Abstract
Objective
There is no consensus on whether a gastroscopic biopsy is necessary during the emergency treatment of gastrointestinal (GI) diseases such as gastric ulcer bleeding. In this study, we examined the clinical utility and safety of an emergency gastroscopic biopsy for the assessment of gastric ulcer bleeding.
Methods
We enrolled 150 patients with a single bleeding gastric ulcer after emergency gastroscopy (EG) from April 2020 to April 2022. The patients were randomly divided into the biopsy and no biopsy groups, and they were followed-up until June 2022 to examine whether recurrent gastric ulcer bleeding had occurred.
Results
Re-bleeding occurred in 15 out of 150 (10%) patients. We diagnosed malignancies in 17 (11.3%) patients and validated 14 (9.3%) of them during the initial gastroscopy procedure. Factors that could predict the occurrence of gastric ulcer re-bleeding with biopsy during EG included an absence of ischemic heart disease (odds ratio [OR] = 0.395, confidence interval [CI]: 0.24–0.65, p ≤ .005), renal disease (OR = 1.74, CI: 0.77–1.59, p ≤ .005), and using warfarin or oral anticoagulants (OR = 11.953, CI: 3.494–39.460, p ≤ .005). No significant differences were observed in 60-day bleeding (p = .077) and the duration of hospitalization (p = .700) between the two groups.
Conclusions
Patients undergoing biopsy during EG did not exhibit an increased risk of re-bleeding compared with those who did not undergo a biopsy. An early biopsy facilitates an early pathologic diagnosis, early clinical intervention, safe discharge of low-risk patients, and improved outcomes in high-risk patients.
Introduction
Gastrointestinal bleeding from gastrointestinal disorders is one of the most common causes of hospitalization in the United States, accounting for more than 507,000 hospitalizations per year at an annual cost of $4.85 billion [Citation1]. Upper GI bleeding (i.e., bleeding that occurs from the duodenum, stomach, or esophagus) is responsible for more than a half of all hospitalizations [Citation2]. Over the recent 20 years, a decrease in case fatality rates has been observed among the hospitalized patients with upper GI bleeding, ranging from 2.1% to 2.5% as reported in the nationwide database studies in the US [Citation3,Citation4] to 10% as reported in large, prospective observational studies in Europe [Citation5,Citation6]. The death rate among the patients who are already hospitalized for other diseases is approximately 3–4 times higher than that in patients who are hospitalized for upper GI bleeding [Citation7].
Peptic ulcers account for 28%–59% of nonvariceal upper gastrointestinal (GI) hemorrhage cases, with duodenal ulcers being responsible for 17%–37% of the cases and gastric ulcers for 11%–24% of the cases. Among these, duodenal bulb ulcers are observed to be mostly benign. Most patients with a duodenal bulb ulcer do not undergo malignant progression, and the incidence of gastric ulcer cancer is approximately 1%–3% [Citation8]. Recent findings of a national epidemiological study involving 1006 cases [Citation9] showed that 43.4% of all hemorrhagic ulcers constituted Forrest grade Ia–IIb ulcers in China, and endoscopic hemostasis was used for the treatment of only 25.2% of these cases.
Emergency gastroscopy (EG) is essential for treating upper GI bleeding. A biopsy should be conducted under direct supervision to examine lesions detected by endoscopic examination and those suspected to be malignant [Citation10]. An analysis of the effects of the wide application of proton pump inhibitors in clinical practice has shown that some malignant ulcer lesions, especially in patients with ulcerative gastric cancers, which can heal in a manner similar to that observed for benign ulcers, are prone to pseudo-healing [Citation11]. This increases the complexity of the endoscopic diagnostic process and necessitates a pathological examination of the results for the confirmation of the results. To our knowledge, conventional medicines are usually ineffective in treating malignancies and may induce recurrence or life-threatening bleeding in the patients; hence, surgery is the best treatment option for these patients. However, a few studies have reported whether a biopsy is necessary for gastric ulcer bleeding lesions during EG, and it is unclear whether biopsy increases the risk of re-bleeding lesions [Citation12,Citation13].
Guidelines and the recommendations provided by meta-analyses regarding the effectiveness of an emergency endoscopic gastric ulcer lesion biopsy have been inconsistent. Therefore, high-quality evidence is required to justify whether it is feasible to conduct a routine biopsy of the gastric mucosa during emergency endoscopy. Here, data regarding possible outcome predictors were extracted and used as a reference for clinical endoscopic diagnosis and treatment.
Methods
Study design
We conducted this investigator-initiated, prospective, and randomized clinical trial from April 2020 to April 2022 at the 900th hospital of the Joint Support Force in China. The institutional review board at the participating center approved the procedures used in the experiment.
