Abstract
Objectives
Meticulous inspection of the mucosa during colonoscopy, represents a lengthier withdrawal time, but has been shown to increase adenoma detection rate (ADR). We investigated if artificial intelligence-aided speed monitoring can improve suboptimal withdrawal time.
Methods
We evaluated the implementation of a computer-aided speed monitoring device during colonoscopy at a large academic endoscopy center. After informed consent, patients ≥18 years undergoing colonoscopy between 5 March and 29 April 2021 were examined without the use of the speedometer, and with the speedometer between 29 April and 30 June 2021. All colonoscopies were recorded, and withdrawal time was assessed based on the recordings in a blinded fashion. We compared mean withdrawal time, percentage of withdrawal time ≥6 min, and ADR with and without the speedometer.
Results
One hundred sixty-six patients in each group were eligible for analyses. Mean withdrawal time was 9 min and 6.6 s (95% CI: 8 min and 34.8 s to 9 min and 39 s) without the use of the speedometer, and 9 min and 9 s (95% CI: 8 min and 45 s to 9 min and 33.6 s) with the speedometer; difference 2.3 s (95% CI: −42.3–37.7, p = 0.91). The ADRs were 45.2% (95% CI: 37.6–52.8) without the speedometer as compared to 45.8% (95% CI: 38.2–53.4) with the speedometer (p = 0.91). The proportion of colonoscopies with withdrawal time ≥6 min without the speedometer was 85.5% (95% CI: 80.2–90.9) versus 86.7% (95% CI: 81.6–91.9) with the speedometer (p = 0.75).
Conclusions
Use of speed monitoring during withdrawal did not increase withdrawal time or ADR in colonoscopy.
ClinicalTrials.gov Identifier
NCT04710251
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death and the third most common cancer worldwide [Citation1]. Colonoscopy is one of the most commonly used screening tests for prevention and early detection of CRC [Citation2].
High adenoma detection rates (ADR) are associated with low risk of CRC after colonoscopy. Therefore, ADR is a key performance indicator in colonoscopy [Citation3]. Withdrawal time has been shown to be a surrogate indicator of ADR [Citation4]. A withdrawal time of at least 6 min is recommended to maintain high ADR and thus high-quality colonoscopy. A recent publication showed that withdrawal time even longer than 6 min and up to 13 min might increase ADR [Citation5]. However, there is still considerable variation between colonoscopists in withdrawal time and suboptimal withdrawal time below 6 min [Citation6–8].
We investigated the implementation of a novel artificial intelligence (AI)-aided speedometer that monitors the withdrawal speed and warns the colonoscopist whenever a pre-defined ‘speed limit’ is exceeded in real time during colonoscopy [Citation9–11]. To our knowledge, there is no study which have investigated the specific role of a speedometer in achieving high-quality colonoscopy [Citation9–12].
Material and methods
Study design and oversight
We conducted a prospective clinical implementation trial at the Center for Advanced Endoscopy at Beth Israel Deaconess Medical Center (BIDMC), Boston, USA. The study was approved by the local institutional review board and registered at ClinicalTrials.gov (NCT04710251). We obtained written informed consent from all participants before enrolment.
Between 5 March 2021 and 29 April 2021, all participants were examined without the use of a speedometer (pre-implementation period). The speedometer was implemented on 29 April 2021, and until 30 June 2021, participants were examined with the use of the speedometer (post-implementation period). Nine participants had colonoscopy without the use of the speedometer in the post-implementation period because two endoscopists (out of the nine who participated in the study) joined the study later than the others.
Study population
All patients aged 18 years or older scheduled for colonoscopy at the study center were eligible for enrollment. Exclusion criteria were known CRC present before colonoscopy, hereditary colorectal polyposis, inflammatory bowel disease or history of colorectal resection.
Data management
Data obtained in the study were registered using REDCap electronic data capture tools hosted at Beth Israel Deaconess Medical Center [Citation13,Citation14]. The registered data included patient age, sex and ethnicity, indication for colonoscopy, quality of bowel preparation assessed by the Boston bowel preparation scale (BBPS) [Citation15] cecum intubation, insertion time, withdrawal time, polypectomy (yes/no), number of adenomas and complications.
Colonoscopy procedures
Preparations and performance of colonoscopy, including bowel preparation, pre-procedure assessment and sedation practices followed ordinary clinical routines at the study center. Colonoscopies were performed using Olympus CLV 180-series or CLV 190-series colonoscopes. Patients underwent sedation at the discretion of the colonoscopist, using either a combination of benzodiazepine and opioids or propofol, both under supervision of a trained anesthesiologist.
