Abstract
Background and aims
The recommended treatment duration of hepatitis C virus (HCV) genotype 1a (GT1a) infection with elbasvir/grazoprevir (EBR/GZR) in the presence of a high baseline viral load and resistance associated substitutions (RAS) is 16 weeks with ribavirin added. The objective of this study was to evaluate the real-world effectiveness of 12 weeks of EBR/GZR without ribavirin and regardless of baseline viral load and RAS testing.
Method
This retrospective, observational cohort study was performed at five Norwegian hospitals that did not systematically utilize RAS testing. All adult patients with chronic HCV GT1a and compensated liver disease who had received 12 weeks of EBR/GZR without ribavirin and baseline RAS testing, were included. The primary endpoint was sustained virologic response at week 12 (SVR12), or if not available, at week 4 (SVR4).
Results
We included 433 patients and attained SVR data on 388. The mean age was 45.7 years (22–73 years). 67.2% were male. HIV co-infection was present in 3.8% (16/424) and cirrhosis in 4% (17/424). The viral load was >800 000 IU/mL in 55.0% (235/427) of patients. Overall SVR was achieved in 97.2% (377/388). SVR was achieved in 98.3% (169/172) of those with viral load ≤800 000 IU/mL and in 96.2% (202/210) of those with viral load >800 000 IU/mL.
Conclusion
We observed high SVR rates among patients with HCV GT1a infection treated with EBR/GZR for 12 weeks without ribavirin, with no regard to baseline viral load and no RAS testing.
Introduction
Elbasvir (EBR) is an inhibitor of the NS5A protein essential for replication and viral assembly, and grazoprevir (GZR) is an inhibitor of the hepatitis C virus NS3/4A protease enzyme responsible for cleaving the hepatitis C polypeptide [Citation1].
In a phase III trial, the combination was effective in treatment-naïve patients with genotype 1a-infection as sustained viral response rates (SVR) was achieved in 92% [Citation2]. In the study, virologic failure was associated with baseline NS5A resistant associated substitutions (RAS) as SVR12 was achieved in 11 of 19 (58%) of patients with NS5A RAS compared with 133 of 135 (99%) patients without baseline NS5A RAS. The association between baseline RAS and SVR was only observed among patients with viral load >800 000 IU/mL. A pooled analysis of phase II and phase III studies of EBR/GZR showed a 100% SVR rate among those with genotype 1a and NS5A RAS at baseline if treatment was given for 16 weeks and ribavirin was added [Citation3].
Based on these data, the European Association for the Study of the Liver (EASL) in 2016 recommended that patients with genotype 1a-infection with a baseline viral load above 800 000 IU/mL and NS5A RAS conferring resistance to elbasvir, or viral load above 800 000 IU/mL and no NS5A resistance testing performed, should receive the combination of elbasvir and grazoprevir for 16 weeks in combination with daily weight-based ribavirin [Citation4].
In its 2018 update, EASL stated that resistance testing before therapy was not recommended due to a lack of standardized tests, which implied that all patients with a high viral load and genotype 1a-infection treated with EBR/GZR should receive prolonged treatment with the addition of ribavirin [Citation5]. In its last update [Citation6], EBR/GZR was not recommended for the treatment of HCV genotype 1a infection altogether. However, the guidelines refer to earlier versions for guidance if regional conditions mandate the use of EBR/GZR.
In 2017, the estimated prevalence of people living with hepatitis C (HCV-RNA positives) in Norway was 0.20–0.31% representing 11 000–17 000 persons, where 80% of infections were related to intravenous drug use, IDU [Citation7]. From early 2017, after a tender competition, EBR/GZR became the primary option in Norway for treatment of HCV genotype 1 and 4 in patients with compensated liver disease, fully covered by the Public Health Maintenance organizations, which gave impetus to a considerable expansion of treatment of genotype 1 and 4 to people who inject drugs (PWID). At the time, hepatis C treatment was offered by out-patient clinics at public hospitals supervised by the head of the clinic. At several of these clinics, it was independently judged that the need for elbasvir resistance testing would lead to a delay in the initiation of treatment, and more importantly, that the prolonged regime of ribavirin given in addition to EBR/GZR would increase the risk of serious side effects requiring more frequent monitoring, and would increase the pill burden. In many clinicians view these problems would be acting as important barriers to treatment for many PWID.
Some hepatitis C treatment centers in Norway eventually prioritized simplifying the treatment to a single tablet regime, initiated without delay, and in disregard of possible resistance to elbasvir. This approach was expected to lead to a slightly higher relapse rate than what would be expected if treatment was tailored according to the result of RAS testing. However, the recommended second line, single tablet option of sofosbuvir/velpatasvir/voxilaprevir is readily available in Norway for retreatment in case of virologic relapse [Citation6]. This acts as a safeguard that allows for the simplified treatment approach with 12-week EBR/GZR.
