Abstract
Introduction
Although sporadic non-ampullary duodenal adenomas (SNADA) are rare, with the risk of progression to cancer, they deserve therapy. Endoscopic therapy of SNADA is effective, but with the increased risk of complications, endotherapy should be performed in high-volume units. The results of endotherapy of SNADA in our unit are presented.
Patients and methods
A total of 97 patients with SNADA had endoscopic resection in 2005–2021 and control endoscopies between 3 and 24 months. Snare polypectomy, endoscopic mucosal resection (EMR), endoscopic band ligation (EBL) and endoloop were used (en bloc 37% and piecemeal 63%). In cases of residual/recurrent adenomas, endotherapy was repeated.
Results
The median size of the adenoma was 12 (5–60) mm and most polyps were sessile (25%) or flat (65%). Primary endotherapy eradicated adenomas in 57 (59%) cases. Residual and recurrence rates were 24% (n = 23) and 17% (n = 16) with successful endotherapy in 16 (70%) and 13 (81%) patients. Endotherapy was successful in 86 (89%) patients after a median (range) follow-up of 23 (1–166) months. Four out of 11 patients with failed endotherapy had surgery; seven patients were not fit for surgery. There were no disease-specific deaths or carcinoma. Eleven patients (11%) suffered from complications: perforation requiring surgery (n = 1), sepsis (n = 1), postprocedure bleeding (n = 7), cardiac arrest (n = 1) and coronary infarct (n = 1). The thirty-day mortality was zero. Colonoscopy was performed on 67 (69%) patients with neoplastic lesions in 33% patients during follow-up.
Conclusions
Endotherapy of SNADA is effective and safe. Repeat endotherapy in residual and recurrent adenomas is successful. Careful patient selection is mandatory. Abbreviations: ASA: American Society of Anesthesiologist classification; BMI: body mass index; CT: computed tomography; EBL: endoscopic band ligation; EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; ET: endotherapy; FAP: familial adenomatous polyposis; F: female; LST: laterally spreading tumours; M: male; SD: standard deviation; SNADA: sporadic nonampullary duodenal adenoma
Background
Duodenal polyps are found in 0.6–5% of patients undergoing gastroscopies [Citation1–3]. Duodenal adenomas were present in 0.03–0.1% of gastroscopies [Citation2,Citation3], mostly in asymptomatic patients. In 40% of patients, duodenal adenomas are sporadic and in 60% associated with familial adenomatous polyposis (FAP) or other genetic syndromes [Citation4,Citation5]. FAP, an autosomal dominant disease is associated with periampullary and duodenal adenomas in up to 80% of patients and the estimated lifetime risk of cancer with these genetic syndromes is up to 4% [Citation4,Citation5]. Most (75–81%) duodenal adenomas are sessile or flat [Citation6,Citation7] and are located in the descending duodenum. Altogether 25–30% of sporadic duodenal adenomas are located in the ampulla region [Citation8,Citation9] Sporadic non-ampullary duodenal adenomas (SNADA) are rare. In adenoma patients with low-grade dysplasia the large initial tumour size and location on the oral side of the papilla of Vater are risk factors of progression to high-grade dysplasia [Citation10].
As duodenal adenoma may progress to carcinoma similar with colonic adenoma-carcinoma sequence it therefore deserves treatment [Citation11,Citation12]. Endoscopic resection primarily by endoscopic mucosal resection (EMR) [Citation13] is safe and less invasive compared to surgery that has major morbidity.
A limited number of studies present data on the outcome of endoscopic therapy of SNADA. The aim of this study is to describe the success of endoscopic therapy, as well as the rate of complications and mortality in patients with SNADA at Helsinki University hospital.
Patients and methods
This study is a retrospective analysis of all the patients with endoscopic therapy for SNADA or ICD-10 code D13.2 during 2005–2020 at Helsinki University Hospital. The patient list from the electronic database was completed by manually checking the appointment lists and files of performed procedures.
