Abstract
Introduction
Novel therapeutic options have improved prognosis for patients with colonic liver metastases (CLM) over the last decades. Despite this, the challenge to select and stratify patients for optimal treatment regimen persists. This study aimed to evaluate established and novel histopathological features and investigate the impact on overall survival (OS) and recurrence in patients undergoing surgery for CLM.
Methods
Two hundred and sixty patients who underwent resection of CLM with curative intent 2006–2017 were included in the study. Clinicopathological characteristics were retrieved from patient medical records. The following histopathological parameters were investigated: vascular/lymphatic invasion, perineural invasion, tumor regression grade (TRG), tumor growth pattern, pseudocapsule and acellular mucin. Histopathological traits were correlated to OS.
Results
Vascular and lymphatic invasion, as well as perineural invasion, significantly correlated with an adverse prognosis hazard ratio (HR) = 1.7, 95% confidence interval (CI) 1.23–2.40 and HR = 1.7, 95% CI 1.20–2.51, respectively. Results retrieved from the study could not propose any novel explorative histopathological features (TRG, tumor growth pattern, pseudocapsule and acellular mucin) to be of significant value as comes correlation with patient OS.
Discussion
Classical histopathological characteristics of previously reported influence on survival were confirmed, while more novel factors that has been proposed, like tumor growth pattern, tumor regression and grade and presence of a pseudocapsule, were not. Further studies are thus needed to identify better ways of understanding the impact of tumor microenvironment and tumor biology on patient outcome and not at least for stratification and improved treatment response.
Introduction
Colorectal cancer (CRC) is a common disease and represents a leading cause of death in cancer worldwide. As of 2020, primary CRC is the third most common cause of cancer-related death globally and similar numbers are expected 2022 [Citation1,Citation2]. Over 50% of patients with CRC will develop colorectal liver metastases (CRLM) at some point during the course of their disease [Citation3]. Surgical resection is the only curative treatment option, but only 20% of patients with CRLM can undergo potentially curable surgery [Citation4]. Surgery is the preferred treatment option, but is only advised in patients where all tumor can be removed (R0 resection), and where functional residual liver volume yet can be maintained [Citation4–7]. In patients undergoing surgery, five-year survival average at least about 40% [Citation8–11]. Surgical mortality rates are less than 2% [Citation12,Citation13].
In addition to tumor resection, modern treatment of CRLM involve systemic chemotherapy, often in both neoadjuvant and adjuvant settings, as well as ablative therapies [Citation4–6,Citation14]. Correct selection of treatment (surgical approach and chemotherapy regimen) is critical to ensure optimal patient outcomes. The process involve both patient and tumor-derived factors [Citation6]. Therefore, patients undergo extensive preoperative evaluation, which is assessed by a multidisciplinary specialty team of medical providers [Citation6].
Even if survival of patients with CRLM has increased substantially over the last decades, we still lack tools for selecting patients who actually benefit from treatment [Citation6]. To enable stratification of patients and increase knowledge of factors that indicate a favorable or an adverse prognosis, risks scores have been developed [Citation15,Citation16]. In addition, histopathological examination of tumor specimens of both the primary CRC and the CRLM provides important information on postoperative outcomes. Current knowledge of factors influencing outcome does not provide precise information to guide patient health care for individual patients or subgroups of patients [Citation6]. The term personalized medicine has consequently evolved as a concept of offering patients optimal treatment regimens, often based on genetic and proteomic analysis [Citation17,Citation18]. Pathology is central in personalized medicine in cancer management, as detailed information from patient tissue samples can be used to identify subgroups of patients. Histopathological reports of CRLM generally focuses on a handful of factors that are believed to influence patient outcome. In CRLM, these factors are typically tumor size and resection margin. Vascular/lymphatic invasion and perineural invasion in the primary tumor are also accounted for as they present important prognostic information [Citation19,Citation20]. Additionally, numerous histopathological characteristics have been studied, such as tumor growth pattern, tumor regression grade (TRG) and presence of a pseudocapsule, though often in more limited patient series [Citation21–28]. The precise impact on patient outcome of these novel histopathological patterns is yet to be validated.