This Norwegian experience provides an opportunity to study a pragmatic use of EBR/GZR. The primary objective of this study was to evaluate the real-world effectiveness of 12-week EBR/GZR treatment without ribavirin and baseline RAS testing and without regard to viral load in patients with HCV GT1a.
Materials and methods
Study population and design
This is a retrospective, observational, multicentre, cohort study. All adult patients (≥18 years) treated with 12 weeks of EBR/GZR for chronic genotype 1a-infection without baseline elbasvir resistance testing, and with no consideration of baseline viral load, were included. Patients with previous known all-oral DAA treatment were excluded. The patients were prescribed 12 weeks of treatment during the period from late 2016 when EBR/GZR was first made available in Norway, up to mid-2019, so that the treatment was completed at least 4 weeks prior to data closure on 1 September 2019.
Data collection and analysis
Data were extracted from local HCV databases if available, or directly from electronic medical records at hospitals without local databases, applying ICD-10 codes, and specific codes of the Public Health Maintenance Organizations for elbasvir and grazoprevir prescription, for search. The data acquired at each participating hospital were anonymized, encrypted, and sent electronically as separate data files to Link Medical in Oslo for analysis.
Assessment
Assessments at enrolment included HCV RNA load, HCV genotype, standard laboratory and clinical assessments, and transient elastography. The viral RNA was performed at each local hospital with commercially available equipment.
Outcomes
The main endpoint was sustained virologic response at week 12 (SVR12) defined as undetectable HCV RNA 12 weeks after end of treatment, or if not available, at week 4 (SVR4).
Ethics
The study was approved by the Regional Ethical Committee – South-East, Oslo.
Statistical analysis
The sample size was determined and limited by the availability of patients for inclusion. Thus, the statistical analysis is purely descriptive.
The results are presented as a modified intention-to-treat analysis (mITT), where patients without assessment of HCV RNA at least 4 weeks after the end of treatment were considered lost to follow-up and excluded from the analysis. The SVR results are presented as percentages with 95% confidence intervals.
Continuous variables are reported as mean values with 95% confidence intervals, and as median values with minimum–maximum ranges. Categorical variables are presented as absolute numbers and frequency counts (percentages).
Cirrhosis was defined by liver stiffness measurement of ≥12.5 kPa or an APRI score >2.
Results
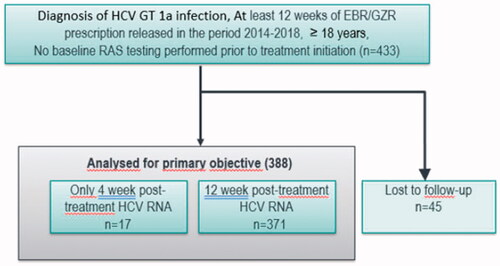
We included 433 patients with genotype 1a, and obtained post-treatment HCV RNA from 388, seventeen from week 4 to 11 post-treatment, and 371 from week 12 or later (). Forty-five patients were lost to follow-up. Among 427 patients with baseline viral load assessed, 235 (55%) had high baseline viral load (>800 000 IU/mL), and 192 (45%) low viral load (≤800 000 IU/mL) (). The mean age was 45.7 years (range 22–73 years), 291 (67.2%) were male, and 27.4% reported recent drug use ().
Table 1. Baseline characteristics (according to availability of data).
Sustained viral response
In an mITT analysis, SVR was achieved in 377 of 388 patients (97.2%), . Among 172 patients with a low baseline viral load, the SVR was 98.3%, and among 210 patients with a high viral load, the SVR was 96.2%. Four in five patients with cirrhosis and viral load above 800 000, and 8 in 9 with low viral load and cirrhosis, achieved SVR ().
Figure 2. Outcome among 388 patients included in the mITT population and treated for 12 weeks of EBR/GZR without ribavirin and with no baseline RAS testing.
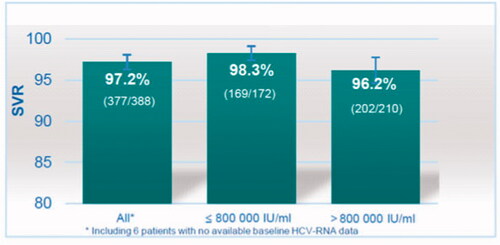
Table 2. Sustained viral response (SVR). HCV RNA results at week 12, or at week 4 to 11 if not available at week 12. Outcomes for all 433 patients together, and for 427 patients apportioned to baseline viral load above, and at or below 800 000 IU/mL, respectively. The results are presented as modified Intention-To-Treat (mITT) and Intention-To-Treat (ITT) analysis with 95% confidence intervals (95% CI).