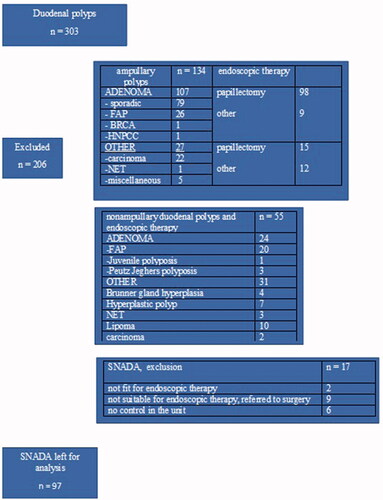
Enrolled patients with duodenal polyps and further excluded patients are presented in .
After the exclusion, there were 97 patients remaining for analysis.
We reviewed endoscopic images and Paris classification [Citation14] and classification of laterally spreading tumours (LST) [Citation15] were used for morphologic classification of the polyp.
In the endoscopy unit, a polyp removal procedure by six experienced endoscopists was performed under conscious sedation provided by an anaesthesiologist and anaesthesia nurse. Patients were either in a prone or in a left lateral cubitus position. Glucagon (GlucaGen®, Novo Nordisk Farma, Espoo, Finland) or hyoscine-N-butylbromide (Buscopan®, Sanofi, Espoo, Finland) were administered to inhibit duodenal motility. Snare polypectomy or EMR was primarily used. Saline or Sigmavisc®, were injected for lifting. When snare polypectomy was not technically possible (scarring or position of the adenoma) an endoscopic band ligation (EBL) device (6 shooter® Cook Medical, Helsinki, Finland) was used, and an endoloop (Polyloop ligating device, Olympus, Espoo Finland) placed in cases of pedunculated polyps. In cases of periprocedural bleeding, coagulation with flushing monopolar probe, hot biopsy or haemostatic clips were used. Successful endoscopic treatment was defined as the absence of visible residual adenoma at the end of the endoscopic resection. Residual adenoma was defined as visible adenoma remnant at first control and recurrent adenoma was defined as adenoma recurs after a period adenoma could not be detected. Patients were followed up until they recovered from sedation and most patients had same-day discharge. However, next-day discharge was required if the patient did not have an adult caretaker at home for the following 24 h or if the patient suffered from any complications.
Control endoscopy under conscious sedation was performed after 3, 6 and 12 months and after 2 years if no residual adenomas were found. However, if residual adenomas or recurrent adenomas were found, endoscopic therapy (snare polypectomy or EMR, EBL, endoloop and argon plasma coagulation) was performed. After 2 years of follow-up, controls were to be discontinued, though, the follow-up was adapted individually. For study purposes, in addition to scheduled controls, any post-polypectomy upper gastrointestinal endoscopies were counted as controls. After the date of the last endoscopy visit, patient files were reviewed for further events possibly related to adenoma recurrence.
Statistics
The chi-square test was used to test for any differences between categorical variables. A nonparametric Wilcoxon–Mann–Whitney test was used to compare differences in continuous and ordinal variables; p values <0.05 (two-sided) were regarded as statistically significant. SPSS version 15.0 (IBM Corporation, Somers, NY) was used for data analysis.
Results
Patient details, comorbidities and an indication of diagnostic gastroscopy in 97 patients with SNADA are presented in .
Table 1. Patient demographics, comorbidities and indication of diagnostic gastroscopy in 97 patients with sporadic non-ampullary duodenal adenomas.
Anaemia or gastrointestinal bleeding was an indication of gastroscopy in 28 (29%) patients. In patients with incidentally found adenomas, gastroscopy was performed for screening of Barrett, for oesophageal varices or for a suspicion of B12 deficiency and in one case the CT scan revealed incidental adenoma.