The overall goal of this study is to identify additional tools that are needed to make histopathological reports even more useful to match the patients tumor characteristics with adequate and prognostic information on the optimal treatment rather than the treatment that happens to be the generally recommended. This study aims to investigate both established and more novel explorative histopathological markers in resected colonic liver metastases (CLM) to identify factors that may influence on patient survival.
Methods
Patient selection and data collection
All patients undergoing liver resection of CRLM at Skåne University Hospital, Sweden, from 2006 to 2017 were identified. Patient characteristics were collected from patient medical records, as presented in . Patients with primary rectal cancers were excluded. Corresponding resected tumor specimen were assembled from Regionalt biobankscentrum, Södra sjukvårdsregionen, i.e., the regional biobank. Follow-up data was retrieved from patient medical records and PASiS. Date of death is imported into the PASiS program from the Swedish population register. Patients were eligible for this study if postoperative histopathological findings were consistent with metastasis from adenocarcinoma. Mucinous cancers (defined according to WHO criteria and local routines as cut off 50% extracellular mucin that contain malignant epithelium) were excluded to make the patient material as homogenous as possible [Citation29]. Patients that died shortly (within 14 days) after surgery due to postoperative complications were excluded. The manuscript is presented according to the STROBE guidelines [Citation30].
Table 1. Baseline characteristics of included patients collected from medical journals.
The following patient data were collected from the medical records: Date of surgery, age at liver resection, date of primary cancer diagnosis, date of CRC resection, gender, localization of primary tumor (right = proximal of splenic flexure, left = splenic flexure or more distal), TNM-stage (TNM8), R0 resection (defined as 1 mm margin or more), lymphovascular invasion in the primary tumor, perineural invasion in the primary tumor, synchronous/metachronous (synchronous defined as CRLM diagnosed within 12 months of primary cancer diagnosis), presence of lung metastases (at time of liver resection), number of liver metastases, largest liver tumor size, neoadjuvant chemotherapy (defined as chemotherapy within 3 months of hepatectomy, type of neoadjuvant chemotherapy), preoperative carcinoembryonic antigen (CEA), adjuvant chemotherapy (defined as chemotherapy within 3 months of hepatectomy, type of adjuvant chemotherapy), recurrence and date for recurrence or last follow-up for recurrence, death and date for death or last follow-up for death.
Histopathological analysis
Samples of tumorous tissue from resected liver metastases were fixed, paraffin-embedded and stained with hematoxylin and eosin. Sections were analyzed on slides by a senior pathologist. The pathologist was blinded for data collected from the medical records. Histopathological analysis included verification of malignant cells originating from the colon, exclusion of mucinous cancers and assessment of TRG, tumor growth pattern, fibrosis, necrosis, presence of pseudocapsule and presence of acellular mucin. TRG was classified according to AJCC/CAP using a 4-grade system: TRG 0 = no residual tumor cells; TRG 1 = single cells or small group of cells; TRG 2 = residual cancer with desmoplastic response; and TRG 3 = minimal evidence of tumor response [Citation31]. Tumor growth pattern were defined according to Vermeulen et al. [Citation32]. By this classification, three different growth patterns are identified. The desmoplastic growth pattern is characterized by no contact between tumor cells and hepatocytes, separated by fibrous tissue. In the pushing growth pattern, the tumor cells are separated from liver parenchyma by a thin layer of reticulin fibers and the liver cells are pushed aside. A replacing growth pattern is characterized by tumor cells replacing the liver parenchyma and with contact between tumor cells and hepatocytes. Mixed tumor growth pattern was used to classify tumors displaying more than one growth pattern. Representative material of described growth patterns are presented in . Presence of a pseudocapsule was defined as whether a fibrous band was present between the tumor cells and the surrounding hepatocellular parenchyma. Acellular mucin was defined as presence of mucin pools lacking neoplastic epithelium.