Sample size
This was a parallel-group randomized controlled study. The intervention group was the biopsy group, and the control group was the no-biopsy group. The main outcome index was the occurrence of rebleeding at 60 days. As previously reported in the provisional literature, the rate of rebleeding in the biopsy group was set as 0.25, and the incidence of the control group was set as 0.5. We used two-sided test with α = 0.05 (bilateral) and β = 0.10. The sample sizes of the intervention and control groups were calculated using the PASS 15 software, N1 = N2 = 70 cases. Assuming that the loss to follow-up rate was 5%, the sample size was N1 = N2 = 70/0.95 = 74 cases. In this study, 75 patients were finally included in each group.
Patients
Patients with age >18 years who successfully underwent emergency gastroscopy (EG) for a single bleeding gastric ulcer within 24 h of emergency treatment were included in this study. We included those who were hemodynamically stable upon admission in the trial. We excluded patients with bleeding who needed the initial endoscopy procedure but refused to provide informed consent, patients who exhibited bleeding while they were already hospitalized because of another illness, and those in whom bleeding occurred from a known stomach carcinoma, non-ulcerative lesion (e.g., Dieulafoy’s lesion), or mechanical factors (i.e., the pressure necrosis induced by the gastrostomy tube in the gastric mucosa).
We included 150 patients in this trial; 75 patients were randomized into each of the two groups, the routine biopsy endoscopy group and the control group. Each of the patients provided written informed consent.
We collected data related to patient demographics, clinical presentation, re-bleeding incidence, and endoscopy and histology-related findings after the initial and subsequent gastroscopies.
The risk of recurrent bleeding and mortality in patients with acute bleeding in the upper GI tract was estimated using the Glasgow–Blatchford score.
Bleeding peptic ulcers
Bleeding peptic ulcers were graded according to the Forrest classification system . During the initial endoscopy, we examined bleeding ulcers exhibiting endoscopic stigmata of acute bleeding (Forrest grades Ia and Ib), visible vessels (Forrest grade IIa), adherent clots (Forrest grade IIb), flat and pigmented spots (Forrest grade IIc), or clean ulcers (Forrest grade III). We measured the diameters of peptic ulcers by comparing the size of the lesions with that of a fully open biopsy forceps (6 mm in size). Biopsy was performed in patients in the biopsy group at 1–2 points in the apical or medical mucosa elevated at the edge of the the ulcer. The depth of the biopsy was the muscularis mucosa.
Endoscopy
We used a GIF-XQ290 gastroscope (Olympus, Tokyo, Japan) to perform endoscopy. To achieve endoscopic hemostasis, we performed fibrin-glue injection therapy, hemoclip application, or thermal coagulation or/and 1:10,000 epinephrine injection. These methods were also used during the scheduled second-look esophagogastroduodenoscopy (EGD) procedure if endoscopic stigmata of Forrest classification grades Ia, Ib, IIa, IIb, IIc, or III was observed to persist. Three endoscopists with experience of more than 200 endoscopic hemostases performed all the endoscopic procedures.
Primary endpoint
The 60-day all-cause re-bleeding rate was considered the primary endpoint. The secondary outcomes were as follows: (1) need to repeat endoscopic intervention within 60 days, (2) transfusion of units of blood within 60 days, (3) in-hospital mortality rate, (4) duration of hospitalization, (5) hospitalization cost, (6) conversion to laparotomy, and (7) pathological findings.
Follow-up
The follow-up of the patients was performed through regular gastroenterological examinations, clinic visits, review of Wechat records, and/or telephonically conducted interviews. Physicians conducted telephonic interviews with the patients; in case the patients were unavailable or dead, interviews were conducted with their spouses or first-degree relatives.
Time to rebleeding was defined as the length of time from performing an emergency gastroscopy until the occurrence of rebleeding. Kaplan–Meier (KM) analyses were performed to examine the 60-day no rebleeding of the patients who underwent or did not underwent a biopsy, and the log-rank test was conducted to assess differences in outcomes among the patients.
Outcome definitions
The clinical parameters that were used to define re-bleeding from peptic ulcers are as follows: (1) hematemesis or bloody nasogastric aspirate >6 h following the initial endoscopy, (2) melena following stool color normalization, (3) hematochezia following stool color normalization or melena, (4) development of tachycardia (heart rate: 110 beats per minute) or hypotension (systolic blood pressure: 90 mm Hg) 1 h after hemodynamic stability, without an alternative explanation for hemodynamic instability, which could have been caused by factors such as medication, cardiogenic shock, or sepsis, (5) reduction of the hemoglobin level to 2 g/dL after obtaining 2 consecutive stable hemoglobin values or a decrease of <0.5 g/dL 3 h apart, and (6) a constant reduction in hemoglobin levels to >3 g/dL in 24 h, along with persistent melena or hematochezia [Citation14].