Virologic failures
Eleven patients did not achieve SVR (). Among these, eight had high viral load. Only two had cirrhosis. The specific fibrosis score of the remaining nine patients was unfortunately not extracted from the data set. Four of the patients had recorded recent intravenous drug use within the 6 month period prior to start of treatment, but there was no information recorded of on-going drug use during or after treatment.
Table 3. Virologic failures. Eleven patients did not achieve SVR. Of these 5 were successfully retreated with alternative DAA-regimes, and 1 were lost to follow up. There was no information retrieved on 5 patients (N.D, not determined).
Discussion
In this retrospective, multicenter observational study of patients with chronic HCV genotype 1a-infection, the overall SVR rate in a modified Intention-To-Treat (mITT) analysis was 97.2% after 12 weeks of EBR/GZR without baseline elbasvir resistance testing or any regard of baseline viral load (). It is noteworthy that among patients with a high baseline viral load, SVR was observed in 96.2%.
A quarter of the patients had ongoing or recent injecting drug use which probably was the main reason for the lost to follow-up category. In our view, this justifies the use of mITT analysis as the basis for the discussion of the SVR rates achieved by 12 week EBR/GZR.
The study population was mainly treatment naïve as only 8.4% had received previous treatment. In addition only 4% had cirrhosis, 3.9% diabetes mellitus, and most had normal weight and Body Mass Index (BMI).
This is one of very few European assessments of the effect of EBR/GZR in a real world setting and our findings contrasts with the findings in the phase III trial of EBR/GZR that showed an SVR of 92% in patients with genotype 1a infection treated for 12 weeks without RBV [Citation2]. However, our findings are well in line with a metanalysis of six RCTs of 12 weeks treatment with EBR/GZR without RBV that demonstrated a SVR rate of 95.7% among patients with genotype 1a infection [Citation8].
The prevalence of baseline elbasvir specific RAS of clinical importance is difficult to assess due to the lack of standardized tests. Jacobson et al. [Citation3] demonstrated that the identification of NS5A RAS associated with virologic failure after 12 weeks of EBR/GZR was highly dependent on the definition of RAS and the methodology used for the detection.
The most significant baseline RASs conferring reduced activity of NS5A inhibitors (DAA) in genotype 1a-infection are mutations leading to substitutions of amino acids in positions 30, 31 and 93 in the NS5A protein, with the likelihood of achieving SVR12 reduced to 61–65% when RASs are detected with Next-Generation Sequencing at a 1% sensitivity threshold (ST), and 38.5–62.5% when detected with population sequencing methods [Citation3]. Changes in positions 28 and 58 impacts activity of NS5A inhibitors to a lesser degree [Citation3]. These highly significant amino acid positions form part of the NS5A protein DAA binding site [Citation9].
Jacobson found an overall prevalence of specific elbasvir RAS in genotype 1a of 5.8 to 6.7% in the EBR/GZR phase II and III-studies, which recruited patients from centers in Asia, Australia, Europe, and Central and North America. In line with this, Palladino et al. reported a prevalence of specific elbasvir RAS of 6.2% among a Spanish cohort of 617 patients with genotype 1a-infecton. Of these, 55% showed a reduced sensitivity to elbasvir in a geno2pheno-analysis [Citation10], while Jacobson observed an SVR 12 rate of 58% among a group of genotype 1a patients with highly specific elbasvir RAS [Citation3].
Sahlgrenska Academy at the University of Gothenburg, Sweden, where most Norwegian genotype 1a-samples are screened for NS5A RASs uses Next-Generation Sequencing (NGS) with a 10% ST and reports changes corresponding to positions 28, 30, 31, 58 and 93 (Martin Lagging: personal communication).
Sahlgrenska performed RAS screening of 447 genotype 1a >800 000 samples from Western Sweden, and Norway in 2018, and 526 in 2019. RASs were detected in 56 (12.5%) of the samples from 2018 and in 80 (15.2%) from 2019, with a combined RAS prevalence of 14%. This gives an overall RAS prevalence of 7% in all genotype 1a-infections in this Scandinavian region, assuming that the viral load cohorts above and below 800 000 are of equal size.
By using data from Jacobson as a basis for the modelling of an overall SVR rate (prevalence of highly specific EBR RAS = 5.8–6.7%, and SVR 58% in the presence of EBR RAS), the expected SVR would be 97.2–97.6%. In a similar modelling of the Spanish cohort, the expected SVR would be 97.4%, and for the Scandinavian cohort 97.1%. All three of these modelled SVR rates compares well to the observed overall SVR rate of 97.2% in our study.