Statistical analysis
Statistical analyses were performed using R (3.5.2). The primary outcome was overall survival (OS) after surgery. Secondary outcome was recurrence after surgery. Values of hazard ratio (HR) were interpreted as the average for the studies interval. 95% confidence intervals were used in cox proportional hazard models. p values <.05 was considered statistically significant. Variables with p < .05 in univariate analysis were further studied in multivariate analysis.
Results
Participants
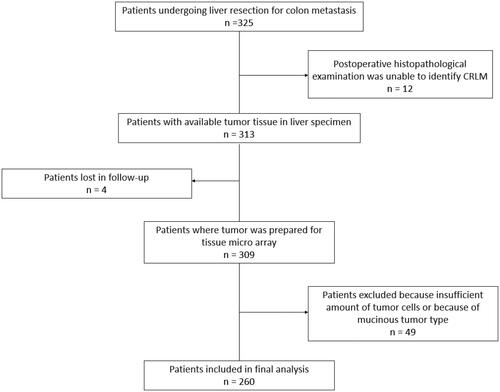
A total of 325 patients were identified that fulfilled the inclusion criteria, i.e., subjected to resection for CLM with the primary cancer located in the colon. Twelve patients undergoing neoadjuvant chemotherapy treatment were excluded as postoperative histopathological examination could not identify any residual tumor cells following neoadjuvant chemotherapy treatment (disappearing liver metastases). Four patients were lost for follow-up. A further 49 patients were excluded during the histopathological examination process, either because the specimen could not be collected from the biobank, because only single cells or a small group of cells remained in the resected specimen, or because the tumor were of the mucinous type. A total of 260 patients remained after all exclusion criteria were considered (). Baseline characteristics of included patients collected from the medical records are presented in . Survival data generated using Cox proportional hazard model are presented in . Recurrence data generated using Cox proportional hazard model are presented in .
Figure 2. Tumor growth patterns. (A) Pushing growth pattern. (B) Desmoplastic growth pattern. (C) Replacement growth pattern.
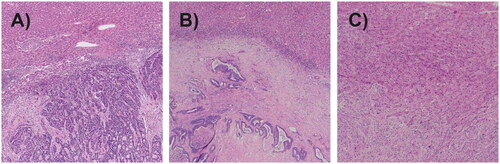
Table 2. Survival data generated by using Cox proportional hazard model.
The median patient age at the time of liver resection was 70 years (range 41–87). The study group consisted of 150 males (58%) and 110 females (42%). The ‘liver first’ approach was applied in 30 patients (11.5%), whereas 226 patients (87%) had their primary cancer resected before the liver resection. Furthermore, four patients (1.5%) underwent resection of colon cancer (CC) and CLM, simultaneously. One hundred and eighty-three (73%) patients received neoadjuvant chemotherapy and 184 patients (74%) had adjuvant chemotherapy. Median OS was 4.6 years. Median follow-up time was 5.1 years for individuals that did not die during follow-up. Median time to recurrence was 1.4 years. Median follow-up time was 3.8 years for recurrence free individuals.
