Abstract
Background
Remote ischemic preconditioning (RIPC) is reported to reduce ischemia-reperfusion injury (IRI) in many vital organs by inhibiting a systemic inflammatory response. Inflammation also plays an essential role in the pathophysiology of prolonged post-operative ileus (PPOI) in patients undergoing colorectal cancer (CRC) surgery. However, the role of RIPC is unclear in reducing the incidence of PPOI in patients undergoing CRC surgery.
Methods
This was a prospective, randomized trial of RIPC vs. placebo-controlled in patients undergoing elective laparoscopic CRC surgery. Eighty patients were randomized to either a RIPC group or a control group (40 per arm), with computer-generated randomization. The aim was to determine whether RIPC improved the recovery of gut function. The primary outcomes assessed were time to gastrointestinal tolerance and incidence of PPOI.
Results
Median time to stool of the RIPC group was significantly lower than that of the control group [RIPC vs. control, 4.0 (3.0, 6.0) vs. 5.0 (4.0, 7.8) days, p = 0.027]. Median time to gastrointestinal tolerance and incidence of PPOI in the RIPC group were lower than the control group; however, there were no statistical differences between the two groups [RIPC vs. control: 5.0 (3.0, 7.0) vs. 6.0 (4.0, 8.8) days, p = 0.178; 15 vs. 30%, p = 0.108].
Conclusion
RIPC could shorten the median time to stool in patients undergoing laparoscopic CRC surgery, but did not improve the overall recovery time of gut function or reduce the incidence of PPOI.
Registration number
ChiCTR2100043313 (http://www.chictr.org.cn).
Question: In patients undergoing laparoscopic CRC surgery, does RIPC improve time to the overall recovery of gut function and reduce the incidence of PPOI?
Findings: In this randomized clinical trial that included 80 patients undergoing elective laparoscopic CRC surgery, no significant difference was found between the RIPC group and the control group concerning median time to gastrointestinal tolerance and incidence of PPOI.
Meaning: RIPC did not improve the time for overall recovery of gut function or reduce the incidence of PPOI in patients undergoing laparoscopic CRC surgery.
Key points
Introduction
Post-operative ileus (POI) is a predominant cause of delayed hospital discharge following elective colorectal cancer (CRC) surgery [Citation1,Citation2]. From the perspective of pathophysiology, POI includes an early neuromodulation stage and a late inflammatory reaction stage [Citation3]. The neuromodulation stage appears early and lasts for a short time, often lasting only a few hours after surgery. The inflammatory phase appears ∼3–6 h after the procedure begins, but can last several days. Prolonged post-operative ileus (PPOI), when POI lasts four days or more, is one of the most common post-operative complications of CRC surgery [Citation3–5]. The incidence of PPOI is ∼3–32% in colonic or rectal surgery [Citation3,Citation6]. Notably, more than 50% of patients with PPOI develop further related complications [Citation4]. As a result, the post-operative hospital stay for patients with PPOI is approximately doubled, and the inpatient economic burden significantly increased [Citation7]. The health care costs are estimated to be 1.4–1.5 billion dollars annually for PPOI prevention and control in the USA alone [Citation4,Citation8]. Therefore, reducing the incidence of PPOI in clinical practice would have great social and economic benefits.
One of the critical problems with PPOI is that few effective preventative measures or treatments exist which would alleviate the healthcare burden [Citation9]. Remote ischemic preconditioning (RIPC) refers to the prevention or reduction of ischemia-reperfusion injury (IRI) of the target organ by performing multiple transient ischemia-reperfusion (IR) cycles of distal tissues or organs, far from the target organ [Citation10,Citation11]. Recent evidence suggests that RIPC can activate the autonomic nervous system through interactions between nerves, body fluids, and systemic pathways, stimulating the production of anti-inflammatory protective factors. The stimulation inhibits the systemic inflammatory response and produces anti-oxidative stress outcomes, protecting many vital organs (including the heart, liver, kidney, brain, and lungs) from IRI [Citation11–15].
The inflammatory response is considered to play a critical role in the pathophysiological mechanism of PPOI [Citation16]. Currently, no clinical study has investigated whether RIPC can reduce the incidence of PPOI in CRC surgery. This study is a randomized controlled trial aiming to investigate whether RIPC can improve the overall recovery time of gut function, and reduce the incidence of PPOI in patients undergoing elective laparoscopic CRC surgery.
