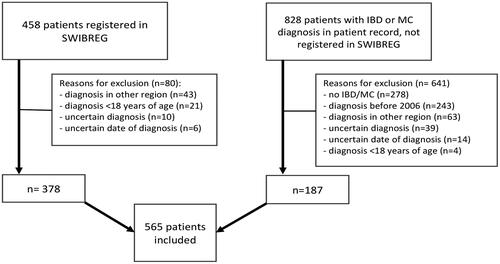
In SWIBREG, we identified 458 patients from RJH diagnosed with IBD or MC between 1 January 2006 and 31 December 2019. A search in the electronic patient records found 828 additional patients with an IBD or MC diagnosis during this period. After validation of the collected data from SWIBREG and patient records, 565 patients fulfilled the inclusion criteria and were included in the study. Common causes for exclusion were diagnosis in another region, diagnosis before 2006, and uncertain or incorrect diagnosis. A flow chart for this process and causes for exclusion is presented in .
Figure 1. Cohort selection process. Patients diagnosed with inflammatory bowel disease (IBD) or microscopic colitis (MC) (ICD K50.0–52.9) in Region Jämtland Härjedalen between 1 January 2006 and 31 December 2019. SWIBREG: Swedish IBD register.
The analyzed cohort consisted of 253 (45%) male patients. The overall median age at diagnosis was 52 years (interquartile range 31–66.5), and a majority of patients were diagnosed with UC (50.8%). When stratified for type of diagnosis, we found no age or sex differences between the pre- and post-outbreak groups. As expected, younger individuals were more prone to UC, whereas older patients were at higher risk of MC. A total of 155 individuals were diagnosed with MC, evenly divided between collagenous and lymphocytic colitis. Seventy-five percent of patients diagnosed with collagenous colitis post-outbreak were female, as were 70% of those with lymphocytic colitis. More group characteristics are presented in .
Table 1. Mean annual incidence and demographic characteristics of the study population presented by diagnosis.
Incidence of IBD and MC
Mean annual incidences increased in absolute numbers for all diagnoses after the outbreak (). Regression analysis showed a trend towards an increased incidence post-outbreak for IBD (IRR 1.39, CI 0.99–1.94), but not when stratified for UC or CD (). However, in the group aged ≥40 years, incidence increased for IBD (RR1.69, CI 1.13–2.51) and CD (2.23, 1.08–4.62) post-outbreak ( and ). The incidence of MC increased 6-fold after the outbreak (RR 6.43, 2.78–14.87) (). No differences were found between collagenous colitis and lymphocytic colitis.
Figure 2. Annual incidence of inflammatory bowel disease and microscopic colitis in individuals ≥40 years of age in Region Jämtland Härjedalen.
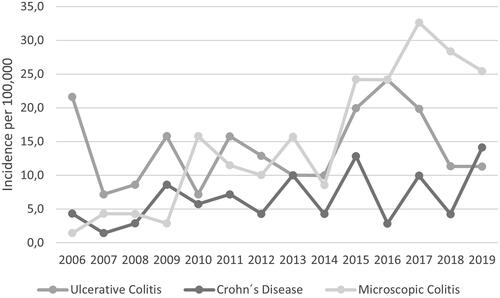
Table 2. Negative binomial regression analysis of inflammatory bowel disease.
Table 3. Negative binomial regression analysis of inflammatory bowel disease in the population ≥40 years of age.
Table 4. Negative binomial regression analysis of microscopic colitis.
Discussion
Our observational study shows a chronological connection between the Cryptosporidium outbreak in Östersund in 2010 and an increased incidence of IBD and MC afterwards. We noted a trend towards an increased incidence of IBD in the entire adult population and a clearly increased incidence of late-onset IBD (i.e., in individuals aged ≥40 years at diagnosis) and MC.
Results in context
In this study, we found post-outbreak incidences of 21 per 100,000 adult inhabitants for UC and 8 per 100,000 for CD. Internationally, IBD incidence varies widely depending on geographical location. The incidence is increasing in developing countries and stabilizing in many high-income countries [Citation4,Citation30]. In Europe, the incidence of CD ranges from 0.4 to 22.8 per 100,000 and the incidence of UC between 2.4 and 44.0 per 100,000 [30]. The post-outbreak UC incidence in RJH is higher than in other Swedish cities, such as Linköping (16 per 100,000), Örebro (16 per 100,000), or Uppsala (20 per 100,000), whereas the post-outbreak incidence of CD is probably more comparable, with incidences of 8–10 per 100,000 in the other cities [Citation30–32].