Histopathological analysis
In primary tumors, lymphovascular invasion and perineural invasion were found in 90 (35%) and 64 (25%), respectively, of tumor specimen. Both were found to be statistically significant for OS by univariate analysis (HR = 1.7, 95% confidence interval [CI] 1.23–2.4 and HR = 1.7, 95% CI 1.2–2.51, respectively). Lymphovascular invasion was also significant for OS following multivariate analysis (HR = 1.6, 95% CI 1.07–2.25). Perineural invasion was not significant for OS by multivariate analysis. R0 resection was reported in 196 (88%) of cases but was not found to significantly associate with OS in univariate analysis (HR = 0.7, 95% CI 0.42–1.12). TRG 1 was found in 4 (2%), TRG 2 in 180 (69%) and TRG 3 in 76 (29%) of patients. When comparing TRG 2 and TRG 3, no statistically significant relation to survival was observed (HR = 0.85, 95% CI 0.59–1.24). Tumor growth pattern A was found in 25 (10%) tumors, B in 169 (65%) tumors and C in 63 (25%) tumors. Tumor growth pattern did not exhibit a statistically significant association with OS when comparing groups A to B (HR = 1.1, 95% CI 0.54–2.1), A to C (HR = 1.4, 95% CI 0.69–3.0) or B to C (HR = 1.3, 95% CI 0.9–1.92). Presence of a pseudocapsule was found in 35 (14%) of tumors. When comparing OS in cohorts divided by presence of pseudocapsule, no statistically significant correlation was found (HR = 0.95, 95% CI 0.59–1.55). Acellular mucin was detected in 44 patients (17%). No statistically significant correlation was found when comparing OS between patients where presence of acellular mucin was detected and in patients where no acellular mucin was present (HR = 1.4, 95% CI 0.92–2.14).
Histopathological factors found to significantly affect recurrence after surgery in univariate analysis was node positive primary tumor (HR = 2.0, 95% CI 1.35–2.86), perineural invasion (HR = 1.8, 95% CI 1.30–2.52) and vascular invasion (HR = 2.2, 95% CI 1.64–3.02). Of these, only vascular invasion was found to significantly affect recurrence in multivariate analysis ().
Discussion
In this study, with a large cohort of resected CLM, we utilized histopathological analysis to explore patterns in resected CLM that could possess a potential as prognostic parameters after colonic surgery. We found lymphovascular invasion and perineural invasion in CC to be independent markers for survival after liver resection. Lymphovascular invasion was also an independent marker for recurrence after surgery, while perineural invasion was not. No significant correlation to OS or recurrence were found in explorative histopathological factors like TRG, tumor growth pattern, presence of a pseudocapsule or acellular mucin. Only a limited number of studies have previously investigated these histopathological features in correlation to survival or recurrence (). To our knowledge, this is the first study to report the role of all these markers and to investigate their potential impact on survival.
Table 3. Recurrence data generated by using Cox proportional hazard model.
Table 4. Literature overview of studied histopathological markers.
Consistent with other studies, the results of our data propose lymphovascular invasion and perineural invasion in CC to be markers for survival and recurrence after liver resection. TRG is mainly used as a measurement of preoperative therapy response [Citation33]. Its influence on prognosis in CLM is less studied. We found no significant difference when comparing survival in TRG groups. Different scoring systems of TRG, variations in statistical methods and in patient cohorts between studies complicates comparisons between publications. Because of the significant differences between studies analyzing TRG, this marker was not meaningful to include in . Our investigation found no significant difference in survival between any tumor growth pattern. Prior studies of tumor growth pattern have yielded inconsistent results (). Interpretation of these studies are also somewhat difficult because of differences in terminology and classifications, highlighted by a recent systematic review [Citation24]. The meta-analysis was based on 33 studies including total of 4641 patients reported a very low certainty of evidence that tumor growth patterns (presented as ‘tumour borders’) impacted on survival [Citation24]. The same meta-analysis found three previous studies where the presence of a pseudocapsule in resected CRLM specimen were investigated in correlation to OS. The authors declare that absence of a pseudocapsule was associated with a decrease in OS, with moderate certainty due to risk of bias, inconsistency and imprecision amongst studies [Citation24]. This differs from our findings, where we found no relationship between absence or presence of a pseudocapsule on survival. Only one previous study could be identified that investigated the role of acellular mucin in CRLM [Citation34]. That study presents survival data as disease-specific survival for patients with primary tumors both from the rectum and colon. In similarity to our findings, the study could not identify acellular mucin to significantly impact on prognosis [Citation34].