Patients received bowel preparation with either 2-liter or 4-liter polyethylene glycol-based preparations, or sodium sulfate-based preparations, or a 30-ounce magnesium citrate-based preparation was used. All bowel preparations were administered as split doses, with the first half of the prescribed bowel preparation in the evening before the procedure and the second half in the morning of the procedure, according to current clinical practice at the study center.
The participating colonoscopists performed all colonoscopies according to clinical routines at the study center. One observer (IB) was present during all colonoscopy procedures during the study to register data and activate the speedometer. The same group of colonoscopists enrolled patients in both the pre- and post-implementation periods. Study colonoscopists were all board-certified endoscopists. As the study center is a major teaching hospital for endoscopy training, trainees participated in some procedures under direct supervision by the study colonoscopists. The exact involvement of trainees in every colonoscopy procedure was not recorded. We did not offer any pre-trial training of the speedometer before implementation.
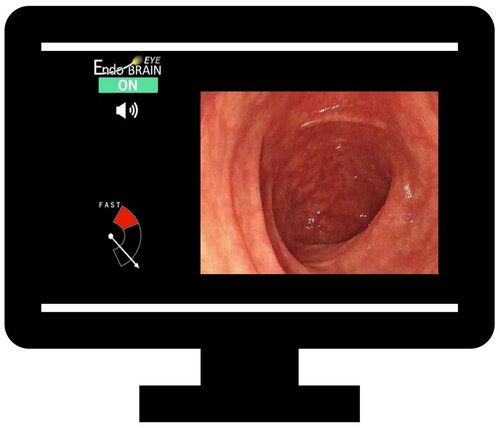
The speedometer
For withdrawal time monitoring, we used a speedometer device developed by Cybernet System Corporation (Tokyo, Japan). This device was developed and validated in Japan (unpublished data). Because morphology of the colonic mucosa has not been shown to differ between ethnic groups, we believe that the device also produces valid measurements for the North-American population studied in this trial [Citation16].
The algorithm of the speedometer is based on the Lucas–Kanade method [Citation17] a differential method for optical flow estimation that combines information from several nearby pixels in a picture, and estimates which direction an object moves so that local changes in intensity can be measured. This allows measurement of the relative speed of withdrawal during colonoscopy (Supplementary Video).
The speedometer was deployed through a high-specification computer, which was connected to the endoscopy processor. In the trial, the observer present during the whole colonoscopy activated the speedometer immediately after cecum intubation. The device was programmed to activate an acoustic alarm whenever the withdrawal speed exceeded a predefined threshold (). This threshold can be set anywhere from level 1 to 20, with level 1 being the lowest level for alarm and level 20 being the highest level for alarm. For this study we chose level 12, which was the optimal alarm threshold based on unpublished data from both Japan and Norway.
Trial endpoints
The primary endpoint of the trial was difference in withdrawal time between colonoscopies performed with and without the speedometer. Secondary outcome measures included ADR of the participating endoscopists on group level and the proportion of colonoscopies with withdrawal time ≥6 min.
Endpoint assessments
All colonoscopy procedures in the trial (pre- and post-implementation) were video-recorded. Before analysis, all video recordings were edited by removing all sound and recording dates to mask whether the speedometer was used. After editing, the videos were given to an independent research assistant who kept a scrambling key containing the information about which video recording corresponded to which study period (pre- or post-implementation). This information was not available to the research team and blinded the endpoint assessors to whether the speedometer had been used.
Two independent, blinded assessors (one experienced endoscopist (IB) and one physician who received pre-trial training on colonoscopy videos (TW)) assessed all recordings and registered insertion time, withdrawal time, bowel preparation using BBPS, number of polyps detected and complications.
Withdrawal time was measured as the duration from the identification of the base of the cecum until exit from the rectum. Withdrawal time often include time spent on maneuvers such as biopsy and polypectomy during the withdrawal phase [Citation7]; however, we subtracted time spent on biopsy and polypectomy performed during the withdrawal phase of the examination in order to obtain the true withdrawal time [Citation9–11]. All polyps were submitted to histopathology. The histopathology diagnosis was used to categorize polyps into adenomas, nonadenomas or cancer. Any complication during the procedure was registered by the observer present during the colonoscopy procedures.
After completion of the video assessments and registration of the data, the database was locked to prevent modification on 29 November 2021. The endpoint assessors provided the annotated data, and they were merged with the group labels (pre- or post-implementation) for analyses.