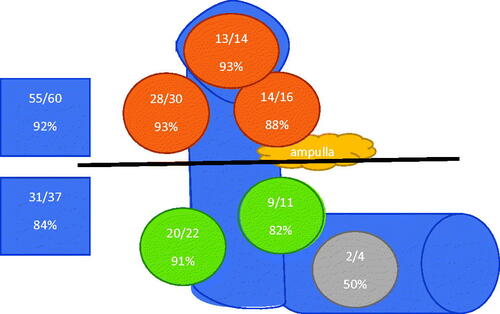
The time from diagnostic gastroscopy to endoscopic removal of duodenal adenoma was a median of 2(0 − 123) months. In 84 (87%) patients, polyp removal was performed in 12 months, after a median of 2 (0–11) months after diagnosis. Thirteen patients had repeated gastroscopy controls with biopsies in cases of adenomas and mild dysplasia in their local hospitals until they were referred for endoscopic therapy, which was undertaken after a median of 23 (12–123) months after the diagnosis. Most of the adenomas were located in the descending part (80/83%) or in the duodenal bulb (13/13%), while four adenomas located in the horizontal part. Though most of the patients had single duodenal adenomas (94/97%), two patients had two adenomas and one patient had four adenomas. In total, there were 102 duodenal adenomas in 97 patients. In case of several adenomas, the diameter of the largest adenoma was counted. The median diameter of the adenoma was 12 (5–60) mm. shows the location of adenomas (duodenal bulb, descending duodenum in four location, horizontal duodenum; oral and distal from papilla Vater) and final endoscopic treatment success, respectively.
Figure 1. The final treatment success of endoscopic therapy of sporadic non-ampullary duodenal adenomas in different adenoma locations, oral and distal from ampulla of Vateri.
Endoscopic therapy
The first session of endotherapy took a median of 23 (5–64) min and was performed with a duodenoscope in 48 (50%) cases, and with a gastroscope in 29 (30%); both endoscopes were used in 20 (21%) cases. The median (range) length of stay was 1 (1–20) day among 30 patients, while 67 patients were treated as outpatients.
Data for polyp size, Paris classification, LST classification in total and in final endotherapy success and failure are presented in , while details of endoscopic procedure are presented in . More than one method was used significantly more often when larger adenomas were treated, compared to adenomas less than 2 cm in diameter (17 (49%) vs. 13 (21%); p = 0.024).
Table 2. Polyp morphology: size, Paris classification, laterally spreading tumour (LST) classification in total and in final endotherapy success and failure.
Table 3. Endotherapy (ET), result and complications of 97 patients with SNADA.
Histology in diagnostic endoscopy and after the first therapeutic endoscopy is presented in . In two patients, a biopsy was taken before placing an endoloop or EBL. In 12 patients, EBL was performed without histologic samples, while in seven patients after snaring; the specimen was lost due to propulsive peristalsis.
Table 4. Histology in diagnostic gastroscopy and after endoscopic therapy.
Dysplasia was upgraded from low to high grade in 21 (22%) patients and downgraded from high to low in 12 (12%; biopsies vs. resection specimen) patients.
Follow-up
During a mean (SD)/median (range) follow-up of 30 (30.7)/23 (1–166) months, patients had altogether 329 follow-up procedures after an initial polyp removal procedure and mean (SD)/median of 3.4 (1.9)/3 (1–11) duodenoscopies. If endotherapy for residual or recurrent adenomas was required, it took a median of 20 (5–45) min and control endoscopy with biopsies of 9 (2–39) min.
When primary and control endoscopies were combined, snare polypectomy or EMR was used in 83 (86%) patients, EBL in 32 (33%), an endoloop was placed in 8 (8%), while coagulation (monopolar or argon) was applied in 27 (28%) patients. Only one method was used in 67 (69%) patients, two methods in 22 (23%), three methods in seven cases (7%) and four different methods were required in one patient.
Multiple endotherapy sessions indicating residual or recurrence were performed significantly more often in larger adenomas (≥ 2 cm in diameter) compared to smaller ones (22 (63%) vs. 24 (39%); p = 0.022), and in high-grade dysplasia compared to low-grade dysplasia (22 (63%) vs. 24 (39%); p = 0.022) when the highest dysplasia grade was regarded. In addition, more sessions were required if the lesion was located in other parts of the duodenum compared to lesions located in the duodenal bulb or greater curvature of the descending duodenum (20 (67%) vs. 26 (39%); p = 0.011).