For MC, the incidence in RJH increased from 4 to >14 per 100,000 adult inhabitants after the outbreak. Interestingly, a meta-analysis showed a stable incidence of MC in Sweden from 2000 to 2010 [11]. In addition, the mean annual incidence post-outbreak seems high compared to, for example, Uppsala, with an incidence of nearly 12 per 100,000 for MC [Citation33]. An overall MC incidence of 10.5 per 100,000 was reported in Sweden from 2006 to 2015 [Citation34]. However, it is difficult to compare our incidence numbers with other studies because we only have data for the adult population of RJH and other studies have included pediatric diagnoses.
Factors that could contribute to increasing incidences of IBD and MC are more extended clinical use of feces-calprotectin as an inflammatory marker during the last decade, the introduction of standardized diagnostic pathways for potential colorectal cancer in 2016, and an increased awareness of MC. Even colorectal cancer screening programs could affect incidence numbers, but such a screening program is not yet in place in RJH. Nevertheless, the effect of these factors on the incidence is considered to be small given the stable incidence rates in Northern Europe for IBD and MC [Citation11,Citation15,Citation30,Citation35] The increasing availability of endoscopy could be another explanation for increasing incidences, but in RJH a stable number of colonoscopies were performed during the study period.
When looking at individuals aged ≥40 years, we found an increased incidence of IBD and CD. This can be explained by the theory that environmental factors play a bigger role in late-onset IBD, compared to younger patients, in whom genetics play a more prominent role [Citation36]. These findings strengthen the hypothesis that the Cryptosporidium outbreak is the cause of the observed increased incidence. They could also be in favor of the hygiene hypothesis. This hypothesis suggests that increased hygiene leads to less microbial stimulation, causing abnormal immunological responses as a reaction to microbiota. But recent studies show this theory might be relevant mainly for developing countries or following migration from developing countries [Citation37,Citation38].
In several studies, gastrointestinal infections have been linked to an increased risk of developing IBD later in life, though there have been conflicting results [Citation8,Citation9,Citation39–41]. One study found that gastrointestinal infection was not a risk factor for CD, but causes a 3-fold elevated risk of UC [Citation42]. A large Swedish study, on the other hand, reported elevated risks for both UC and CD after gastroenteritis, with the highest risk for CD (aOR 1.67 versus 1.55) [Citation10]. Also, increased stool testing has been mentioned as an explanation for a connection between gastrointestinal infections and IBD [Citation39].
The numbers of included parasitic infections in published studies are usually low, which makes generalizability of the results uncertain. One study included 3105 individuals, only 39 of whom were affected by a parasite and none were infected with Cryptosporidium [Citation10]. Despite low numbers, they were able to show a pronounced risk of CD after parasitic infection, which is in agreement with our findings.
The role of pathological microorganisms in the development of MC is unclear [Citation13]. However, a recently published cohort study shows a high risk of MC after positive stool cultures with Campylobacter consisus, indicating that previous gastrointestinal infections could contribute to the development of MC [Citation14]. Furthermore, a nationwide case-control study in Sweden found an association between gastrointestinal infection, particularly with Clostridioides difficile, and an increased risk of subsequent MC [Citation15]. These results are consistent with previous findings regarding classical IBD and may explain why the incidence of MC in RJH has increased since the outbreak.
Strengths and limitations
A strength of this study is that RJH, especially its capital Östersund, is quite isolated, which gives unique opportunities to investigate the long-term effects of a Cryptosporidium outbreak. Relatively few people move to and from the region compared to more urbanized areas, and even commuting to other regions is limited. In addition, all colonoscopies are performed in the region’s only hospital.
Another strength is that we have not relied on registry data only, but conducted a review and validated all patients with an IBD diagnosis in their medical records. Therefore, we can be assured that all adult individuals with a pathology-confirmed IBD diagnosis from RJH within the investigated period have been identified.
This study has several limitations. Despite the region’s favorable location and an established chronological connection, we cannot claim that there is a causal link between cryptosporidiosis and the development of IBD, as only a small fraction of all affected inhabitants had their Cryptosporidium infection confirmed by fecal microscopy. On the other hand, none of the 149 stool samples positive for C. hominis contained other gastrointestinal pathogens [Citation25].
Our study showed a nearly significant increase in IBD incidence in the entire adult population after the outbreak. The lack of significant results may be due to reduced statistical power, as it was not possible to select patients living or working in the Östersund area only. Therefore, our analyses were based on the entire region’s population, whereas those affected by the outbreak primarily lived or worked in Östersund.