Methods
A prospective, randomized clinical trial was undertaken to investigate whether RIPC improves the overall recovery time for gut function and reduces the incidence of PPOI. The study was approved by the Research Ethical Committee of the Yongchuan Hospital of Chongqing Medical University. Written informed consent was obtained from all patients and their families involved in the trial. Patients and their families did not participate in this study’s design or implementation. The trial was registered prospectively at the Chinese Clinical Trial Registry (http://www.chictr.org.cn, ChiCTR2100043313). There was no change to the trial methods following commencement.
Patients
The study included 80 patients undergoing elective laparoscopic CRC surgery between December 2020 and June 2022. Inclusion criteria were: (1) patients undergoing laparoscopic CRC surgery under general anesthesia; (2) ASA grade of I–III; and (3) age 45–75 years. Exclusion criteria were: (1) ASA grade of IV or higher; (2) patients with active inflammatory bowel disease; (3) patients receiving pre-operative intravenous nutrition (IVN); (4) moderate-to-severe renal impairment or severe hepatic impairment; (5) patients scheduled for enterostomy; (6) gut dysmotility due to endocrine, cardiovascular and cerebrovascular, or neurological causes system diseases; (7) language difficulties, delirium or cognitive impairment; and (8) vascular disease affecting the implementation of RIPC.
Patients were recruited consecutively and randomized to the control or RIPC group by study investigators not involved in patient care. On the afternoon before the surgery, patients were randomized into each group sequentially, in a 1:1 allocation, and numbered sequentially. Participants and clinical staff were all blinded to the assigned treatment regimen. Patients undergoing elective laparoscopic colorectal surgery at Yongchuan Hospital of Chongqing Medical University follow a structured enhanced recovery after surgery (ERAS) protocol. This protocol includes pre-operative oral carbohydrate drinks, early introduction of a post-operative diet, multimodal analgesia and opiate use, early removal of drainage tubes or catheters, and early patient activity or subcutaneous thromboprophylaxis.
It should be noted that if there is no choking, nausea, vomiting, abdominal distension, or dizziness 2 h post-operatively, the patient should try to drink water and try to resume oral eating within 24 h after surgery. A combination of enteral and parenteral nutrition is also recommended, anticipating that oral and enteral feeding will remain at <50% of the calculated caloric requirement for >7 days. Any sedative medication was prohibited before surgery. Passive mobilization was required 2–4 h after surgery, and ambulatory attempts were allowed on the first post-operative day. The multimodal analgesic protocol of this study was Local Wound Infiltration (LWI) plus patient-controlled intravenous analgesia (PCIA) with weak opioids. Patients were operated on following LWI with 40 ml 0.33% ropivacaine after the closure of the anterior peritoneum, and local anesthetic agents were injected to infiltrate subcutaneous and muscle layers of the incision. The formula of PCIA contained tramadol 800 mg, ketorolac tromethamine 90 mg, and dexamethasone 5 mg, all of which were dissolved in saline to form a 100-ml solution. The loading and bolus dose of PCIA was set as 2 ml with an infusion rate of 1 ml/h, and the locking time was set at 15 min. For breakthrough pain, the patients received the loading dose of the PCIA pump either with or without ketorolac tromethamine 30 mg intravenously and PCIA pump parameters were adjusted when necessary.
Intervention
All patients did not receive pre-operative medication. The electrocardiogram, arterial pressure, oxygen saturation, temperature, and bispectral index were routinely monitored after entering the surgical theatre. Anesthesia was induced using midazolam (0.05 mg/kg), etomidate (0.2 mg/kg), sufentanil (0.3 μg/kg), and rocuronium bromide (0.6 mg/kg). After endotracheal intubation, it was maintained with intravenous pumping of propofol 4–8 mg kg−1h−1 and sufentanil 0.3–0.4 μg kg−1h−1, and inhalation of sevoflurane 2%. During the operation, rocuronium bromide (0.15 mg/kg, 40–50 min intervals) was injected intermittently to maintain muscle relaxation. End-expiratory carbon dioxide was managed as 35–45 mmHg, and a bispectral index was maintained at 40–50. The comprehensive fluid management was integrated according to blood loss, urine volume, and arterial blood pressure. The intraoperative fluid replacement protocol was 1:1 of compound sodium chloride and a polygelatin peptide. A blood transfusion was considered when hemoglobin levels were <70 g/L, vasopressors were considered when the mean arterial pressure was <60 mmHg, and furosemide was used when the urine volume was <1 ml kg−1h−1.