In light of the WHO hepatitis C elimination strategy [Citation11,Citation12] there is a demand for effective and well-tolerated HCV treatments with a low pill burden and low cost, for targeting the population of people who inject drugs (PWID). This is a highly vulnerable population when it comes to initiation of and adherence to treatment [Citation7,Citation13,Citation14]. While there are now pan-genotypic treatment options available, cost considerations may still confer a need for genotype-specific options like EBR/GZR. The delay to start treatment incurred by elbasvir resistance testing, and the prolonged multi-tablet option with increased risk of side effects, by our experience represent significant treatment barriers. In this perspective, the satisfactory overall SVR rates of our real-world study are important.
Conclusion
We observed high SVR rates among patients with HCV GT1a infection treated with EBR/GZR for 12 weeks without ribavirin, with no regard to baseline viral load and no RAS testing.
| Abbreviations | ||
| DAA | = | Direct-acting antiviral agents |
| EBR | = | elbasvir |
| EBR/GZR | = | elbasvir/grazoprevir, proprietary name Zepatier |
| Elbasvir resistance | = | NS5A RAS with highly specific resistance to elbasvir |
| GT | = | Genotype |
| GZR | = | grazoprevir |
| HCV | = | Hepatitis C virus |
| pegIFN-riba | = | Peginterferon alfa 2a or 2b, and ribavirin |
| PWID | = | People Who Inject Drugs |
| NGS | = | Next-Generation Sequencing |
| NS5A RAS | = | Resistance associated substitutions in the NS5A gene |
| RAS | = | Resistance associated substitutions |
| RNA | = | Ribonucleic acid |
| SVR | = | Sustained virologic response |
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bell AM, Wagner JL, Barber KE, et al. Elbasvir/grazoprevir: a review of the latest agent in the fight against hepatitis C. Int J Hepatol. 2016;2016:3852126. https://www.hindawi.com/journals/ijh/2016/3852126/
- Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir–elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection. A randomized trial C-EDGE treatment naive trial of grazoprevir–elbasvir. Ann Intern Med. 2015;163(1):1–13. https://doi.org/10.7326/M15-0785.
- Jacobson IM, Asante-Appiah E, Wong P, et al. Prevalence and impact of baseline NS5A resistance-associated variants (RAVs) on the efficacy of elbasvir/grazoprevir (EBR/GZR) against GT1a infection – 16 weeks vs 12 weeks. 66th Annual Meeting of the American Association for the Study of Liver Diseases. Boston, MA 2015. https://www.natap.org/2015/AASLD/AASLD_47.htm
- Recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66(1):153–194. https://doi.org/10.1016/j.jhep.2016.09.001.
- Recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511. https://doi.org/10.1016/j.jhep.2018.03.026.
- Recommendations on treatment of hepatitis C 2020. J Hepatol. 2020;73(5):1170–1218. https://doi.org/10.1016/j.jhep.2020.08.018.
- Midgard H. Management of hepatitis C virus infection among people who inject drugs: Treatment uptake, reinfection and risk behaviours [PhD thesis]. University of Oslo; 2017. https://www.duo.uio.no/handle/10852/61510.
- Ahmed H, Abushouk AI, Menshawy A, et al. Meta-analysis of grazoprevir plus elbasvir for treatment of hepatitis C virus genotype 1 infection. Ann Hepatol. 2018;17(1):18–32. https://www.medigraphic.com/pdfs/hepato/ah-2018/ah181e.pdf
- Knops E, Sierra S, Kalaghatgi P, et al. Epistatic interactions in NS5A of hepatitis C virus suggest drug resistance mechanisms. Genes (Basel). 2018;9(7):343. https://doi.org/10.3390/genes9070343.
- Palladino C, Esteban-Cartelle B, Mate-Cano I, et al. Prevalence of relevant NS5A resistance-associated substitutions to elbasvir in genotype 1a hepatitis C virus patients in Spain. Enferm Infecc Microbiol Clin (Engl Ed). 2018;36(5):262–267. https://doi.org/10.1016/j.eimc.2017.03.008.
- WHO. Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis. World Health Organization; 2016. https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/
- Wisløff T, White R, Dalgard O, et al. Feasibility of reaching world health organization targets for hepatitis C and the cost-effectiveness of alternative strategies. J Viral Hepat. 2018;25(9):1066–1077. https://doi.org/10.1111/jvh.12904.
- Toresen KH, Salte IM, Skrede S, et al. Clinical outcomes in a cohort of anti-hepatitis C virus-positive patients with significant barriers to treatment referred to a Norwegian outpatient clinic. Scand J Gastroenterol. 2014;49(4):465–472. https://doi.org/10.3109/00365521.2013.863965.
- Sølund C, Hallager S, Pedersen MS, DANHEP group, et al. Direct acting antiviral treatment of chronic hepatitis C in Denmark: factors associated with and barriers to treatment initiation. Scand J Gastroenterol. 2018;53(7):849–856. https://doi.org/10.1080/00365521.2018.1467963.