Statistics
We used SPSS (version 25.0) software to obtain random numbers and divide patients into two groups and for all data analyses. Data related to measurement and counts were expressed in terms of X ± s and percentages, respectively. Data related to measurement and counts for the two groups were compared by performing the t-test and the χ2 test, respectively.
Odds ratios (ORs) with confidence intervals (CIs) of 95% were calculated for evaluating the incidence of re-bleeding. We performed multivariate analyses to determine the predictive factors associated with re-bleeding by using the method of backwash elimination with variables such as p < .05 in univariate analyses and the risk factors previously reported for re-bleeding. A p value <.05 represents statistical significance for all statistical analyses.
Results
Patient characteristics
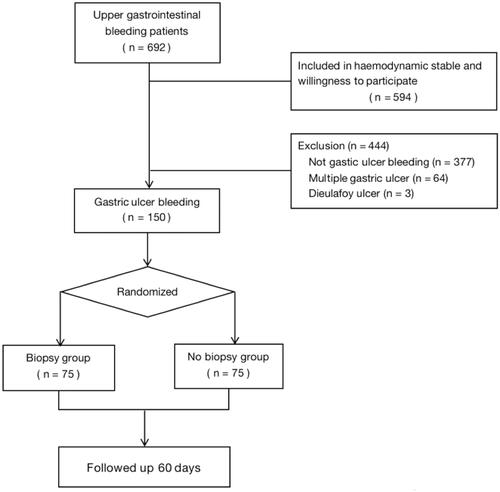
A total of 692 patients were considered for upper gastrointestinal bleeding, excluding 98 patients who were hemodynamically unstable or unwilling to participate in the trial. After excluding 377 patients without gastric ulcer bleeding, 64 patients with multiple gastric ulcers, and 3 patients with Dieulafoy ulcer, 150 patients eligible subjects were finally enrolled in the study. Of the total, 125 were men and 25 were women. We randomized these patients and assigned an equal number of patients to the biopsy and no biopsy groups (n = 75 for each group). No significant difference was detected between the groups in the baseline characteristics including age, sex, comorbidity, medication history, indication, Glasgow–Blatchford score, and vital signs (p > .05 for all), as shown in .
Table 1. Baseline Characteristics of the Patients.
The mean patients’ age in the no biopsy group was 56.8 ± 19.3 years, whereas that of patients in the biopsy group was 59.1 ± 17.7 years. Sixty-two (77.5%) of the patients were male, whereas 63 (84.0%) were male. shows the flowchart for this study. In no biopsy group, melena (n = 72), hematemesis (n = 17), syncope (n = 9), and hematemesis + melena (n = 72) were mainly attributable for patient admission.
In the biopsy group, the percentage of patients with ischemic heart disease, renal disease, postoperative gastric cancer, and liver cirrhosis was 11 (14.7%), 14 (18.7%), 5 (6.7%), and 5 (6.7%), respectively. Additionally, in the biopsy group, the number of patients using non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, clopidogrel, and warfarin or direct oral anticoagulants was 9, 13, 10, and 3, respectively.
Different correlations between parameters associated with endoscopy had a minor effect on ulcers in the two groups, as shown in . Ulcer distribution, size, and morphology in the two groups were approximately the same.
Table 2. Differences between ulcers in patients subjected or not subjected to a biopsy at initial gastroscopy.
Endoscopic examination in patients who underwent a biopsy indicated that 4 (5.3%), 8 (10.7%), 14 (18.7%), 10 (13.3%), 8 (10.7%), and 31 (41.3%) patients had lesions of Forrest grade Ia, Ib, IIa, IIb, IIc, and III, respectively. The distribution of ulcers was as follows: gastric corporeal ulcer, antral gastric ulcer, incisura ulcer, fundic ulcer, and anastomotic ulcers occurred in 19 (25.3%), 24 (32.0%), 17 (22.7%), 10 (13.3%), and 5 (6.7%) patients, respectively. Two (2.7%) patients received epinephrine spray, whereas 41 (54.7%), 6 (8.0%), and 15 (20.0%) patients received injection therapy, thermal coagulation, and mechanical therapy, respectively.