Patients in the RIPC group received RIPC for a total of about 30 min (three cycles of 5 min of ischemia and 5 min reperfusion in the upper limb with cuff pressure at 26.7 kPa [200 mm Hg]) before the start of surgery. An effective intervention program was defined as when the distal radial artery pulse oxygen saturation could not be measured. Patients in the control group received sham RIPC for ∼30 min (the cuff was not inflated). Finally, the study participants included 80 patients (51 males and 29 females). The same team of anesthesiologists and surgeons performed all the operations.
Demographics and data collection
The demographic characteristics of the patients were collected, including age, sex, body mass index (BMI), ASA grade, comorbidities, and intraoperative data. Fixed researchers blind-evaluated patients twice daily (at 8:00 and 20:00) until the patient reached gastrointestinal tolerance. If the patient did not reach gastrointestinal tolerance at discharge, the time was determined by telephone. Primary and secondary outcome measures, blood biochemical data, and complications were recorded on pre-prepared data collection tables. The primary outcomes of this study were to investigate the time taken for gastrointestinal tolerance and the incidence of PPOI. Secondary outcomes included: time to flatus, time to stool, time to diet, post-operative duration of hospital stay, nasogastric tube (NGT) required, intravenous nutrition (IVN) required, results of blood biochemical data, and post-operative complications.
All patients were resuscitated in the post-anesthesia recovery unit after surgery. Specifically, gastrointestinal tolerance was defined as transanal or stoma defecation and oral dietary tolerance. In contrast, oral dietary tolerance was defined as the ability to eat a solid or semisolid diet of 25% or more of the pre-operative intake without significant nausea or vomiting over two or more consecutive meals [Citation4]. PPOI was diagnosed if patients met two or more of the following conditions on or after post-operative day 4: inability to tolerate the oral diet over the past 24 h, nausea or vomiting, without flatus over the past 24 h, abdominal distension or radiological evidence of intestinal distension without mechanical intestinal obstruction [Citation17]. Pre-operative and post-operative blood biochemical test results from days 1 to 5 included: white blood cell (WBC), central granulocyte percentage, tumor necrosis factor-α (TNF-α) levels, and C-reactive protein (CRP) levels. The post-operative complications were recorded using the Clavien–Dindo classification system and included: nausea or vomiting, abdominal distension, anastomotic leakage, new pulmonary infection, poor wound healing, cognitive dysfunction, unplanned reoperation, and 30-day readmission rate.
Statistical analysis
A prior power calculation was performed using retrospective data from 60 patients who underwent laparoscopic CRC surgery in the center during the past 6 months. The pilot data showed a mean (s.d.) time to gastrointestinal tolerance of 6.1 (1.8) days. The statistical significance level was set at 0.05, with a study power of 0.9. The hypothesis being investigated is that RIPC would reduce the time to gastrointestinal tolerance by a clinically meaningful 25%. The PASS software (version 11.0; NCSS, LLC) was used to assign a 1:1 two-tailed test and calculated that 31 patients were required in each group. An expected 20% drop-out rate was assumed, with 80 patients recruited.
Intentionality analysis was conducted using the SPSS statistical software (version 26.0; IBM Corp., Armonk, NY, USA). Data were assessed for normal distribution using the Shapiro–Wilk test. Measurement data with normal distributions were expressed as mean ± standard deviation (SD). Normally distributed data were analyzed using one-way analysis of variance (ANOVA) or repeated measures ANOVA and the LSD t-test was used within the group. Measurement data with skewed distributions were expressed as medians (range) and compared between groups using the Mann–Whitney U test. The categorical data were expressed as rates or constituent ratios and the χ2 test was used. Two-sided p-values <0.05 were deemed significant.
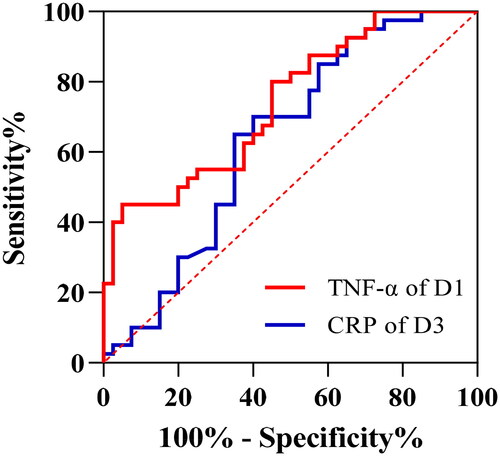
In response to peer review, the receiver operating characteristic curve from TNF-α values obtained in patients undergoing elective laparoscopic colorectal surgery was performed post-hoc to differentiate the RIPC group and the control group. The area under the receiver operating characteristic curve (95% CI) was calculated and the optimal cutoff was defined by maximization of the Youden index. The same analysis was performed for CRP and the areas under the receiver operating characteristic curve were compared using DeLong’s method. Meanwhile, a multivariate analysis was also conducted to identify if any of the outcome factors were associated with the intervention after the results of the univariable analysis.