Out of 97 patients, 14 (14%) had a follow-up of less than six months. Colonoscopy revealed metastatic colon carcinoma in two, one patient died and one moved to another district, while five morbid patients had anaemia/GI-bleeding ± anticoagulation therapy and underwent endotherapy to eradicate adenoma with short follow-up. In four elderly patients, controls were stopped on the discretion of the endoscopist, while one patient refused control endoscopy. Altogether, 75 (77%) patients had at least a 12-month endoscopic follow-up and 45 (46%) patients a ≥ 24-month follow-up.
In 67 (69%) patients undergoing a colonoscopy, a neoplastic lesion was found in one third (adenomas in 20 and cancer in three patients).
Treatment success and failure
Primary endotherapy eradicated adenomas in 57 (59%) cases. Residual and recurrence rates were 24% (n = 23) and 17% (n = 16) with successful endotherapy in 16 (70%) and 13 (81%) patients with a final success of endoscopic therapy in 86 (89%) patients. Although the size of the adenoma had an effect on the success of the treatment, the Paris classification or LST status did not (). In 11 patients, endotherapy failed, four of whom were operated on. In seven patients unfit for surgery, residual or recurrent SNADA therapy was later discontinued. Three of these patients died due to causes not related to adenomas, while four patients who were still alive had no hospital admissions related to adenoma. Details of patients with failed endotherapy are presented in .
Table 5. Patients with a final failure of endoscopic therapy.
When patients with treatment failure were compared to patients with final treatment success, they had more often anaemia and/or gastrointestinal bleeding symptoms for index gastroscopy (8/73 vs. 20/23%; p = 0.002), were on oral anticoagulants (6/55 vs. 13/15%; p = 0.007), had higher ASA (ASA 3–4: 10/91 vs. 1/3%, p = 0.035). Moreover, compared to patients with final treatment success, patients with treatment failure had larger adenomas (mean size in mm/SD: 28.2/18.9 vs. 14.8/7.6 mm; p < 0.041), were frequently with high-grade dysplasia (8/73 vs. 27/31%; p = 0.007), and multiple endoscopic methods were used regularly (7/64 vs. 23/27%; p = 0.005). In most cases, treatment failed with EBL (n = 5; 28%) compared to other methods (n = 6; 7%), p = 0.029.
Surgery
Four patients had surgery after failed endoscopic therapy, two of whom underwent a pancreaticoduodenectomy. Despite repeated endoscopic therapy, in one of these patients, residual adenomas with high-grade dysplasia were growing in the bulbar area and pyloric ring. Eventually, this patient had surgery five years after the first polyp removal. The other patient had adenomas with high-grade dysplasia, filling half of the circumference in transverse duodenum that could not be removed in two sessions. The other two patients had local resection. One comorbid patient had circumferential adenomas and mild dysplasia. After polyp removal attempts (ligature and snare methods), the patient was referred to surgery, but this was primarily rejected as too risky. Thereafter, several duodenoscopies with biopsies and dilatation of the duodenal stricture were undertaken and finally the patient had local resection with an uneventful recovery. The fourth patient who had residual high-grade dysplastic adenoma in the transverse duodenum after ligation procedures had local resection without complications.
Complications
In total, complications occurred in 3% of 426 procedures; these complications are listed in . There was no significant difference between the rate of complications among those with final treatment success vs. failure: 8 (9%) vs. 3 (27%); p = 0.07, respectively.
Mortality
Thirty-day and one-year mortality was zero. During follow-up, 20 patients died in a median of three [Citation1–13] years after endoscopic therapy at a median age of 75 (60–93) years. Two patients with ASA 1–2 (5%) died after a mean (SD) age of 10.5 (3.5) years, compared to 18 patients in ASA 3–4 (30%) patients who died after a mean age of 2.9 (1.8) years, p < 0.001. There were no deaths related to adenomas.
Patients with ASA 3–4
Low-grade dysplasia rate (46/77 vs. 30/8%, p = 0.5) and complication rate (6/10 vs. 3/8%; p = 0.8) were similar between ASA 3–4 and ASA 1–2 groups, respectively. Compared to the ASA 1–2 group, the ASA 3–4 group, had anaemia or gastrointestinal bleeding more often (23/38 vs. 5/14%; p = 0.009), were on oral anticoagulants (17/28 vs. 2/5%; p = 0.006) and had a higher rate of mortality during follow-up (18/30 vs. 2/5%; p = 0.004).