Re-bleeding occurred in 7 and 8 patients from the biopsy and no biopsy groups, respectively, during EG. In the biopsy group, 7 (9.3%) patients () exhibited re-bleeding. Of these, 5 and 1 patients underwent repeating endotherapy and laparotomy, respectively. The mean hospitalization duration and mortality rate for the patients were 11.68 ± 2.39 (minimum: 1, maximum: 22) days and 0% (n = 0), respectively. Helicobacter pylori infection occurred in 65 (86.7%) patients. The secondary end points for both the groups were significantly different. These included the need for repeated endoscopic interventions, hospitalization cost, and H. pylori infection incidence (p < .05) (). No statistically significant difference was detected in 60-day bleeding incidence and the duration of hospitalization between the two groups. We observed that there was no increase nosocomial bleeding (p = .27) and further bleeding (p = .12) in the no biopsy group compared with that in the biopsy group. The results of multivariate analysis revealed that no re-bleeding incidence occurred in the patients who underwent biopsy. We observed that the H. pylori infection incidence (p =.021) and cancer incidence (p = .019) differed significantly between the two groups.
Table 3. Primary and secondary endpoints.
Significant differences were observed in the incidence of ischemic heart disease and renal disease between patients exhibiting and not exhibiting recurrent bleeding (p < .001 for both) (). No significant difference was detected in the re-bleeding rates between the biopsy and no biopsy groups (p = .609).
Table 4. Univariate analyses for the risk factors linked to re-bleeding.
Factors linked to a high bleeding risk in the biopsy group included ischemic heart disease, renal disease, NSAID use, warfarin or direct oral anti-coagulant use, H. pylori infection, urea levels, creatine levels, and albumin (ALB) levels.
The descriptive statistics for the aforementioned risk factors are as follows: absence of ischemic heart disease (OR = 0.395 [0.24–0.65], p ≤ .005), renal disease (OR = 1.74 [0.77–1.59], p ≤ .005), NSAID use (OR = 2.828 [1.852–4.317], p ≤ .005), warfarin or direct oral anti-coagulant use (OR = 11.953 [3.494–39.460], p ≤ .005], creatine levels ≥265 mmol/L (OR = 9.308 [3.752–23.088], p ≤ .005), urea levels ≥12 mmol/L (OR = 1.028 [1.008–1.05], p ≤ .005), and ALB levels ≤ 30 g/L (OR = 1650.349, p ≤ .005). Significant factors identified using the multivariate analysis were ischemic heart disease, renal disease, and NSAID use or warfarin or direct oral anticoagulant use, as well as creatine levels ≥ 265 mmol/L, urea levels ≥ 12 mmol/L, and ALB levels ≤ 30 g/L (p < .05) ().
Table 5. Multivariate analysis.
The multivariate and univariate analyses indicated that the use of anti-thrombotic drugs was not a significant factor for rebleeding incidence. However, we observed that the risk of re-bleeding was significantly increased in patients who were using anticoagulants (p < .05).
Analysis of time to rebleeding
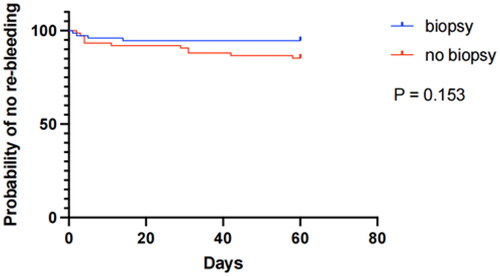
The relationship between the time of days and the end point of re-bleeding was investigated further. A KM curve for 60-day no rebleeding of patients subjected and not subjected to a biopsy has been shown in . No significant difference was detected in the re-bleeding probabilities between the biopsy and no biopsy groups (p = .153 by log-rank test).
Discussion
The prospective, randomized, controlled trial has been conducted for the first time to investigate the risk of re-bleeding from gastric ulcers via emergency endoscopic hemostasis and lesion biopsy. The trends in the incidence of gastric ulcer bleeding during emergency endoscopy and the association between the factors that cause gastric ulcer re-bleeding after a biopsy were investigated in this study. No significant difference was found in the re-bleeding risk in patients who were subjected or not subjected to a biopsy. Significantly lower hospitalization cost was associated with patients who underwent a biopsy during EG, compared with that for those who did not undergo a biopsy.