Results
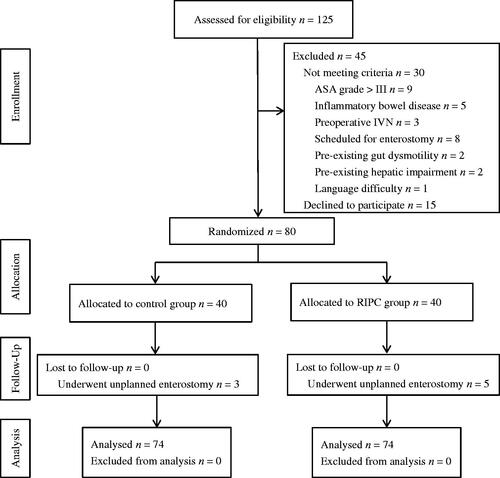
One hundred and twenty-five patients were evaluated for eligibility to participate, with 45 being excluded. Eighty patients were randomized into two groups, with 40 participants per group (). All subjects completed data collection, and no patients were lost to follow-up. Three patients in the control group and five in the RIPC group underwent unplanned enterostomy procedures (7.5 and 12.5%, respectively). However, according to the study protocol, all eight patients were included in the intention-to-treat analysis.
Figure 1. CONSORT diagram for the trial. CONSORT: CONsolidated Standards Of Reporting Trials; ASA: American Society of Anesthesiologists; RIPC: remote ischemic preconditioning.
Demographic data
No significant differences were observed between the two groups in patient characteristics, pre-operative blood biochemical results, and intraoperative data ().
Table 1. Demographic data for the eighty participants.
Outcome indicators
The median time to gastrointestinal tolerance and incidence of PPOI in the RIPC group were lower than in the control group. However, there were no statistical differences between the two groups [RIPC vs. control: 5.0 (3.0, 7.0) vs. 6.0 (4.0, 8.8) days, p = 0.178; 15 vs. 30%, p = 0.108] (). The median time to stool within the RIPC group was significantly lower than the control group [RIPC vs. control, 4.0 (3.0, 6.0) vs. 5.0 (4.0, 7.8) days, p = 0.027] (). However, there were no statistical differences in the median time to flatus, time to diet, post-operative duration of hospital stay, NGT required, IVN required, and post-operative complications (all p > 0.05) ().
Table 2. Results of intention-to-treat analysis.
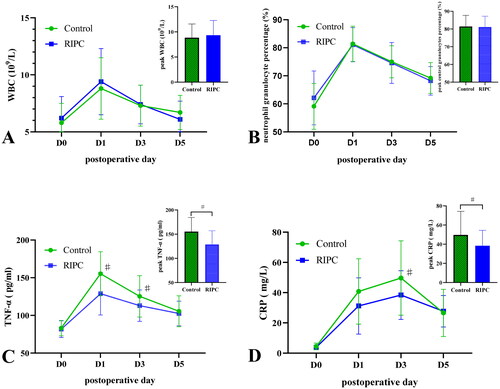
There were no significant differences in WBC, neutrophil granulocyte percentage, TNF-α levels, and CRP levels between the control and RIPC groups pre-operatively on day 1 (all p > 0.05) (). The peak WBC, neutrophil granulocyte percentage, and TNF-α occurred post-operatively on day 1. The peak CRP occurred post-operatively on day 3. There were no statistical differences in peak WBC and neutrophil granulocyte percentage between the two groups (F = 0.649, p = 0.423; F = 0.034, p = 0.855) (), but the peak TNF-α and CRP in the RIPC group were significantly lower than the control group (F = 16.943, p < 0.001; F = 7.514, p = 0.009) (). Repeated experimental ANOVA results showed that TNF-α levels had significant interaction (F = 17.076, p < 0.001) and main treatment effects (F = 54.910, p < 0.001) between the control and RIPC groups. TNF-α levels in the RIPC group were lower than the control group post-operatively on days 1 and 3 (F = 16.943, p < 0.001; F = 5.149, p = 0.026) (). CRP levels had significant interaction (F = 3.433, p = 0.033) and main treatment effects (F = 7.375, p = 0.010) between the control and RIPC groups, and CRP levels in the RIPC group were lower than the control group post-operatively on day 3 (F = 7.514, p = 0.009) (). For WBC and neutrophil granulocyte percentages, there was no significant interaction (F = 2.336, p = 0.114; F = 1.920, p = 0.130), and main treatment effects (F = 0.443, p = 0.052; F = 0.126, p = 0.725) between the control and RIPC groups, but a significant time effect (F = 70.783, p < 0.001; F = 163.148, p < 0.001) was demonstrated ().