Sample size calculation
Our sample size rationale was based on two sources of information: firstly, a randomized trial from China [Citation9] which showed a withdrawal time difference between standard colonoscopies and colonoscopies with a speedometer in a combination with a blind spot detector was 1.6 min, with an SD of 2.5 min, and secondly unpublished data collected from 125 colonoscopy cases performed by expert colonoscopists at the study center, which revealed a withdrawal time of 5.9 min with an SD of 4.8 min. Because the Chinese trial [Citation9] used a nonparametric test to compare withdrawal time between the groups, we assumed that time distribution was not normal, and chose to account for that with ± 5% to our sample size estimates.
Thus, with an SD of 4.8 min in withdrawal time in both groups, statistical power of 80%, an alpha of 5% and no intra-cluster correlation coefficient among colonoscopists, the required sample size was 299 patients in total. Expecting a 10% drop out, we planned to enroll 332 patients; 166 patients in the pre-implementation period and 166 patients in the post-implementation period.
Statistical analysis
All analyses were based on modified intention-to-treat analyses, defined as patients with a complete colonoscopy (cecum intubated) and video recording technically assessable. Data were presented as frequencies and proportions for categorical variables and medians and interquartile ranges (IQR) for continuous variables. Histograms were used to assess the distribution of the variables. Normally distributed variables (age) were compared using t test. Variables with non-normal or ordinal distribution (insertion time, number of adenomas) were compared using nonparametric Wilcoxon’s test. Chi-square test or exact Fisher’s tests were used to compare the proportions.
For the main endpoint (withdrawal time), mean and 95% confidence intervals were reported. In sensitivity analysis, we used multivariable linear regression models with the withdrawal time as dependent variable to adjust for imbalance in baseline characteristics between the pre-implementation period (without speedometer) and post-implementation period (with speedometer). Only variables for which a significant difference between the groups was observed were included in the model. We calculated adjusted withdrawal time assuming mean value of the confounders. We defined statistical significance if p < 0.05. All p values are two-sided. All analyzes were performed using Stata version 16.0 (Texas, USA).
Results
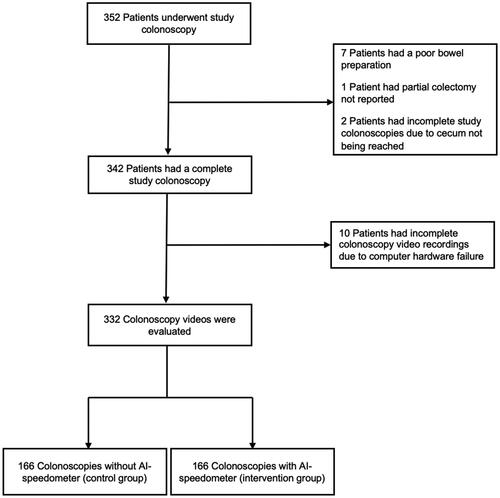
Out of 352 patients who consented to participate in the trial () and underwent colonoscopy during the trial period from 5 March 2021 to 30 June 2021, 342 patients were eligible for analyses, while 10 patients were excluded; 7 due to poor bowel preparation (too poor quality to complete the colonoscopy), 1 because of colonic resection and 2 due to cecum intubation failure.
Out of the 342 eligible patients, 10 patients had incomplete colonoscopy recordings due to computer hardware failure. Thus, 332 patients were included in the analyses; 166 without the speedometer and 166 with the speedometer. All colonoscopies were performed by 9 colonoscopists.
The median patient age was 61 years, and 53% were women (). The indication for colonoscopy was polyp surveillance in 48%, screening colonoscopy in 40% and clinical signs or symptoms in 11%. The median colonoscopy insertion time was 6.2 min (IQR 4.1–9.2) (). We did not observe any complications related to the colonoscopy.
Table 1. Baseline characteristics of the included patients and detected polyps.
Table 2. Withdrawal time without and with AI-speedometer in minutes.
Table 3. ADR and proportion of exams with >6 min withdrawal time (95% CI) without and with AI-speedometer.
Speedometer effects
Mean withdrawal time was 9 min and 6.6 s (9.11 min) (95% CI: 8 min and 34.8 s to 9 min and 39 s) without the use of the speedometer, and 9 min and 9 s (9.15 min) (95% CI 8 min and 45 s to 9 min and 33.6 s) with the speedometer, for a difference of 2.3 s (95% CI: 42.3–37.7, p = 0.91) ( and Supplementary Figures 1 and 2).