To achieve a pathological diagnosis of GI disease, it is crucial to acquire and process adequate levels of tissue samples during endoscopy. Two biopsies of the antrum and body of the stomach need to be performed for patients suspected to have H. pylori infections and for determining the staging of gastritis, according to the European Society for Gastrointestinal Endoscopy (ESGE) [Citation15] guidelines. However, no guidelines have specified whether patients with gastric ulcers should undergo a biopsy during EG. It is complicated to perform a biopsy during emergency endoscopic treatment of gastric ulcer hemorrhage, and clinicians mostly operate based on their experience. In clinical practice, biopsy is performed during repeated endoscopy, because of concerns that a biopsy during EG may increase the risk of hemorrhage.The British Society of Gastroenterology guidelines recommend that repeated gastroscopy and biopsy should be conducted for all gastric ulcers within 8 weeks [Citation16]. The Asia-Pacific Working Group [Citation17] recommends that the EGD re-examination should be conducted only if patients are at high risk of recurrent bleeding; hence, clinicians are recommended to perform a regular secondary endoscopic review, to enable them to intervene as early as possible during hemostasis and detect malignant ulcers at an early stage [Citation12]. However, low patient compliance results in a delayed diagnosis and worsening of the disease. Moreover, a previous study [Citation18]indicated that clinical outcomes were not improved by repeatedly performing endoscopy procedures for persistent GI malignancy-associated bleeding. Presently, there is a limited amount of data available regarding whether a biopsy of the gastric mucosa during EG is required in all patients with gastric ulcers. In addition, there is a lack of cases available for selection, and no established protocols are available for the management of ulcer biopsy. Therefore, we aimed to examine the clinical utility and safety of emergency endoscopic biopsy for the evaluation of bleeding from gastric ulcers.
Our follow-up data suggest that repeat gastroscopy is expensive and is associated with low patient compliance. Moreover, it adds to the difficulty associated with the treatment of a large patient population. This indicates that there is a lack of durable endoscopic therapies that could be used for the treatment of tumor-related bleeding events. In the present study, the pathological results for the biopsy group showed that malignancies had occurred in 14 patients. In one patient, the outcome was benign, although the lesion was considered malignant during emergency endoscopic treatment. In this study, secondary endoscopy was performed for only 42 (56%) patients in no biopsy group. Three patients with pathologic findings were found to exhibit malignant progression during the 60-day follow-up period. A possible reason for this difference is that it was not found without secondary endoscopic review, or that malignant patients in the non-biopsy group were older, making it difficult to review endoscopy, and the treatment goal for these patients is only to stop bleeding successfully. Fortunately, three patients were diagnosed and treated at an early stage. However, it is possible that malignant ulcers could develop at a later stage in 33 patients who did not undergo biopsy and endoscopic re-examination.
Acute upper GI bleeding that occurred directly from a tumor accounted for around 5% of all the upper GI bleeding cases [Citation19,Citation20]. Tumor bleeding is not considered a rare complication that can occur in patients with a tumor in their upper GI tract; instead, it is the leading cause of upper GI bleeding [Citation21,Citation22]. Based on the endoscopic examination of patients with tumor bleeding, the active bleeding rate of 21%–40% has been reported [Citation19,Citation20,Citation22], and the urgent hemostatic interventions (e.g., surgery, transarterial embolization, and endoscopic therapy) might be required control bleeding. However, the extent of tumor bleeding might not be high enough to induce hemodynamic instability [Citation23]. The findings of a recently conducted retrospective study by Zhao et al. [Citation24] showed that the risk of recurrent bleeding does not increase in patients with Forrest I acute non-variceal upper gastrointestinal bleeding and suspected malignant gastric ulcer who had undergone a biopsy during EG, as compared with those who had not undergone a biopsy. A retrospective study reported that chronic tumor bleeding occurred in approximately 50% of patients [Citation23]. Thus, it might be difficult to make decisions regarding urgent hemostasis-related interventions in patients with gastric cancer presented with upper GI bleeding. Young-Il Kim et al. [Citation25] carried out a retrospective study of 357 cases in South Korea and showed that the proportion of high-risk ulcers with Forrest IIa and IIb grade tumor hemorrhage in patients with inoperable gastric cancer was 34.5%. Unlike the study by Zhao et al. the inclusion criteria in the present study were not limited to patients with Forrest I grade malignancies.