Figure 2. Blood biochemical data. The midpoints and error bars showed mean and SDs. RIPC: remote ischemic preconditioning; WBC: white blood cell; TNF-α: tumor necrosis factor-α; CRP: C-reactive protein; D0: pre-operative day 1; D1: post-operative day 1; D3: post-operative day 3; D5: post-operative day 5. Repeated measures analysis of variance was used and multiple comparisons were analyzed after Bonferroni correction and compared with the control group, ♯p < 0.013.
The receiver operating characteristic curves of the TNF-α and the CRP levels between the control and RIPC groups are shown in . There was no significant difference in the area under the receiver operating characteristic curve (95% CI) between TNF-α levels of post-operative day 1 and CRP levels of post-operative day 3 [0.741 (0.634–0.847) vs. 0.637 (0.513–0.761); p = 0.206]. The best TNF-α cutoff value for identifying the control and RIPC groups was 118.3 pg/ml [sensitivity, 0.450 (0.307–0.602); specificity, 0.950 (0.835–0.991)]. The best CRP cutoff value for identifying the control and RIPC groups was 46.8 mg/L [sensitivity, 0.700 (0.546–0.819); specificity, 0.600 (0.446–0.737)].
Per-protocol analysis
A per-protocol, subgroup analysis was performed that excluded eight patients who underwent an unplanned enterostomy, including three (7.5%) patients in the control group and five (12.5%) patients in the RIPC group. The results were consistent with the intention-to-treat analysis results. The time to stool of patients in the RIPC group was shorter than the control group [4.0 (3.0, 6.0) vs. 5.0 (4.0, 6.5) days, p = 0.021] but they did not have a shorter time to gastrointestinal tolerance [5.0 (3.0, 6.0) vs. 5.0 (4.0, 7.5) days, p = 0.122], post-operative duration of hospital stay [9.0 (8.0, 9.0) vs. 9.0 (7.0, 10.3) days, p = 0.055], or a lower incidence of PPOI (14.3 vs. 26.5%, p = 0.208). Consistently, TNF-α levels of the RIPC group were significantly lower than the control group post-operatively on day 1 (129.3 ± 27.6 vs. 156.1 ± 29.2 pg/ml, p < 0.001).
Analysis of patients with chronic gastrointestinal diseases or not
A post-hoc analysis was performed including only patients with chronic gastrointestinal diseases (34 in the control group, 35 in the RIPC group). The time to stool of patients in the RIPC group was shorter than in the control group [4.0 (3.0, 6.0) vs. 5.0 (4.0, 8.0) days, p = 0.006]. However, there were no differences in the time to gastrointestinal tolerance [5.0 (3.0, 7.0) vs. 6.0 (4.0, 9.3) days, p = 0.055] and post-operative hospital stay [9.0 (8.0, 9.0) vs. 9.0 (7.0, 10.3) days, p = 0.055]. The incidence of PPOI (control vs. RIPC, 26.5 vs. 14.3%, p = 0.208) was similar in the two groups. The TNF-α levels in the RIPC group were significantly lower than in the control group post-operatively on day 1 (124.7 ± 26.9 vs. 150.7 ± 28.7 pg/ml, p < 0.001). However, there were no differences in the time to flatus, time to diet, NGT/IVN required, and post-operative complications (all p > 0.05).
An analysis of patients without chronic gastrointestinal diseases (six in the control group, five in the RIPC group) showed no difference between control and RIPC groups in time to stool [6.0 (4.0, 8.5) vs. 4.0 (3.8, 5.8) days, p = 0.240], time to gastrointestinal tolerance [7.0 (4.5, 10.0) vs. 5.0 (3.5, 6.0) days, p = 0.251], post-operative hospital stay [9.0 (7.5, 10.0) vs. 9.0 (8.5, 11.0) days, p = 0.058], the incidence of PPOI (50 vs. 20%, p = 0.545), the TNF-α on post-operative day 1 (157.8 ± 20.4 vs. 181.2 ± 16.3 pg/ml, p = 0.064), or the CRP on post-operative day 3 (48.0 ± 24.4 vs. 38.6 ± 16.9 mg/L, p = 0.050). There was also no difference between the control and RIPC groups in the time to flatus, time to diet, NGT/IVN required, and post-operative complications (all p > 0.05).