Up to now, no effective tool is accessible to sub-categorize patients with CRLM based on biological markers. The continuously ongoing challenge of stratifying patients the best possible treatment for CLM still persists due to lack of information of the individual tumor. This study did not identify any novel histopathological factor to be of value in predicting prognosis after resection of CLM. Molecular transformation of CRC is described to involve key mutations (including oncogenes APS, KRAS, DDC and tumor suppressor p53); however, studies have shown that the traditionally reported mutation sequence only occurs in 10% of tumors [Citation18]. A variety of molecular mutation pathways could explain heterogeneity observed in biological studies and patient outcomes. There are data suggesting that CC and CLM has an advanced evolutionary relationship, as both the primary tumor and the metastatic lesions continue to accumulate mutations after that the metastatic processes have been initiated [Citation18]. Generalizability of biological conclusions of CRC to CRLM should though consequently be cautious. It is therefore important to not only investigate tumor biology of CRC, but also of the CRLM when future research of new biological traits is conducted. Of the well-studied biomarkers, most remain unavailable for clinical decision making. Difficulties in interpreting study results between biological markers and clinical outcomes arise as many studies are small and inter-study differences are substantial. Integration of pre-clinical biological data in CRLM with clinical outcomes in translational projects could, however, have a great potential of yielding meaningful results. Biological markers of CRLM may thus predict prognosis, predict response to therapy and potentially serve as targets for new treatments if effort is put into yielding more scientific data. This represents a great unmet need.
Some limitations in our study must be acknowledged. As histopathological samples were saved over a long time period, there is a risk of potential differences in material assessment. To counteract risks of variations in histopathological evaluation, all resected colonic tumor specimens of CLM were re-evaluated by a senior pathologist with extensive experience in analyzing malignancies from resected liver specimens. During the study period, it became gradually more common to use neoadjuvant chemotherapy, as well as the liver-first surgery approach. Most patients received 5-fluorouracil based chemotherapy both in the neoadjuvant and the adjuvant settings, but combination therapy with Oxaliplatin, Irinotecan or both became more frequent during the later period of the study. Combinational approaches of liver resection and local ablative procedures also became more frequent during the later period of the study which further limits interpretation of our study results.
Conclusion
The present study could not identify any novel explorative histopathological factors influencing postoperative survival or recurrence after resection of CLM. Further studies are needed to further investigate these findings. Effective markers depicting tumor biology and tumor microenvironment are warranted in order to provide a more personalized medicine approach. Such markers may tailor future management in an improved way, predicting prognosis and outcome both after surgery and chemotherapy. Proteogenomic studies of tumor specimens have a potential to define methods for patient risk stratification and novel therapies.
Ethical approval
This project has an ethical approval as of Swedish law (2003:460 §3-4). The research plan was tested and approved by the Regional Ethical Review Board of Lund University (2018/1113). Tumor specimens were collected after approval of collection from the regional biobank representatives, Regionalt biobankscentrum Södra sjukvårdsregionen (SC2879). The study was completed in accordance with the Helsinki Declaration of the World Medical Association. Management of data was in consistency with GDPR.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Zarour LR, Anand S, Billingsley KG, et al. Colorectal cancer liver metastasis: evolving paradigms and future directions. Cell Mol Gastroenterol Hepatol. 2017;3(2):163–173.
- Garden OJ, Rees M, Poston GJ, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55 Suppl 3(3):iii1–iii8.
- Berri RN, Abdalla EK. Curable metastatic colorectal cancer: recommended paradigms. Curr Oncol Rep. 2009;11(3):200–208.
- Chow FC, Chok KS. Colorectal liver metastases: an update on multidisciplinary approach. World J Hepatol. 2019;11(2):150–172.
- Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol. 2005;23(33):8490–8499.
- Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109(4):718–726.
- Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125–135.
- Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg. 2004;240(3):438–447; discussion 47–50.
- Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97(7):1110–1118.
- Mavros MN, de Jong M, Dogeas E, et al. Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg. 2013;100(5):711–718.