A subgroup of ASA 3–4 patients without anaemia or gastrointestinal bleeding and with low-grade dysplasia, were significantly younger than those ASA 3–4 patients with symptoms of bleeding or high-grade dysplasia (67.5 (SD9.2) vs. 72.6 (SD7.6); p = 0.023), respectively, and had a significantly lower rate of mortality (5 (15%) vs. 13(46%); p = 0.012, respectively.
There were eight patients with ASA 4 and low-grade dysplasia, with no signs of anaemia or gastrointestinal bleeding possibly eligible for non-treatment.
Discussion
This study reports our experience in endoscopic resection of SNADA at a single tertiary hospital. In this retrospective study with large patient material and a long follow-up, we could show that endoscopic therapy is safe and effective, with final treatment success in 89% of the SNADA patients.
According to ESGE guidelines, all duodenal adenomas should be considered for endoscopic resection as progression to adenocarcinoma is highly likely [Citation16]. In a Japanese study of 84 superficial nonampullary duodenal epithelial tumours in 73 patients, 26% were upgraded in final diagnosis. The sensitivity, specificity and accuracy of biopsy diagnoses of duodenal adenocarcinoma were 72, 80 and 74%, respectively [Citation17]. In our study, dysplasia was upgraded from low to high grade in 21 (22%) patients and downgraded from high to low in 12 (12%; biopsies vs. resection specimen). Similarly, in a French study of 134 SNADA, 32% of the lesions were upgraded and 11% downgraded [Citation18], while in an Italian study, histology was upgraded in 4% [Citation19]. Therefore, it seems that resection gives more accurate histology.
Because 20% of duodenal adenomas undergo malignant transformation, an evaluation for surgical or endoscopic excision is necessary. High-grade dysplasia diagnosed in the first biopsy and a lesion diameter of ≥ 20 mm are factors significantly predictive of progression to adenocarcinoma [Citation12]. Several follow-up endoscopies without adenoma removal in patients fit for endotherapy may just increase costs. Instead, a patient should be referred to a high-volume unit. On the other hand, elderly frail patients most probably do not benefit from endoscopic adenoma removal. In a Japanese multicentre follow-up study with a median observation period of 2.7 years and 101 duodenal neoplasia patients without polyp removal, the lesion size did not change in 50% of the patients, the lesions disappeared in 27%, shrank in 10% and grew in 13% of the cases, though four patients developed adenocarcinoma. The authors concluded that a policy of not resecting adenomas could be considered for those older patients with poor prognosis, or for small lesions [Citation20]. In our material, ASA 3–4 patients with low-grade dysplasia and without anaemia or gastrointestinal bleeding were younger and had lower mortality than those with high-grade dysplasia or anaemia or gastrointestinal bleeding. However, adenomas should be eradicated from patients on oral anticoagulants and bleeding or anaemia symptoms. In these cases, removing most of the adenoma tissue may be sufficient to stop gastrointestinal bleeding symptoms. In our study, there were eight SNADA patients with ASA 4, low-grade dysplasia without anaemia or gastrointestinal bleeding, who could have been suitable for not to resect policy. Therefore, careful patient selection is necessary especially in incidentally found adenomas and a multidisciplinary meeting discussion is advisable.