We found a significant difference in the distribution between men and women enrolled in the pre-implementation period and the post-implementation period (58.4% men and 41.6% women vs. 47% men and 53% women, p = 0.04) (). Thus, we performed sensitivity analyses to adjust for this, but found no differences to the outcomes (see Supplementary Tables 3–6).
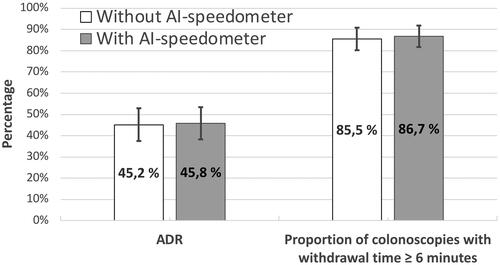
ADRs were 45.2% (95% CI: 37.6–52.8) without the speedometer as compared to 45.8% (95% CI: 38.2–53.4) with the speedometer (p = 0.91) ().
The proportion of colonoscopies with withdrawal time ≥6 min without the speedometer was 85.5% (95% CI: 80.2–90.9) versus 86.7% (95% CI: 81.6–91.9) with the speedometer (p = 0.75) ().
The mean number of adenomas per colonoscopy was 0.71 (95% CI: 0.553–0.857) without the speedometer versus 0.73 (95% CI: 0.577–0.881) with the speedometer (p = 0.83) (Supplementary Figure 3).
One endoscopist (endoscopist no 1) performed almost one-third of the colonoscopies (). Thus, we performed sensitivity analyses (see Supplementary Tables 1–6) to adjust for this by looking at the results separately for endoscopist no 1 and the rest of the endoscopists combined (endoscopist no. 2-9). Endoscopist no. 1 had >1 min longer withdrawal time with AI than without AI which was a significant difference (p = 0.02), but we found no differences to the overall outcomes (Supplementary Table 1) and no differences were observed for the other variables (Supplementary Table 2).
Discussion
Missed adenomas are the main contributing factors for interval CRC after colonoscopy screening [Citation18,Citation19]. Standardized minimum withdrawal times have been shown to correlate with improved adenoma detection. A speedometer for withdrawal time monitoring could help in maintaining an ideal withdrawal speed even more consistently throughout the duration of the procedure, and thus reduce both recognition errors of polyps and exposure errors of colorectal surface during colonoscopy. However, our study found no benefit from using the speedometer to increase withdrawal time. Although several clinical trials have investigated the effectiveness of the combined use of speedometer and blind-spot detection AI tools, no previous studies have investigated the isolated impact of a speedometer [Citation9–12].
Several factors may explain the discordant results between our trial and other studies that have evaluated speedometers. First, our study was performed at a teaching hospital with colonoscopies performed by endoscopists with different experience levels. Unfortunately, we did not have detailed information about the level of experience (number of performed colonoscopies) for each participating endoscopist. A similar study from China [Citation9] involving less experienced endoscopists with only 1–3 years of endoscopy training found a significant withdrawal time difference with the use of a combined device (p < 0.0001). Thus, by involving only nonexpert endoscopists, the difference in withdrawal time might be larger. Additionally, in US centers, where colonoscopy is part of national screening guidelines, withdrawal time is often already proactively recorded, which may encourage standardization of withdrawal time compared to countries where there is no national colonoscopy screening program.
Another factor that distinguishes our study from the previously RCTs is the sole use of speedometer. The previous RCTs evaluated two or more AI technologies at the same time. Three randomized trials investigated the combined use of speedometer and computer-aided detection [Citation9–11]. Two studies also used a blind spot detector in addition [Citation9,Citation11,Citation12]. Two trials showed a significant increase in withdrawal time [Citation9,Citation10] and the trial which showed the largest increment in withdrawal time, included a blind spot detector [Citation9]. Sole use of the speedometer in our study may have resulted in no difference in withdrawal time.
The Hawthorne effect may have also contributed to our findings [Citation20]. Since our trial design was nonblinded and an observer was present during procedures in both the pre- and post-intervention period, operational bias might have influenced the result. Withdrawal time in unmonitored endoscopists has been shown to be shorter compared to withdrawal time in endoscopists that are aware of the monitoring, and ADR increases with the awareness of monitoring [Citation6]. Trials that evaluated the efficiency of AI-devices with a blinded design could address this bias.