- Egeland C, Rostved AA, Schultz NA, et al. Morbidity and mortality after liver surgery for colorectal liver metastases: a cohort study in a high-volume fast-track programme. BMC Surg. 2021;21(1):312.
- Takahashi H, Berber E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg Nutr. 2020;9(1):49–58.
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309–318; discussion 18–21.
- Ansari D, Torén W, Andersson R. Primary lymph node ratio and hepatic resection for colorectal metastases. Hepatobiliary Surg Nutr. 2018;7(2):149–150.
- Ansari D, Torén W, Zhou Q, et al. Proteomic and genomic profiling of pancreatic cancer. Cell Biol Toxicol. 2019;35(4):333–343.
- Torén W, Ansari D, Andersson R. Immunohistochemical investigation of prognostic biomarkers in resected colorectal liver metastases: a systematic review and meta-analysis. Cancer Cell Int. 2018;18:217.
- Liebig C, Ayala G, Wilks J, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27(31):5131–5137.
- Lim SB, Yu CS, Jang SJ, et al. Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis Colon Rectum. 2010;53(4):377–384.
- Ben Slama S, Bacha D, Sassi A, et al. Pathological response of colorectal liver metastases treated after induction treatment. Tunis Med. 2017;95(10):854–858.
- Serrablo A, Paliogiannis P, Pulighe F, et al. Impact of novel histopathological factors on the outcomes of liver surgery for colorectal cancer metastases. Eur J Surg Oncol. 2016;42(9):1268–1277.
- Fonseca GM, de Mello ES, Faraj SF, et al. Prognostic significance of poorly differentiated clusters and tumor budding in colorectal liver metastases. J Surg Oncol. 2018;117(7):1364–1375.
- de Oliveira CVC, Fonseca GM, Kruger JAP, et al. Histopathological prognostic factors for colorectal liver metastases: a systematic review and meta-analysis of observational studies. Histol Histopathol. 2020;36(2):159–181.
- Berardi G, De Man M, Laurent S, et al. Radiologic and pathologic response to neoadjuvant chemotherapy predicts survival in patients undergoing the liver-first approach for synchronous colorectal liver metastases. Eur J Surg Oncol. 2018;44(7):1069–1077.
- Serayssol C, Maulat C, Breibach F, et al. Predictive factors of histological response of colorectal liver metastases after neoadjuvant chemotherapy. World J Gastrointest Oncol. 2019;11(4):295–309.
- Eefsen RL, Vermeulen PB, Christensen IJ, et al. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metastasis. 2015;32(4):369–381.
- Nielsen K, Rolff HC, Eefsen RL, et al. The morphological growth patterns of colorectal liver metastases are prognostic for overall survival. Mod Pathol. 2014;27(12):1641–1648.
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188.
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–S34.
- Langer R, Becker K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch. 2018;472(2):175–186.
- Vermeulen PB, Colpaert C, Salgado R, et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001;195(3):336–342.
- Ryan R, Gibbons D, Hyland JMP, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47(2):141–146.
- Poultsides GA, Bao F, Servais EL, et al. Pathologic response to preoperative chemotherapy in colorectal liver metastases: fibrosis, not necrosis, predicts outcome. Ann Surg Oncol. 2012;19(9):2797–2804.
- Okano K, Yamamoto J, Kosuge T, et al. Fibrous pseudocapsule of metastatic liver tumors from colorectal carcinoma. Clinicopathologic study of 152 first resection cases. Cancer. 2000;89(2):267–275.
- Wiggans MG, Shahtahmassebi G, Malcolm P, et al. Extended pathology reporting of resection specimens of colorectal liver metastases: the significance of a tumour pseudocapsule. HPB. 2013;15(9):687–694.
- Yamamoto J, Shimada K, Kosuge T, et al. Factors influencing survival of patients undergoing hepatectomy for colorectal metastases. Br J Surg. 1999;86(3):332–337.
- Van den Eynden GG, Bird NC, Majeed AW, et al. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin Exp Metastasis. 2012;29(6):541–549.