A flat or sessile morphology is most common in SNADA, in our study too, and submucosal injection of a lifting solution may help polyp removal. In our study, we used mostly saline, that is cheap and easy to use. Snare polypectomy and EMR were the most common methods in our unit. EMR has shown complete resection rates ranging from 70 to 100% [Citation21–23]. In a case series of 10 patients, the "band and slough" technique was effective and no complications occurred [Citation24]. In addition, the EBL method was used in our unit if there was difficulty to snare polyp. EBL was safe, but related to technically difficult cases, and had worse success rate compared to other methods. We neither used an underwater technique [Citation25] nor a cap-assisted technique [Citation26]. En bloc resection is possible with smaller polyps and piecemeal method is necessary with larger ones [Citation23,Citation26,Citation27]. Our en bloc resection rate was 37%, similar to studies by Probst et al. [Citation8] and Valli et al. [Citation22]. As in a study by Tomizawa and Ginsberg [Citation23], we mostly used a duodenoscope in polyp removal in order to identify papilla and utilise elevator of the duodenoscope. Further, a gastroscope is used with a cap-assisted technique and EBL methods [Citation24–27]. Our residual adenoma rate is high, 24%, but repeated endotherapy was successful in 70% of them. Later adenoma recurrence is comparable to literature (), and our final endotherapy success rate, 89% is acceptable when 42% of the adenomas were larger than 2 cm. Failure of endotherapy was associated with high-grade dysplasia, higher ASA grades, larger adenomas, and EBL technique. The success rate and complications of endoscopic therapy from earlier studies and this study are presented in .
Table 6. Results of endoscopic therapy (success rate, recurrence, and complications) of sporadic non-ampullary duodenal adenomas in different studies.
To find out an incomplete resection or recurrence, a followup is necessary. ESGE recommends first control after 3 months and then after one year of removal: controls should be adjusted individually [Citation16]. In this study, we controlled all the patients in 3 months and 77% of the patients had at least a 1-year follow-up.
Post-procedure bleeding rates vary between 11 and 18%, while perforation occurs in 0–3% [Citation10–12,Citation20]. Closing the mucosal defect with clips has been attempted to decrease complication rate. However, clips and powders increase costs and may not be helpful. In a study by Probst et al., delayed bleeding (17%) and perforations (4%) occurred despite preventive measures [Citation8]. Despite preventive measures during polyp removal, bleeding and perforation occurred and the authors concluded that techniques and preventive measures should be improved. Only in 10% of the cases, did we use preventive haemostastic methods, but our post-procedure rebleeding rate of 7% and perforation rate of 1% are acceptable.
When EMR and ESD were performed for duodenal adenomas, complications occurred only in the ESD group [Citation27], or the complication rate was 10-fold compared to EMR [Citation28]. We did not perform duodenal ESD, as it is difficult to perform, the risk of complication is high and therefore it should be reserved for highly experienced experts only.
A colonoscopy was performed on 67 (69%) of our patients with colonic neoplasia findings in one third of them. Similarly, in an Italian study, 53% of SNADA patients underwent a colonoscopy, one third of whom also had colonic neoplasia [Citation7]. In a French study, 78% of SNADA patients had screening colonoscopy and the adenoma rate was 59%, while the cancer rate was 13% [Citation6]. Therefore, a colonoscopy should be scheduled for SNADA patients.
The limitations of this study are that this is a retrospective study and has a single centre setting. In addition, procedures were performed at the discretion of the endoscopist, not by fixed protocol. Further, a colonoscopy was not performed on all the patients. Nevertheless, the strengths of this study are the number of patients with large polyps, careful monitoring, a long follow-up and six endoscopists performing procedures.
In conclusion, endoscopic therapy of SNADA is effective and safe, and most residual or recurrent adenomas can be treated endoscopically. Moreover, a larger sample size gives a more accurate histologic diagnosis compared to biopsies. If endotherapy fails, surgery is still possible. Careful patient selection is mandatory especially when treating incidental adenomas in elderly patients with comorbidities. Lastly, patients with duodenal adenomas have an increased risk of colonic neoplasia, and a colonoscopy should be scheduled for them.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Jepsen JM, Persson M, Jakobsen NO, et al. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29(6):483–487.
- Jung SH, Chung WC, Kim EJ, et al. Evaluation of non-ampullary duodenal polyps: comparison of non-neoplastic and neoplastic lesions. World J Gastroenterol. 2010;16(43):5474–5480.
- Alkhatib AA. Sporadic nonampullary tubular adenoma of the duodenum: prevalence and patients’ characteristics. Turk J Gastroenterol. 2019;30(1):112–113.
- Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2(8666):783–785.
- Lepistö A, Kiviluoto T, Halttunen J, et al. Surveillance and treatment of duodenal adenomatosis in familial adenomatous polyposis. Endoscopy. 2009;41(6):504–509.