Multivariable analysis of the association between the control and RIPC groups
After univariate analysis of the results, all outcome measures suitable for multivariate analysis were analyzed to determine whether they were associated with the intervention. The results of the multivariate analysis were similar to those of the protocol analysis. The median time to stool was decreased by one day (F = 4.230, p = 0.043) in the RIPC group compared to the control group. The TNF-α of post-operative day 1 and day 3 in the RIPC group were significantly lower than in the control group (F = 16.943, p < 0.001; F = 5.149, p = 0.026). The CRP of post-operative day 3 in the RIPC group was also significantly lower than in the control group (F = 5.905, p = 0.017).
Discussion
After CRC surgery, neurogenic and inflammatory factors interact to promote the occurrence and development of PPOI [Citation2,Citation16,Citation18]. Clinically, the inhibitory effect of gastrointestinal peristalsis is not only manifested in the local intestine but also has a general inhibitory effect on the whole digestive tract. The mechanism is closely related to the intestinal inflammatory reaction and the activation of inhibitory neural pathways [Citation19,Citation20]. The mechanism of RIPC organ protection also involves the interaction of neural, humoral, and systemic pathways [Citation11]. Studies investigating whether RIPC demonstrates a gut protective effect on PPOI caused by post-operative intestinal inflammation of CRC surgery have not been found. This prospective, randomized, placebo-controlled trial found that, compared with control patients, RIPC did not improve time to gastrointestinal tolerance or reduce the incidence of PPOI in patients undergoing elective laparoscopic CRC surgery. Although RIPC shortened the median time to stool and reduced the post-operative TNF-α and CRP levels, there were no significant improvements in time to flatus or diet tolerance. RIPC did not shorten the post-operative duration of hospital stays but was found to be safe and did not increase the rate of PPOI, post-operative complications, or NGT/IVN required.
RIPC significantly improved the median time to stool by one day and reduced post-operative TNF-α levels among patients without chronic gastrointestinal disease who underwent laparoscopic CRC surgery. There is a potential advantage of RIPC during laparoscopic surgery for patients without a chronic gastrointestinal disease. However, it neither significantly improved gut function recovery nor shortened the post-operative hospital stay. These outcomes were derived from a post-hoc analysis, not part of the original statistical analysis plan. However, most patients in this study did not suffer from a chronic gastrointestinal disease (86.3%), so there is sufficient data to suggest that these results may be clinically meaningful. Intestinal inflammation plays a vital role in the development of PPOI, delaying the recovery of gut function. The intestinal inflammatory response of patients without chronic gastrointestinal diseases is significantly lower than patients with chronic gastrointestinal diseases. Therefore, the increased intestinal inflammatory response caused by chronic gastrointestinal diseases may have led to the ineffectiveness of RIPC in patients with chronic gastrointestinal diseases.
The highlight of this study is that RIPC treatment occurs 30 min before surgery, so RIPC is effective during surgery when the intestinal inflammatory response commencement is mediated by nerve and inflammatory factors. Although there is no measured indicator of RIPC adequacy, applying RIPC in three cycles of 5 min of ischemia and 5 min of upper limb reperfusion with a cuff pressure at 26.7 kPa (200 mm Hg) resulted in clinically significant outcomes. Notably, the definition of effective RIPC in the upper limb was that the distal radial artery pulse oxygen saturation could not be measured, which adequately assured the effectiveness of RIPC intervention.
The present study does contain some limitations. Firstly, the study has a single-center design, which may have led to uncertainty of selection bias. Secondly, in the post-hoc analysis, patients with chronic gastrointestinal diseases accounted for only 13.7% of the total study subjects. Further studies are planned to evaluate the clinical efficacy of RIPC in patients with and without chronic gastrointestinal diseases. Finally, although three patients in the control group and 5 in the RIPC group were excluded during the study, the authors do not believe this would have affected the primary outcomes, as the proportion was lower than the 20% dropout rate set by the study. The results of the pre-protocol subgroup analysis (excluding patients who underwent unplanned enterostomy) were consistent with the intention-to-treat analysis.