The malignancy incidence identified by urgent endoscopy ranged from 0.8% to 5% [Citation26–29]. In a previous single-center retrospective study that was conducted for 6 years in the Netherlands, 225 (70%) out of 321 participants with gastric ulcers who underwent a repeat gastroscopy, malignancies were detected only in 5 (2%) patients during repeat procedures [Citation30]. Twenty-seven (6%) out of 432 gastric ulcer cases were found to be malignant in a multi-center retrospective study conducted in the United Kingdom [Citation27]. However, 25 (93%) of these cases were diagnosed after an initial gastroscopy and biopsy, with a low malignancy yield (0.9%) following repeat gastroscopy. Our results showed that the risk of re-bleeding did not increase in patients with gastric ulcers after they underwent a biopsy during the emergency treatment of bleeding via endoscopy (p = .077); however, it resulted in an increased rate of detection of malignant tumors (p = .019) and significantly different incidence of H. pylori infection (p = .021). During the acute-phase peptic ulcer (PU) hemorrhage, a gastric biopsy often results in false negative results in examinations for the detection of H. pylori infections, as stated in the Maastricht V/Florence consensus report [Citation31]. Wong et al. [Citation8] conducted a randomized, prospective, placebo-controlled study in China in 2003 and reported that eradicating H. pylori did not reduce the incidence of gastric cancer. By contrast, a prospective study by Susumu et al. [Citation32] involving 1342 patients showed that the eradication of H. pylori prevented gastric cancer. The role of eradication of H. pylori in preventing gastric cancer incidence has been debated. However, it is evident from the results of this study that if a patient with gastric cancer does not undergo a biopsy, the diagnosis could be delayed, which may lead to a poor prognosis. Generally, patients diagnosed at an earlier stage receive complete chemoradiation or surgical treatment.
Because biopsy is a low-risk procedure and the hemorrhage yields observed with and without biopsy are similar, an immediate biopsy is recommended because it allows benign and malignant ulcers to be diagnosed at an earlier stage and enables the detection of H. pylori infection. However, the available data regarding the factors related to re-bleeding risk after a biopsy is performed to examine bleeding from gastric ulcers are limited.
Previous reports have shown that factors associated with re-bleeding because of endoscopic hemostasis in peptic ulcers [Citation33–40] included the patient age, Forrest classification, blood transfusion, use of NSAIDs, and comorbidity indices. Yongkang Lai et al. [Citation36] performed a study in 386 patients and predicted that re-bleeding would occur in high-risk patients with peptic ulcers (PUs) bleeding within three days of emergent endoscopic hemostasis. Predictors of re-bleeding risk such as the ALB levels, prothrombin time, shock, hematemesis/melena, and Forrest grade were included in the nomogram. The model demonstrated good levels of discrimination and calibration, with the C-index of 0.854 (C-index: 0.830 via bootstrapping validation). However, none of these studies described biopsy as a technique that could be used for exploration. Here, we found that risk factors associated with a biopsy during EG included ischemic heart disease, renal disease, NSAID use, warfarin or direct oral anti-coagulant use, creatine levels ≥265 mmol/L, urea levels ≥12 mmol/L, and ALB levels ≤ 30 g/L. Our findings are consistent with those of a study by Yongkang Lai et al. [Citation36]
According to a previous report [Citation8], a re-bleeding rate of 66% in PUs could be observed in Japanese patients fin whom the bacterium H. pylori could not be eliminated. We found that re-bleeding occurred very rarely after the successful curing of H. pylori infection. Our study indicated that H. pylori infection is associated with re-bleeding risk but is not an independent risk factor; this finding is consistent with those of other studies [Citation41,Citation42].
In multivariate regression analysis, we found that using warfarin or oral anticoagulants was associated with an increasing risk of re-bleeding (OR = 11.953, CI: 3.494–39.460, p ≤ .005). A mucosal biopsy was reportedly performed without discontinuing anti-thrombotic drugs in Japan. Ono et al. [Citation43] conducted a prospective, observational study in which severe bleeding was noted within 2 weeks of biopsy and the bleeding time was determined post-biopsy. In this study, 101 mucosal biopsies were performed without the withdrawal of the anti-platelet or anti-coagulant drug; no significant bleeding was observed within 2 weeks of biopsy. In addition, no significant difference was observed in the duration of bleeding after the biopsy was conducted with an anti-platelet or anti-coagulant drug or with 2 or more drugs, and no significant difference was reported with the use of oral warfarin and non-oral warfarin. Fujita et al. [Citation44] and Ara et al. [Citation45] reported that the risk of bleeding was not increased after performing an endoscopic mucosal biopsy using oral anti-thrombotic drugs. However, none of these studies examined the use of biopsy as a technique that facilitated exploration. A biopsy does not increase the risk of bleeding; however, drug use has been linked to the factors associated with re-bleeding. The results of our univariate and multivariate analyses showed that the risk of recurrent bleeding was not increased in patients who underwent biopsy during EG and did not take anti-thrombotic drugs, as compared with those who did not use anti-thrombotic drugs. However, the risk of re-bleeding was significantly increased (p < .05) in patients who used anticoagulants.
It is important to acknowledge two main limitations of this study. First, the sample size was relatively small, and all participants were from the same research center; this might have resulted in a selection bias. Second, multi-center prospective studies need to be performed to verify the results of this study.