It is possible that patients diagnosed soon after the Cryptosporidium outbreak probably already had symptoms or pre-clinical signs of their disease before the outbreak. Nevertheless, we find it important to include these patients in the post-outbreak period in order not to miss cases in which Cryptosporidium infection accelerated the development of underlying IBD or MC. Therefore, 1 January 2011, was used as a starting point for the post-outbreak period. Furthermore, the outbreak may have increased vigilance for gastrointestinal diseases, which could lead to increased incidence. However, because the increase in incidence was observed several years after the outbreak, this is unlikely to play a significant role.
Smoking status and use of (over-the-counter) medication was not available for some of the included patients and could not be used as an independent variable in our analyses. Therefore, we cannot rule out that altered smoking habits and medication use affected the outcome of our study. Smoking is a known risk factor for IBD and MC [Citation12,Citation43]. Frequent use of non-steroidal anti-inflammatory drugs increases the risk of developing IBD, and proton pump inhibitors, selective serotonin reuptake inhibitors, statins, and beta-blockers are associated with the development of MC [Citation7,Citation12,Citation44].
Conclusions
This study showed an increased incidence of late-onset IBD after the Cryptosporidium outbreak in Östersund and supports the hypothesis that cryptosporidiosis can trigger IBD. Even the marked increase in incidence of MC could be indicative of cryptosporidiosis as an environmental risk factor. Future research is needed to further prove a connection between (parasitic) gastrointestinal infections and IBD and MC. This study contributes knowledge that we should not consider gastrointestinal infections harmless and that long-term effects can occur. Therefore, accurate filters and water quality monitoring should be considered for all municipal water systems. Globally, we should continue to aim for safe water supplies for all.
Disclosure statement
The authors report no conflict of interest. All authors state compliance to ICMJE recommendations. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Additional information
Funding
References
- Büsch K, Ludvigsson JF, Ekström-Smedby K, et al. Nationwide prevalence of inflammatory bowel disease in Sweden: a population-based register study. Aliment Pharmacol Ther. 2014;39(1):57–68.
- Mak WY, Zhao M, Ng SC, et al. The epidemiology of inflammatory bowel disease: east meets west. J Gastroenterol Hepatol. 2020;35(3):380–389.
- Vegh Z, Burisch J, Pedersen N, EpiCom-Group, et al. Incidence and initial disease course of inflammatory bowel diseases in 2011 in Europe and Australia: results of the 2011 ECCO-EpiCom inception cohort. J Crohns Colitis. 2014;8(11):1506–1515.
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778.
- Ng SC, Bernstein CN, Vatn MH, Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD), et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62(4):630–649.
- Abegunde AT, Muhammad BH, Bhatti O, et al. Environmental risk factors for inflammatory bowel diseases: evidence based literature review. World J Gastroenterol. 2016;22(27):6296–6317.
- Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15(1):39–49.
- Sacco RE, Haynes JS, Harp JA, et al. Cryptosporidium parvum initiates inflammatory bowel disease in germfree T cell receptor-alpha-deficient mice. Am J Pathol. 1998;153(6):1717–1722.
- Rogler G, Zeitz J, Biedermann L. The search for causative environmental factors in inflammatory bowel disease. Dig Dis. 2016;34(Suppl. 1):48–55.
- Axelrad JE, Olén O, Askling J, et al. Gastrointestinal infection increases odds of inflammatory bowel disease in a nationwide case–control study. Clin Gastroenterol Hepatol. 2019;17(7):1311–1322.
- Tong J, Zheng Q, Zhang C, et al. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110(2):265–276.
- Park T, Cave D, Marshall C. Microscopic colitis: a review of etiology, treatment and refractory disease. World J Gastroenterol. 2015;21(29):8804–8810.
- Miehlke S, Verhaegh B, Tontini GE, et al. Microscopic colitis: pathophysiology and clinical management. Lancet Gastroenterol Hepatol. 2019;4(4):305–314.
- Nielsen HL, Dalager-Pedersen M, Nielsen H. High risk of microscopic colitis after Campylobacter concisus infection: population-based cohort study. Gut. 2020;69(11):1952–1958.
- Khalili H, Axelrad JE, Roelstraete B, et al. Gastrointestinal infection and risk of microscopic colitis: a nationwide case–control study in Sweden. Gastroenterology. 2021;160(5):1599–1607.
- Pogreba-Brown K, Austhof E, Armstrong A, et al. Chronic gastrointestinal and joint-related sequelae associated with common foodborne illnesses: a scoping review. Foodborne Pathog Dis. 2020;17(2):67–86.
- Bouzid M, Hunter PR, Chalmers RM, et al. Cryptosporidium pathogenicity and virulence. Clin Microbiol Rev. 2013;26(1):115–134.
- Feng Y, Ryan UM, Xiao L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018;34(11):997–1011.