One of the concerns with a new clinical device is the potential for distraction [Citation21]. An audible alarm from the speedometer, especially if it goes off frequently, may interrupt the endoscopist’s focus leading to errors. Finding the right threshold level is about finding the optimal sensitivity level, one that allows the endoscopists to alter their withdrawal speed, without making them feel distracted by the alarm itself. One of the effects of having the speed set to 1–11 is the possibility of a high false-alert rate, because the endoscopists deem the alarm to be too sensitive and disregard it. As a consequence of this, the endoscopists may not alter their withdrawal speed, but instead feel distracted. Having the speed set to level 13–20, as opposed to 1–11, could lead to an alarm that is too infrequent and thus not allowing the endoscopists enough chances to alter their withdrawal speed. However, more studies are needed to assess whether level 1–11 on the speedometer could increase the proportion of colonoscopies with a withdrawal time ≥6 min or increase ADR. Our results show that there was no difference in performance between these two groups and thus there is no evidence to suggest that withdrawal time monitoring causes distraction during colonoscopy.
Additional sensitivity analysis performed (Supplementary Table 7 and Supplementary Figure 4) showed that the change in withdrawal time was not correlated with the change in ADR neither with nor without AI.
In conclusion, we found no significant increment in withdrawal time difference, ADR or proportion of colonoscopies with withdrawal time ≥ 6 min by comparing standard colonoscopy and colonoscopy performed with a speedometer.
Supplemental Material
Download MS Word (116.8 KB)Disclosure statement
I.B. has received a travel grant from Olympus Norway and has received consultant fees from AbbVie; M.M. is an associate editor at Digestive Endoscopy and has received consultant fees and speaking honoraria from Olympus Corp, and has ownership interest with Cybernet Corporation.; M.B. has received consultant fees from Cybernet Systems Corp. Y.M. has received consultant fees and speaking honoraria from Olympus Corp and has ownership interest with Cybernet Corporation.; T.M.B. has received consultant fees from Wision AI, Fujifilm, Magentiq Eye, and Medtronic; All other authors have nothing to disclose.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3):209–249.
- Helsingen LM, Vandvik PO, Jodal HC, et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a clinical practice guideline. BMJ. 2019;367:l5515.
- Michal Kaminski AF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) quality improvement initiative. Endoscopy. 2017;49(04):378–397.
- Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 362(19):1795–1803.
- Desai M, Rex DK, Bohm ME, et al. Impact OF withdrawal time ON adenoma detection rate: results from a prospective, multi-center trial. Gastrointest Endosc. S0016-5107(22)02039-9.
- Vavricka SR, Sulz MC, Degen L, et al. Monitoring colonoscopy withdrawal time significantly improves the adenoma detection rate and the performance of endoscopists. Endoscopy. 2016;48(3):256–262.
- Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355(24):2533–2541.
- Shaukat A, Rector TS, Church TR, et al. Longer withdrawal time is associated with a reduced incidence of interval cancer after screening colonoscopy. Gastroenterology. 2015;149(4):952–957.
- Gong D, Wu L, Zhang J, et al. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol Hepatol. 2020;5(4):352–361.
- Su JR, Li Z, Shao XJ, et al. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: a prospective randomized controlled study (with videos). Gastrointest Endosc. 2020;91(2):415–424.e4.
- Yao L, Zhang L, Liu J, et al. An artificial intelligence-based quality improvement system significantly improved the efficacy of computer-aided detection system in colonoscopy: a four group parallel study. Endoscopy. 2022;54(8):757–768.
- ClinicalTrials.gov. A single center study on comparing the different function of endoangel in improving the quality of colonscopy. U.S. National Library of Medicine.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
- Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3 Pt 2):620–625.
- Penn E, Garrow D, Romagnuolo J. Influence of race and sex on prevalence and recurrence of Colon polyps. Arch Intern Med. 2010;170(13):1127–1132.
- Lucas BD, Kanade T. An iterative image registration technique with an application to stereo vision. In: Proceedings of the 7th International Joint Conference on Artificial Intelligence—Volume 2; 1981. San Francisco, CA: Morgan Kaufmann Publishers Inc. p. 674–679.
- Zhao S, Wang S, Pan P, et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: a systematic review and meta-analysis. Gastroenterology. 2019;156(6):1661–1674.e11. May 1
- Robertson DJ, Lieberman DA, Winawer SJ, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63(6):949–956.
- McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267–277.
- Barua I, Bretthauer M, Mori Y. Computer-aided quality assessment (CAQ): the next step for artificial intelligence in colonoscopy? Mini-Invasive Surg. 2022;6(5):28.