One feature for consideration is that multiple factors, including demographic characteristics and past medical history, may increase the risk of developing PPOI in laparoscopic CRC surgery patients [Citation6,Citation21]. In a study of determining pre-operative risk factors associated with the development of POI in CRC surgery patients, Watkins et al. [Citation22] found that women were at lower risk of POI compared to men (p = 0.013; RR 0.56; 95% CI 0.36–0.89). Additionally, all previous smokers had a higher risk of POI than lifetime non-smokers (p = 0.0069; RR 1.78; 95% CI 1.17–2.70). In a recent series [Citation23,Citation24], being male and having a smoking history have been identified as independent risk factors for PPOI. It is still unclear whether these patients can benefit from RIPC and further well-designed multicenter studies are necessary.
Another feature for consideration is that the standardized propofol-based anesthetic technique used in all groups may inhibit the role of RIPC. Cho et al. [Citation25] found that the cardioprotective effects of limb RIPC were eliminated under propofol or sevoflurane anesthesia. Although, there is no direct evidence that propofol can block the effects of RIPC [Citation26]. Several clinical trials showed a neutral effect of RIPC on outcomes after hepatectomy, cardiac surgery, and intestinal resection [Citation27–29]. Most patients received propofol anesthesia during RIPC, and the role of propofol was considered to be the main factor leading to the results obtained. This may be due to the protective effect of propofol against ischemia-reperfusion injury as it inhibits NADPH oxidase-mediated oxidative stress after mast cell activation [Citation28]. However, these issues are beyond the scope of the present study and should be investigated in further studies.
In conclusion, the study outcomes show that RIPC did not significantly improve the recovery of intestinal function or reduce the incidence of PPOI following laparoscopic CRC surgery. POI/PPOI remains a significant problem for medical staff and patients after CRC surgery [Citation30,Citation31]. Although RIPC can significantly shorten the time to stool and reduce the post-operative TNF-α and CRP levels in patients undergoing laparoscopic CRC surgery, due to the multiple risk factors for PPOI, more studies are needed to evaluate the clinical application of RIPC.
Ethical approval
The Ethics Committee of Yongchuan Hospital of ChongQing Medical University has reviewed the project (Approval number: 2020.89). The project was undertaken following the codes of practice and the Declaration of Helsinki.
Author contributions
Xiuming Yang helped design the study, acquire the data and biological samples, ensure data were accurate and complete, and draft the manuscript. Chun Tian helped design the study, acquire the data and biological samples, interpret the data, and review the manuscript. Yuansong Gao and Liu Yang helped acquire the data, analyze the statistics, and interpret the data. You Wu and Na Zhang helped acquire the data and supervise the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Namba Y, Hirata Y, Mukai S, et al. Clinical indicators for the incidence of postoperative ileus after elective surgery for colorectal cancer. BMC Surg. 2021;21(1):80.
- Sommer NP, Schneider R, Wehner S, et al. State-of-the-art colorectal disease: postoperative ileus. Int J Colorectal Dis. 2021;36(9):2017–2025.
- Chapman SJ, Pericleous A, Downey C, et al. Postoperative ileus following major colorectal surgery. Br J Surg. 2018;105(7):797–810.
- Milne T, Liu C, O'Grady G, et al. Effect of prucalopride to improve time to gut function recovery following elective colorectal surgery: randomized clinical trial. Br J Surg. 2022;109(8):704–710.
- Wolthuis AM, Bislenghi G, Fieuws S, et al. Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta-analysis. Colorectal Dis. 2016;18(1):O1–O9.
- Quiroga-Centeno AC, Jerez-Torra KA, Martin-Mojica PA, et al. Risk factors for prolonged postoperative ileus in colorectal surgery: a systematic review and meta-analysis. World J Surg. 2020;44(5):1612–1626.
- Traeger L, Koullouros M, Bedrikovetski S, et al. Cost of postoperative ileus following colorectal surgery: a cost analysis in the Australian public hospital setting. Colorectal Dis. 2022;24(11):1416–1426.
- Peters EG, Pattamatta M, Smeets BJJ, et al. The clinical and economical impact of postoperative ileus in patients undergoing colorectal surgery. Neurogastroenterol Motil. 2020;32(8):e13862.
- Mao H, Milne TGE, O'Grady G, et al. Prolonged postoperative ileus significantly increases the cost of inpatient stay for patients undergoing elective colorectal surgery: results of a multivariate analysis of prospective data at a single institution. Dis Colon Rectum. 2019;62(5):631–637.