- Checkley W, White AC Jr, Jaganath D, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect Dis. 2015;15(1):85–94.
- Chalmers RM, Davies AP. Minireview: clinical cryptosporidiosis. Exp Parasitol. 2010;124(1):138–146.
- Lilja M, Widerström M, Lindh J. Persisting post-infection symptoms 2 years after a large waterborne outbreak of Cryptosporidium hominis in northern Sweden. BMC Res Notes. 2018;11(1):625.
- Rehn M, Wallensten A, Widerström M, et al. Post-infection symptoms following two large waterborne outbreaks of Cryptosporidium hominis in northern Sweden, 2010–2011. BMC Public Health. 2015;15:529.
- Hunter PR, Hughes S, Woodhouse S, et al. Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin Infect Dis. 2004;39(4):504–510.
- MacKenzie WR, Schell WL, Blair KA, et al. Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin Infect Dis. 1995;21(1):57–62.
- Widerström M, Schönning C, Lilja M, et al. Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg Infect Dis. 2014;20(4):581–589.
- Adler S, Widerström M, Lindh J, et al. Symptoms and risk factors of Cryptosporidium hominis infection in children: data from a large waterborne outbreak in Sweden. Parasitol Res. 2017;116(10):2613–2618.
- Statistics Sweden. Population of jämtland county, 18 years and older, male/female, 2006–2019. [cited 2020 Oct 12]. Available from: https://www.statistikdatabasen.scb.se/pxweb/en/ssd/
- Ludvigsson JF, Andersson M, Bengtsson J, et al. Swedish inflammatory bowel disease register (SWIBREG) – a nationwide quality register. Scand J Gastroenterol. 2019;54(9):1089–1101.
- Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal world congress of gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A.
- Zhao M, Gönczi L, Lakatos PL, et al. The burden of inflammatory bowel disease in Europe in 2020. J Crohns Colitis. 2021;15(9):1573–1587.
- Sjöberg D, Holmström T, Larsson M, et al. Incidence and natural history of ulcerative colitis in the Uppsala region of Sweden 2005–2009 – results from the IBD cohort of the Uppsala region (ICURE). J Crohns Colitis. 2013;7:351–357.
- Sjöberg D, Holmström T, Larsson M, et al. Incidence and clinical course of Crohn's disease during the first year – results from the ibd cohort of the Uppsala region (icure) of Sweden 2005–2009. J Crohns Colitis. 2014;8(3):215–222.
- Thörn M, Sjöberg D, Ekbom A, et al. Microscopic colitis in Uppsala health region, a population-based prospective study 2005–2009. Scand J Gastroenterol. 2013;48(7):825–830.
- Bergman D, Clements MS, Khalili H, et al. A nationwide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther. 2019;49(11):1395–1400.
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30.
- Afzali A, Katz S. Inflammatory bowel disease in the baby to baby boomer: pediatric and elderly onset of IBD. Curr Treat Options Gastroenterol. 2018;16(3):289–305.
- Noverr MC, Huffnagle GB. The ‘microflora hypothesis’ of allergic diseases. Clin Exp Allergy. 2005;35(12):1511–1520.
- Leong RW, Mitrev N, Ko Y. Hygiene hypothesis: is the evidence the same all over the world? Dig Dis. 2016;34(1–2):35–42.
- Jess T, Simonsen J, Nielsen NM, et al. Enteric Salmonella or Campylobacter infections and the risk of inflammatory bowel disease. Gut. 2011;60(3):318–324.
- Axelrad JE, Cadwell KH, Colombel JF, et al. Systematic review: gastrointestinal infection and incident inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51(12):1222–1232.
- Di Re A, Liang Y, Gosselink MP, et al. Acute gastroenteritis in the etiology of inflammatory bowel disease: systematic review and meta-analysis. Crohn's Colitis 360. 2021;3(4):1–8.
- Porter CK, Welsh M, Riddle MS, et al. Epidemiology of inflammatory bowel disease among participants of the Millennium cohort: incidence, deployment-related risk factors, and antecedent episodes of infectious gastroenteritis. Aliment Pharmacol Ther. 2017;45(8):1115–1127.
- Salih A, Widbom L, Hultdin J, et al. Smoking is associated with risk for developing inflammatory bowel disease including late onset ulcerative colitis: a prospective study. Scand J Gastroenterol. 2018;53(2):173–178.
- Bonderup OK, Nielsen GL, Dall M, et al. Significant association between the use of different proton pump inhibitors and microscopic colitis: a nationwide Danish case‐control study. Aliment Pharmacol Ther. 2018;48(6):618–625.