In conclusion, the use of gastroscopy with biopsy during EG as a method used for the examination and treatment of patients could effectively ensure the safety and reliability of the clinical treatment provided for gastric ulcer bleeding and reduce adverse effects. This novel approach can help clinicians avoid diagnostic delays and make decisions regarding the most suitable therapy, including oncological surgery, whenever feasible.
Ethical approval
This study was approved by the Ethics Committee of the 900th Hospital of PLA. Written informed consent was obtained from all participants (Decision No: 2020-003).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149(7):1731–1741.e3.
- Lanas A, García-Rodríguez LA, Polo Tomás M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104(7):1633–1641.
- Abougergi MS, Travis AC, Saltzman JR. The in-hospital mortality rate for upper GI hemorrhage has decreased over 2 decades in the United States: a nationwide analysis. Gastrointest Endosc. 2015;81(4):882–888.e1.
- Laine L, Yang H, Chang SC, et al. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012;107(8):1190–1195; quiz 1196.
- Hearnshaw SA, Logan RFA, Lowe D, et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60(10):1327–1335.
- Nahon S, Hagège H, Latrive JP, et al. Epidemiological and prognostic factors involved in upper gastrointestinal bleeding: results of a French prospective multicenter study. Endoscopy. 2012;44(11):998–1008.
- Laine L. Upper gastrointestinal bleeding due to a peptic ulcer. N Engl J Med. 2016;375(12):1198.
- Wong BC, Lam S K, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291(2):187–194.
- Bai Y, Du YQ, Wang D, et al. Peptic ulcer bleeding in China: а multicenter endoscopic survey of 1006 patients. J Dig Dis. 2014;15(1):5–11.
- Editorial board of Chinese Journal of Internal Medicinel, editorial board of Chinese Journal of Digestion, etc Guidelines for diagnosis and treatment of acute non-varicose upper gastrointestinal bleeding (2018, hangzhou). Chin J Int Med. 2019;58(3):173–180.
- Shiyan Z, Yunlin W. Attention must be paid to the pseudo-healing of malignant ulcer caused by proton pump inhibitor. Chin J Digest Med Imageol. 2019;9(6):241–243.
- Barkun AN, Almadi M, Kuipers EJ, et al. Management of nonvariceal upper gastrointestinal bleeding: guideline recommendations from the international consensus group. Ann Intern Med. 2019;171(11):805–822.
- Tielleman T, Bujanda D, Cryer B. Epidemiology and risk factors for upper gastrointestinal bleeding. Gastrointest Endosc Clin N Am. 2015;25(3):415–428.
- Laine L, Spiegel B, Rostom A, et al. Methodology for randomized trials of patients with nonvariceal upper gastrointestinal bleeding: recommendations from an international consensus conference. Am J Gastroenterol. 2010;105(3):540–550.
- Pouw RE, Barret M, Biermann K, et al. Endoscopic tissue sampling – Part 1: upper gastrointestinal and hepatopancreatobiliary tracts. European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2021;53(11):1174–1188.
- Beg S, Ragunath K, Wyman A, et al. Quality standards in upper gastrointestinal endoscopy: a position statement of the british society of gastroenterology (BSG) and association of upper gastrointestinal surgeons of great britain and Ireland (AUGIS). Gut. 2017;66(11):1886–1899.
- Sung JJ, Chiu PW, Chan FKL, et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67(10):1757–1768.
- Abu-Sbeih H, Szafron D, Elkafrawy AA, et al. Endoscopy for the diagnosis and treatment of gastrointestinal bleeding caused by malignancy. J Gastroenterol Hepatol. 2022;37(10):1983–1990.
- Savides TJ, Jensen DM, Cohen J, et al. Severe upper gastrointestinal tumor bleeding: endoscopic findings, treatment, and outcome. Endoscopy. 1996;28(2):244–248.
- Sheibani S, Kim JJ, Chen B, et al. Natural history of acute upper GI bleeding due to tumours: short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharmacol Ther. 2013;38(2):144–150.
- Maluf-Filho F, Martins BC, de Lima MS, et al. Etiology, endoscopic management and mortality of upper gastrointestinal bleeding in patients with cancer. United European Gastroenterol J. 2013;1(1):60–67.
- Schatz RA, Rockey DC. Gastrointestinal bleeding due to gastrointestinal tract malignancy: natural history, management, and outcomes. Dig Dis Sci. 2017;62(2):491–501.
- Imbesi JJ, Kurtz RC. A multidisciplinary approach to gastrointestinal bleeding in cancer patients. J Support Oncol. 2005;3(2):101–110.