- Mitsuishi T, Hamatani S, Hirooka S, et al. Clinicopathological characteristics of duodenal epithelial neoplasms: focus on tumors with a gastric mucin phenotype (pyloric gland-type tumors). PLoS One. 2017;12(4):e0174985.
- Ahmad NA, Kochman ML, Long WB, et al. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55(3):390–396.
- Probst A, Freund S, Neuhaus L, et al. Complication risk despite preventive endoscopic measures in patients undergoing endoscopic mucosal resection of large duodenal adenomas. Endoscopy. 2020;52(10):847–855.
- Murray MA, Zimmerman MJ, Ee HC. Sporadic duodenal adenoma is associated with colorectal neoplasia. Gut. 2004;53(2):261–265.
- Ikenoyama Y, Yoshimizu S, Namikawa K, et al. Sporadic non-ampullary duodenal adenoma with low-grade dysplasia: natural history and clinical management. Endosc Int Open. 2022;10(03):E254–E261.
- Seifert E, Schulte F, Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. 1992;87(1):37–42.
- Okada K, Fujisaki J, Kasuga A, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. 2011;106(2):357–364.
- Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol. 2016;22(2):600–617.
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and Colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6):S3–S43.
- Kudo Se Lambert R, Allen JI, Fujii H, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68(4):S3–S47.
- Vanbiervliet G, Moss A, Arvanitakis M, et al. Endoscopic management of superficial nonampullary duodenal tumors: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2021;53(05):522–534.
- Kakushima N, Kanemoto H, Sasaki K, et al. Endoscopic and biopsy diagnoses of superficial, nonampullary, duodenal adenocarcinomas. World J Gastroenterol. 2015;21(18):5560–5567.
- Hoibian S, Ratone JP, Gonzalez JM, et al. Endoscopic mucosal resection of sporadic duodenal nonampullary adenoma: outcomes of 130 patients with a long-term follow up in two tertiary French centers. Ann Gastroenterol. 2021;34(2):169–176.
- Valerii G, Tringali A, Landi R, et al. Endoscopic mucosal resection of non-ampullary sporadic duodenal adenomas: a retrospective analysis with long-term follow-up. Scand J Gastroenterol. 2018;53(4):490–494.
- Kanzaki H, Matsueda K, Nakagawa M, et al. Clinical characteristics and course of sporadic non-ampullary duodenal adenomas: a multicenter retrospective study. Medicine (Baltimore). 2021;100(39):e27382.
- Nonaka S, Oda I, Tada K, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47(2):129–135.
- Valli PV, Mertens JC, Sonnenberg A, et al. Nonampullary duodenal adenomas rarely recur after complete endoscopic resection: a swiss experience including a literature review. Digestion. 2017;96(3):149–157.
- Tomizawa Y, Ginsberg GG. Clinical outcome of EMR of sporadic, nonampullary, duodenal adenomas: a 10-year retrospective. Gastrointest Endosc. 2018;87(5):1270–1278.
- Koritala T, Zolotarevsky E, Bartley AN, et al. Efficacy and safety of the band and slough technique for endoscopic therapy of nonampullary duodenal adenomas: a case series. Gastrointest Endosc. 2015;81(4):985–988.
- Yamasaki Y, Uedo N, Akamatsu T, et al. Nonrecurrence rate of underwater EMR for ≤20-mm nonampullary duodenal adenomas: a multicenter prospective study (D-UEMR study). Clin Gastroenterol Hepatol. 2021;20:1010–1018.e3.
- Jamil LH, Kashani A, Peter N, et al. Safety and efficacy of cap-assisted EMR for sporadic nonampullary duodenal adenomas. Gastrointest Endosc. 2017;86(4):666–672.
- Hara Y, Goda K, Dobashi A, et al. Short- and long-term outcomes of endoscopically treated superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2019;25(6):707–718.
- Na HK, Kim DH, Ahn JY, et al. Clinical outcomes following endoscopic treatment for sporadic nonampullary duodenal adenoma. Dig Dis. 2020;38(5):364–372.