- Bell RM, Basalay M, Bøtker HE, et al. Remote ischaemic conditioning: defining critical criteria for success-report from the 11th hatter cardiovascular workshop. Basic Res Cardiol. 2022;117(1):39.
- Donato M, Bin EP, V DA, et al. Myocardial remote ischemic preconditioning: from cell biology to clinical application. Mol Cell Biochem. 2021;476(10):3857–3867.
- Wu J, Yu C, Zeng X, et al. The hepatoprotective effect from ischemia-reperfusion injury of remote ischemic preconditioning in the liver related surgery: a meta-analysis. ANZ J Surg. 2022;92(6):1332–1337.
- Zheng L, Han R, Tao L, et al. Effects of remote ischemic preconditioning on prognosis in patients with lung injury: a meta-analysis. J Clin Anesth. 2020;63:109795.
- Mollet I, Marto JP, Mendonça M, et al. Remote but not distant: a review on experimental models and clinical trials in remote ischemic conditioning as potential therapy in ischemic stroke. Mol Neurobiol. 2022;59(1):294–325.
- Liu Z, Zhao Y, Lei M, et al. Remote ischemic preconditioning to prevent acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Front Cardiovasc Med. 2021;8:601470.
- Docsa T, Sipos A, Cox CS, et al. The role of inflammatory mediators in the development of gastrointestinal motility disorders. Int J Mol Sci. 2022;23(13):6917.
- Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. J Gastrointest Surg. 2013;17(5):962–972.
- Mallesh S, Schneider R, Schneiker B, et al. Sympathetic denervation alters the inflammatory response of resident muscularis macrophages upon surgical trauma and ameliorates postoperative ileus in mice. Int J Mol Sci. 2021;22(13):6872.
- Schledwitz A, Xie G, Raufman JP. Exploiting unique features of the gut-brain interface to combat gastrointestinal cancer. J Clin Invest. 2021;131(10):e143776.
- Hellstrom EA, Ziegler AL, Blikslager AT. Postoperative ileus: comparative pathophysiology and future therapies. Front Vet Sci. 2021;8:714800.
- Hain E, Maggiori L, Mongin C, et al. Risk factors for prolonged postoperative ileus after laparoscopic sphincter-saving total mesorectal excision for rectal cancer: an analysis of 428 consecutive patients. Surg Endosc. 2018;32(1):337–344.
- Watkins EL, Schellack N, Abraham V, et al. Men and those with a history of smoking are associated with the development of postoperative ileus following elective colorectal cancer resection at a private academic hospital in Johannesburg, South Africa: a retrospective cohort study. Front Surg. 2021;8:667124.
- Koch KE, Hahn A, Hart A, et al. Male sex, ostomy, infection, and intravenous fluids are associated with increased risk of postoperative ileus in elective colorectal surgery. Surgery. 2021;170(5):1325–1330.
- Cribb B, Kollias V, Hawkins R, et al. Increased risk of complications in smokers undergoing reversal of diverting ileostomy. ANZ J Surg. 2021;91(10):2115–2120.
- Cho YJ, Nam K, Kim TK, et al. Sevoflurane, propofol and carvedilol block myocardial protection by limb remote ischemic preconditioning. Int J Mol Sci. 2019;20(2):269.
- Benstoem C, Goetzenich A, Stoppe C. The role of propofol for remote ischaemic preconditioning in the setting of cardiac surgery – a cochrane systematic review. Br J Anaesth. 2017;119(6):1234–1235.
- Cho YJ, Nam K, Yoo SJ, et al. Effects of remote ischemic preconditioning on platelet activation and reactivity in patients undergoing cardiac surgery using cardiopulmonary bypass: a randomized controlled trial. Platelets. 2022;33(1):123–131.
- Gan X, Xing D, Su G, et al. Propofol attenuates small intestinal ischemia reperfusion injury through inhibiting NADPH oxidase mediated mast cell activation. Oxid Med Cell Longev. 2015;2015:167014.
- Teo JY, Ho AFW, Bulluck H, et al. Effect of remote ischemic preConditioning on liver injury in patients undergoing liver resection: the ERIC-LIVER trial. HPB. 2020;22(9):1250–1257.
- Lin Z, Yang C, Wang Y, et al. Comparison of prolonged postoperative ileus between laparoscopic right and left colectomy under enhanced recovery after surgery: a propensity score matching analysis. World J Surg Oncol. 2022;20(1):68.
- Buscail E, Deraison C. Postoperative ileus: a pharmacological perspective. Br J Pharmacol. 2022;179(13):3283–3305.