- Zhao Q, Chi T. Biopsy in emergency gastroscopy does not increase the risk of rebleeding in patients with forrest I acute nonvariceal upper gastrointestinal bleeding combined with suspected malignant gastric ulcer: a multicenter retrospective cohort study. BMC Gastroenterol. 2021;21(1):250.
- Kim YI, Choi IJ, Lee JY, et al. Comparison of the performance of risk scoring systems for tumor bleeding in patients with inoperable gastric cancer. Endoscopy. 2020;52(5):359–367.
- Manas MD, Domper A, Albillos A, et al. Endoscopic follow-up of gastric ulcer in a population at intermediate risk for gastric cancer. Rev Esp Enferm Dig. 2009;101:317–324.
- Selinger CP, Cochrane R, Thanaraj S, et al. Gastric ulcers: malignancy yield and risk stratification for follow-up endoscopy. Endosc Int Open. 2016;4(6):E709–E714.
- Wan JJ, Fei SJ, Lv SX, et al. Role of gastroscopic biopsy of gastric ulcer margins and healed sites in the diagnosis of early gastric cancer: a clinical controlled study of 513 cases. Oncol Lett. 2018;16(4):4211–4218.
- Yang LS, Hartley I, Thompson AJ, et al. Evaluation of endoscopic practices and outcomes in follow-up of gastric ulcers. J Clin Gastroenterol. 2022;56(5):412–418.
- Gielisse EA, Kuyvenhoven JP. Follow-up endoscopy for benign-appearing gastric ulcers has no additive value in detecting malignancy: it is time to individualise surveillance endoscopy. Gastric Cancer. 2015;18(4):803–809.
- Capurso G, Annibale B, Osborn J, et al. Occurrence and relapse of bleeding from duodenal ulcer: respective roles of acid secretion and Helicobacter pylori infection. Aliment Pharmacol Ther. 2001;15(6):821–829.
- Take S, Mizuno M, Ishiki K, et al. The effect of eradicating helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100(5):1037–1042.
- Bitar SM, Moussa M. The risk factors for the recurrent upper gastrointestinal hemorrhage among acute peptic ulcer disease patients in Syria: a prospective cohort study. Ann Med Surg. 2022;74:103252.
- Xiaohua H. Correlation between endoscopic morphology and bleeding of gastric ulcer. J Healthc Eng. 2022;2022:2169551.
- Ponzetto A, Holton J. Risk of rebleeding after hemostasis for peptic ulcer. Dig Dis Sci. 2019;64(1):281–282.
- Lai Y, Xu Y, Zhu Z, et al. Development and validation of a model to predict rebleeding within three days after endoscopic hemostasis for high-risk peptic ulcer bleeding. BMC Gastroenterol. 2022;22(1):64.
- Cho SH, Lee YS, Kim YJ, et al. Outcomes and role of urgent endoscopy in High-Risk patients with acute nonvariceal gastrointestinal bleeding. Clin.Gastroenterol. Hepatol. 2018;16(3):370–377.
- Kubota Y, Yamauchi H, Nakatani K, et al. Factors for unsuccessful endoscopic hemostasis in patients with severe peptic ulcer bleeding. Scand J Gastroenterol. 2021;56(12):1396–1405.
- Romstad KK, Detlie TE, Søberg T, et al. Treatment and outcome of gastrointestinal bleeding due to peptic ulcers and erosions – (BLUE study). Scand J Gastroenterol. 2022;57(1):8–15.
- Fujishiro M, Iguchi M, Kakushima N, et al. Guidelines for endoscopic management of non-variceal upper gastrointestinal bleeding. Dig Endosc. 2016;28(4):363–378.
- Bernica J, Cole R, Flores A, et al. HP QI abstract: improving Helicobacter pylori testing in patients with acute upper GI bleeding due to peptic ulcer disease. Gastroenterology. 2020;159(2):e22–e23.
- Jiang F, Guo C, Cheung KS, et al. Long-term risk of upper gastrointestinal bleeding after Helicobacter pylori eradication: a population-based cohort study. Aliment Pharmacol Ther. 2021;54(9):1162–1169.
- Ono S, Fujishiro M, Kodashima S, et al. Evaluation of safety of endoscopic biopsy without cessation of antithrombotic agents in Japan. J Gastroenterol. 2012;47(7):770–774.
- Fujita M, Shiotani A, Murao T, et al. Safety of gastrointestinal endoscopic biopsy in patients taking antithrombotics. Dig Endosc. 2015;27(1):25–29.
- Ara N, Iijima K, Maejima R, et al. Prospective analysis of risk for bleeding after endoscopic biopsy without cessation of antithrombotics in Japan. Dig Endosc. 2015;27(4):458–464.